CIESC Journal ›› 2022, Vol. 73 ›› Issue (11): 4826-4837.DOI: 10.11949/0438-1157.20221029
• Thermodynamics • Previous Articles Next Articles
Chenyang ZHU1( ), Xiangyang LIU2, Maogang HE2, Guangjin CHEN3(
), Xiangyang LIU2, Maogang HE2, Guangjin CHEN3( )
)
Received:2022-07-26
Revised:2022-09-21
Online:2022-12-06
Published:2022-11-05
Contact:
Guangjin CHEN
通讯作者:
陈光进
作者简介:朱晨阳(1992—),男,博士,讲师,zcyzhu@cup.edu.cn
基金资助:CLC Number:
Chenyang ZHU, Xiangyang LIU, Maogang HE, Guangjin CHEN. Viscosity estimation of fluid mixtures based on Eyring's absolute rate theory[J]. CIESC Journal, 2022, 73(11): 4826-4837.
朱晨阳, 刘向阳, 何茂刚, 陈光进. 基于Eyring绝对速率理论的流体混合物黏度推算[J]. 化工学报, 2022, 73(11): 4826-4837.
Add to citation manager EndNote|Ris|BibTeX
| 作者 | 流动活化能① | 温度/K | 压力/MPa | 物质 | 文献 |
|---|---|---|---|---|---|
| Kincaid等 | ΔG*=ΔUvap/2.45 | 273~353 | 饱和压力 | 纯质 | [ |
| Lei等 | ΔG*=αΔUvap | 77~653 | 饱和压力 | 纯质 | [ |
| Macías-Salinas等 | (ΔG*/RT)=α(ΔUvap/RT) β | 20~623 | 0.1~100 | 纯质 | [ |
| ΔG*E=αGE | 275~350 | 0.1~65 | 二元混合物 | [ | |
| Martins等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| ΔG*E=GE | 283~328 | 0.1 | 二元混合物 | [ | |
| Liu等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| Han等 | ΔG*=0.175NAε | 283~353 | 饱和压力 | 纯质 | [ |
| Tochigi等 | ΔG*E=αGE | 298~370 | 0.1~100 | 二元混合物 | [ |
| Wang等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| He等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| Atashrouz等 | ΔG*E=GE | 278~353 | 0.1 | 二元混合物 | [ |
| Xu等 | ΔG*E=GE | 298~343 | 0.1 | 二、三元混合物 | [ |
Table 1 Summary of absolute rate theory models for the viscosities of fluids
| 作者 | 流动活化能① | 温度/K | 压力/MPa | 物质 | 文献 |
|---|---|---|---|---|---|
| Kincaid等 | ΔG*=ΔUvap/2.45 | 273~353 | 饱和压力 | 纯质 | [ |
| Lei等 | ΔG*=αΔUvap | 77~653 | 饱和压力 | 纯质 | [ |
| Macías-Salinas等 | (ΔG*/RT)=α(ΔUvap/RT) β | 20~623 | 0.1~100 | 纯质 | [ |
| ΔG*E=αGE | 275~350 | 0.1~65 | 二元混合物 | [ | |
| Martins等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| ΔG*E=GE | 283~328 | 0.1 | 二元混合物 | [ | |
| Liu等 | ΔG*-ΔG0*=A-A0 | 65~460 | 0.1~253 | 纯质 | [ |
| Han等 | ΔG*=0.175NAε | 283~353 | 饱和压力 | 纯质 | [ |
| Tochigi等 | ΔG*E=αGE | 298~370 | 0.1~100 | 二元混合物 | [ |
| Wang等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| He等 | ΔG*E=GE | 298~343 | 0.1 | 二元混合物 | [ |
| Atashrouz等 | ΔG*E=GE | 278~353 | 0.1 | 二元混合物 | [ |
| Xu等 | ΔG*E=GE | 298~343 | 0.1 | 二、三元混合物 | [ |
| 物质 | T/K | p/MPa | 参数 | AAD/% | MD/% | 数据点数 | 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a1 | 102a2 | b | c | d | |||||||
| 正戊烷 | 298~373 | 0.1~25 | 0.2455 | -5.1312 | 9.3775 | 5.1283 | -1.9272 | 1.07 | 2.99 | 31 | [ |
| 正己烷 | 273~373 | 0.1~205 | 0.1420 | -1.0610 | 2.1592 | 2.7046 | 0.1682 | 2.28 | 5.10 | 38 | [ |
| 正庚烷 | 298~474 | 0.1~100 | 0.1497 | -1.0627 | -0.5700 | 7.8378 | 0.6419 | 1.30 | 3.23 | 33 | [ |
| 正辛烷 | 273~467 | 0.1~203 | 0.1163 | -0.4310 | 1.1151 | 4.1198 | 0.2715 | 2.61 | 7.26 | 104 | [ |
| 正壬烷 | 300~420 | 0.1~50 | 0.1431 | -0.8425 | 2.6574 | 1.9450 | -0.0004 | 0.44 | 3.04 | 220 | [ |
| 正癸烷 | 293~373 | 0.1~100 | 0.1278 | -0.7410 | 3.4988 | 1.1772 | -0.1271 | 1.93 | 5.19 | 66 | [ |
| 正十二烷 | 293~373 | 0.1~200 | 0.0774 | 0.1410 | 3.7887 | 1.0225 | -0.1582 | 1.97 | 8.62 | 112 | [ |
| 正十三烷 | 293~353 | 0.1~100 | 0.0728 | 0.1678 | 2.8687 | 1.8857 | -0.0281 | 2.12 | 6.76 | 42 | [ |
| 正十四烷 | 313~393 | 0.7~60 | 0.1041 | -0.7250 | 2.7749 | 1.7020 | -0.0872 | 1.84 | 4.44 | 40 | [ |
| 正十六烷 | 298~413 | 0.1~202 | 0.0534 | 0.2920 | 3.6739 | 1.1963 | -0.1388 | 2.30 | 7.94 | 117 | [ |
| 环己烷 | 298~413 | 0.1~100 | 0.2955 | -2.2960 | 2.2599 | 2.1360 | -0.0745 | 2.60 | 7.01 | 120 | [ |
| 2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | 0.1673 | -1.2950 | 3.1482 | 1.4400 | -0.0796 | 2.10 | 6.11 | 98 | [ |
| 七甲基壬烷 | 293~353 | 0.1~100 | 0.0786 | 0.8681 | 4.3655 | 0.8666 | -0.2015 | 0.96 | 2.89 | 42 | [ |
| 甲基环己烷 | 293~353 | 0.1~100 | 0.2426 | -2.0455 | -0.0096 | 5.9618 | 0.3825 | 1.17 | 3.20 | 42 | [ |
| 苯 | 298~393 | 0.1~178 | 0.1615 | 0.8100 | 3.2339 | 1.3008 | -0.1247 | 1.58 | 5.78 | 70 | [ |
| 甲苯 | 293~373 | 0.1~203 | 0.1697 | -1.1530 | 3.2276 | 1.3479 | -0.0745 | 1.33 | 6.28 | 201 | [ |
| 壬苯 | 313~353 | 0.1~40 | 0.1169 | -0.4350 | 0.3116 | 4.3219 | 0.1644 | 1.13 | 2.72 | 15 | [ |
| 1,2,4-三甲基苯 | 293~353 | 0.1~140 | 0.0699 | 0.9220 | 1.9018 | 2.8515 | 0.1272 | 0.57 | 2.40 | 56 | [ |
| 甲基萘 | 293~353 | 0.1~100 | 0.0859 | 0.8097 | 4.8653 | 0.5503 | -0.2135 | 2.11 | 6.14 | 42 | [ |
| 顺式十氢化萘 | 293~353 | 0.1~100 | 0.1482 | 0.3316 | 3.8357 | 1.1811 | -0.1438 | 1.25 | 3.12 | 42 | [ |
| 辛酸乙酯 | 288~362 | 0.1~30 | 0.1466 | -1.1153 | 3.8311 | 0.9913 | -0.1722 | 0.75 | 2.26 | 87 | [ |
| 十二酸甲酯 | 283~353 | 0.1~200 | 0.0483 | 0.5054 | 4.3916 | 0.7843 | -0.1991 | 1.35 | 8.57 | 105 | [ |
| 十二酸乙酯 | 283~354 | 0.1~200 | 0.0407 | 0.5081 | 4.2869 | 0.8756 | -0.1826 | 1.65 | 8.29 | 109 | [ |
| 乙醇 | 293~353 | 0.1~100 | 0.2585 | -2.7712 | 0.5546 | 4.3166 | 0.0860 | 1.13 | 5.25 | 59 | [ |
| 丁醇 | 293~353 | 0.1~140 | 0.2197 | -1.5720 | 1.3790 | 2.8759 | -0.0894 | 1.45 | 6.91 | 98 | [ |
| 异丙醇 | 298~343 | 0.1~118 | 0.2785 | -2.5450 | 2.5620 | 1.5637 | -0.2813 | 1.46 | 3.43 | 44 | [ |
| 二丙酮醇 | 303~343 | 0.1~100 | 0.1243 | 1.3320 | 3.7976 | 1.0327 | -0.2458 | 0.78 | 2.13 | 18 | [ |
| 总体 | 1.54 | 8.62 | |||||||||
Table 2 Calculated viscosity of pure fluids
| 物质 | T/K | p/MPa | 参数 | AAD/% | MD/% | 数据点数 | 文献 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a1 | 102a2 | b | c | d | |||||||
| 正戊烷 | 298~373 | 0.1~25 | 0.2455 | -5.1312 | 9.3775 | 5.1283 | -1.9272 | 1.07 | 2.99 | 31 | [ |
| 正己烷 | 273~373 | 0.1~205 | 0.1420 | -1.0610 | 2.1592 | 2.7046 | 0.1682 | 2.28 | 5.10 | 38 | [ |
| 正庚烷 | 298~474 | 0.1~100 | 0.1497 | -1.0627 | -0.5700 | 7.8378 | 0.6419 | 1.30 | 3.23 | 33 | [ |
| 正辛烷 | 273~467 | 0.1~203 | 0.1163 | -0.4310 | 1.1151 | 4.1198 | 0.2715 | 2.61 | 7.26 | 104 | [ |
| 正壬烷 | 300~420 | 0.1~50 | 0.1431 | -0.8425 | 2.6574 | 1.9450 | -0.0004 | 0.44 | 3.04 | 220 | [ |
| 正癸烷 | 293~373 | 0.1~100 | 0.1278 | -0.7410 | 3.4988 | 1.1772 | -0.1271 | 1.93 | 5.19 | 66 | [ |
| 正十二烷 | 293~373 | 0.1~200 | 0.0774 | 0.1410 | 3.7887 | 1.0225 | -0.1582 | 1.97 | 8.62 | 112 | [ |
| 正十三烷 | 293~353 | 0.1~100 | 0.0728 | 0.1678 | 2.8687 | 1.8857 | -0.0281 | 2.12 | 6.76 | 42 | [ |
| 正十四烷 | 313~393 | 0.7~60 | 0.1041 | -0.7250 | 2.7749 | 1.7020 | -0.0872 | 1.84 | 4.44 | 40 | [ |
| 正十六烷 | 298~413 | 0.1~202 | 0.0534 | 0.2920 | 3.6739 | 1.1963 | -0.1388 | 2.30 | 7.94 | 117 | [ |
| 环己烷 | 298~413 | 0.1~100 | 0.2955 | -2.2960 | 2.2599 | 2.1360 | -0.0745 | 2.60 | 7.01 | 120 | [ |
| 2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | 0.1673 | -1.2950 | 3.1482 | 1.4400 | -0.0796 | 2.10 | 6.11 | 98 | [ |
| 七甲基壬烷 | 293~353 | 0.1~100 | 0.0786 | 0.8681 | 4.3655 | 0.8666 | -0.2015 | 0.96 | 2.89 | 42 | [ |
| 甲基环己烷 | 293~353 | 0.1~100 | 0.2426 | -2.0455 | -0.0096 | 5.9618 | 0.3825 | 1.17 | 3.20 | 42 | [ |
| 苯 | 298~393 | 0.1~178 | 0.1615 | 0.8100 | 3.2339 | 1.3008 | -0.1247 | 1.58 | 5.78 | 70 | [ |
| 甲苯 | 293~373 | 0.1~203 | 0.1697 | -1.1530 | 3.2276 | 1.3479 | -0.0745 | 1.33 | 6.28 | 201 | [ |
| 壬苯 | 313~353 | 0.1~40 | 0.1169 | -0.4350 | 0.3116 | 4.3219 | 0.1644 | 1.13 | 2.72 | 15 | [ |
| 1,2,4-三甲基苯 | 293~353 | 0.1~140 | 0.0699 | 0.9220 | 1.9018 | 2.8515 | 0.1272 | 0.57 | 2.40 | 56 | [ |
| 甲基萘 | 293~353 | 0.1~100 | 0.0859 | 0.8097 | 4.8653 | 0.5503 | -0.2135 | 2.11 | 6.14 | 42 | [ |
| 顺式十氢化萘 | 293~353 | 0.1~100 | 0.1482 | 0.3316 | 3.8357 | 1.1811 | -0.1438 | 1.25 | 3.12 | 42 | [ |
| 辛酸乙酯 | 288~362 | 0.1~30 | 0.1466 | -1.1153 | 3.8311 | 0.9913 | -0.1722 | 0.75 | 2.26 | 87 | [ |
| 十二酸甲酯 | 283~353 | 0.1~200 | 0.0483 | 0.5054 | 4.3916 | 0.7843 | -0.1991 | 1.35 | 8.57 | 105 | [ |
| 十二酸乙酯 | 283~354 | 0.1~200 | 0.0407 | 0.5081 | 4.2869 | 0.8756 | -0.1826 | 1.65 | 8.29 | 109 | [ |
| 乙醇 | 293~353 | 0.1~100 | 0.2585 | -2.7712 | 0.5546 | 4.3166 | 0.0860 | 1.13 | 5.25 | 59 | [ |
| 丁醇 | 293~353 | 0.1~140 | 0.2197 | -1.5720 | 1.3790 | 2.8759 | -0.0894 | 1.45 | 6.91 | 98 | [ |
| 异丙醇 | 298~343 | 0.1~118 | 0.2785 | -2.5450 | 2.5620 | 1.5637 | -0.2813 | 1.46 | 3.43 | 44 | [ |
| 二丙酮醇 | 303~343 | 0.1~100 | 0.1243 | 1.3320 | 3.7976 | 1.0327 | -0.2458 | 0.78 | 2.13 | 18 | [ |
| 总体 | 1.54 | 8.62 | |||||||||
| 物质 | T/K | p/MPa | κ12 | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|---|
| 正戊烷+正辛烷 | 298~373 | 0.1~25 | 0.2225 | 2.95 | 9.13 | 295 | [ |
| 正戊烷+正癸烷 | 298~373 | 0.1~25 | 0.3476 | 2.45 | 8.51 | 312 | [ |
| 正庚烷+正己烷 | 303~323 | 0.1~72 | 0.0735 | 1.14 | 3.15 | 53 | [ |
| 正庚烷+正壬烷 | 303~323 | 0.1~72 | 0.0434 | 1.14 | 3.03 | 57 | [ |
| 正庚烷+正辛烷 | 298~473 | 0.1~10 | -0.0601 | 1.02 | 4.01 | 122 | [ |
| 正辛烷+正癸烷 | 298~373 | 0.1~25 | 0.0872 | 1.90 | 8.78 | 324 | [ |
| 正癸烷+正十六烷 | 313~353 | 0.1~100 | 0.1812 | 1.80 | 4.57 | 54 | [ |
| 正庚烷+甲基萘 | 303~343 | 0.1~100 | -0.4344 | 3.37 | 11.3 | 126 | [ |
| 正己烷+正十二烷 | 298~373 | 0.1~204 | 0.3014 | 3.21 | 5.67 | 15 | [ |
| 正辛烷+正十二烷 | 298~373 | 0.1~203 | 0.0916 | 3.13 | 6.99 | 21 | [ |
| 正十三烷+七甲基壬烷 | 293~353 | 0.1~100 | -0.0641 | 1.96 | 6.65 | 294 | [ |
| 正庚烷+壬苯 | 313~353 | 0.1~40 | 0.3640 | 3.93 | 9.53 | 105 | [ |
| 环己烷+苯 | 313~393 | 0.7~60 | -0.1587 | 3.02 | 9.08 | 160 | [ |
| 甲基环己烷+正庚烷 | 303~343 | 0.1~100 | -0.1338 | 1.38 | 5.53 | 126 | [ |
| 甲基环己烷+顺式十氢化萘 | 293~353 | 0.1~100 | -0.0347 | 1.91 | 6.55 | 294 | [ |
| 甲基环己烷+七甲基壬烷 | 293~353 | 0.1~100 | 0.2681 | 2.34 | 7.20 | 294 | [ |
| 苯+正十四烷 | 313~393 | 0.7~60 | 0.3191 | 3.38 | 10.5 | 160 | [ |
| 甲苯+正己烷 | 298~373 | 0.1~203 | 0.0283 | 4.12 | 12.9 | 84 | [ |
| 甲基萘+甲基环己烷 | 303~343 | 0.1~100 | -0.3382 | 3.62 | 10.0 | 126 | [ |
| 甲基萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.2426 | 2.00 | 6.78 | 294 | [ |
| 顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.0150 | 1.21 | 3.72 | 294 | [ |
| 辛酸乙酯+正十六烷 | 293~362 | 0.1~30 | 0.0050 | 1.99 | 4.77 | 168 | [ |
| 十二酸甲酯+辛酸乙酯 | 303~323 | 0.1~15 | 0.0243 | 1.48 | 3.79 | 162 | [ |
| 丁醇+2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | -0.5304 | 3.42 | 9.94 | 168 | [ |
| 丁醇+1,2,4-三甲基苯 | 293~353 | 0.1~140 | -0.4963 | 2.38 | 8.95 | 168 | [ |
| 十二酸乙酯+乙醇 | 303~323 | 0.1~15 | 0.6092 | 3.58 | 9.97 | 216 | [ |
| 异丙醇+二丙酮醇 | 303~343 | 0.1~100 | -0.0659 | 1.91 | 5.34 | 162 | [ |
| 总体 | 2.35 | 12.9 |
Table 3 Calculated viscosity of binary mixtures
| 物质 | T/K | p/MPa | κ12 | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|---|
| 正戊烷+正辛烷 | 298~373 | 0.1~25 | 0.2225 | 2.95 | 9.13 | 295 | [ |
| 正戊烷+正癸烷 | 298~373 | 0.1~25 | 0.3476 | 2.45 | 8.51 | 312 | [ |
| 正庚烷+正己烷 | 303~323 | 0.1~72 | 0.0735 | 1.14 | 3.15 | 53 | [ |
| 正庚烷+正壬烷 | 303~323 | 0.1~72 | 0.0434 | 1.14 | 3.03 | 57 | [ |
| 正庚烷+正辛烷 | 298~473 | 0.1~10 | -0.0601 | 1.02 | 4.01 | 122 | [ |
| 正辛烷+正癸烷 | 298~373 | 0.1~25 | 0.0872 | 1.90 | 8.78 | 324 | [ |
| 正癸烷+正十六烷 | 313~353 | 0.1~100 | 0.1812 | 1.80 | 4.57 | 54 | [ |
| 正庚烷+甲基萘 | 303~343 | 0.1~100 | -0.4344 | 3.37 | 11.3 | 126 | [ |
| 正己烷+正十二烷 | 298~373 | 0.1~204 | 0.3014 | 3.21 | 5.67 | 15 | [ |
| 正辛烷+正十二烷 | 298~373 | 0.1~203 | 0.0916 | 3.13 | 6.99 | 21 | [ |
| 正十三烷+七甲基壬烷 | 293~353 | 0.1~100 | -0.0641 | 1.96 | 6.65 | 294 | [ |
| 正庚烷+壬苯 | 313~353 | 0.1~40 | 0.3640 | 3.93 | 9.53 | 105 | [ |
| 环己烷+苯 | 313~393 | 0.7~60 | -0.1587 | 3.02 | 9.08 | 160 | [ |
| 甲基环己烷+正庚烷 | 303~343 | 0.1~100 | -0.1338 | 1.38 | 5.53 | 126 | [ |
| 甲基环己烷+顺式十氢化萘 | 293~353 | 0.1~100 | -0.0347 | 1.91 | 6.55 | 294 | [ |
| 甲基环己烷+七甲基壬烷 | 293~353 | 0.1~100 | 0.2681 | 2.34 | 7.20 | 294 | [ |
| 苯+正十四烷 | 313~393 | 0.7~60 | 0.3191 | 3.38 | 10.5 | 160 | [ |
| 甲苯+正己烷 | 298~373 | 0.1~203 | 0.0283 | 4.12 | 12.9 | 84 | [ |
| 甲基萘+甲基环己烷 | 303~343 | 0.1~100 | -0.3382 | 3.62 | 10.0 | 126 | [ |
| 甲基萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.2426 | 2.00 | 6.78 | 294 | [ |
| 顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | -0.0150 | 1.21 | 3.72 | 294 | [ |
| 辛酸乙酯+正十六烷 | 293~362 | 0.1~30 | 0.0050 | 1.99 | 4.77 | 168 | [ |
| 十二酸甲酯+辛酸乙酯 | 303~323 | 0.1~15 | 0.0243 | 1.48 | 3.79 | 162 | [ |
| 丁醇+2,2,4-三甲基戊烷 | 293~353 | 0.1~140 | -0.5304 | 3.42 | 9.94 | 168 | [ |
| 丁醇+1,2,4-三甲基苯 | 293~353 | 0.1~140 | -0.4963 | 2.38 | 8.95 | 168 | [ |
| 十二酸乙酯+乙醇 | 303~323 | 0.1~15 | 0.6092 | 3.58 | 9.97 | 216 | [ |
| 异丙醇+二丙酮醇 | 303~343 | 0.1~100 | -0.0659 | 1.91 | 5.34 | 162 | [ |
| 总体 | 2.35 | 12.9 |
| 物质 | T/K | p/MPa | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|
| 正戊烷+正辛烷+正癸烷 | 298~373 | 0.1~25 | 5.81 | 19.7 | 530 | [ |
| 正庚烷+甲基环己烷+甲基萘 | 303~343 | 0.1~100 | 4.04 | 10.8 | 378 | [ |
| 甲基环己烷+顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | 1.79 | 7.82 | 546 | [ |
| 总体 | 3.86 | 19.7 |
Table 4 Calculated viscosity of ternary mixtures
| 物质 | T/K | p/MPa | AAD/% | MD/% | 数据点数 | 文献 |
|---|---|---|---|---|---|---|
| 正戊烷+正辛烷+正癸烷 | 298~373 | 0.1~25 | 5.81 | 19.7 | 530 | [ |
| 正庚烷+甲基环己烷+甲基萘 | 303~343 | 0.1~100 | 4.04 | 10.8 | 378 | [ |
| 甲基环己烷+顺式十氢化萘+七甲基壬烷 | 293~353 | 0.1~100 | 1.79 | 7.82 | 546 | [ |
| 总体 | 3.86 | 19.7 |
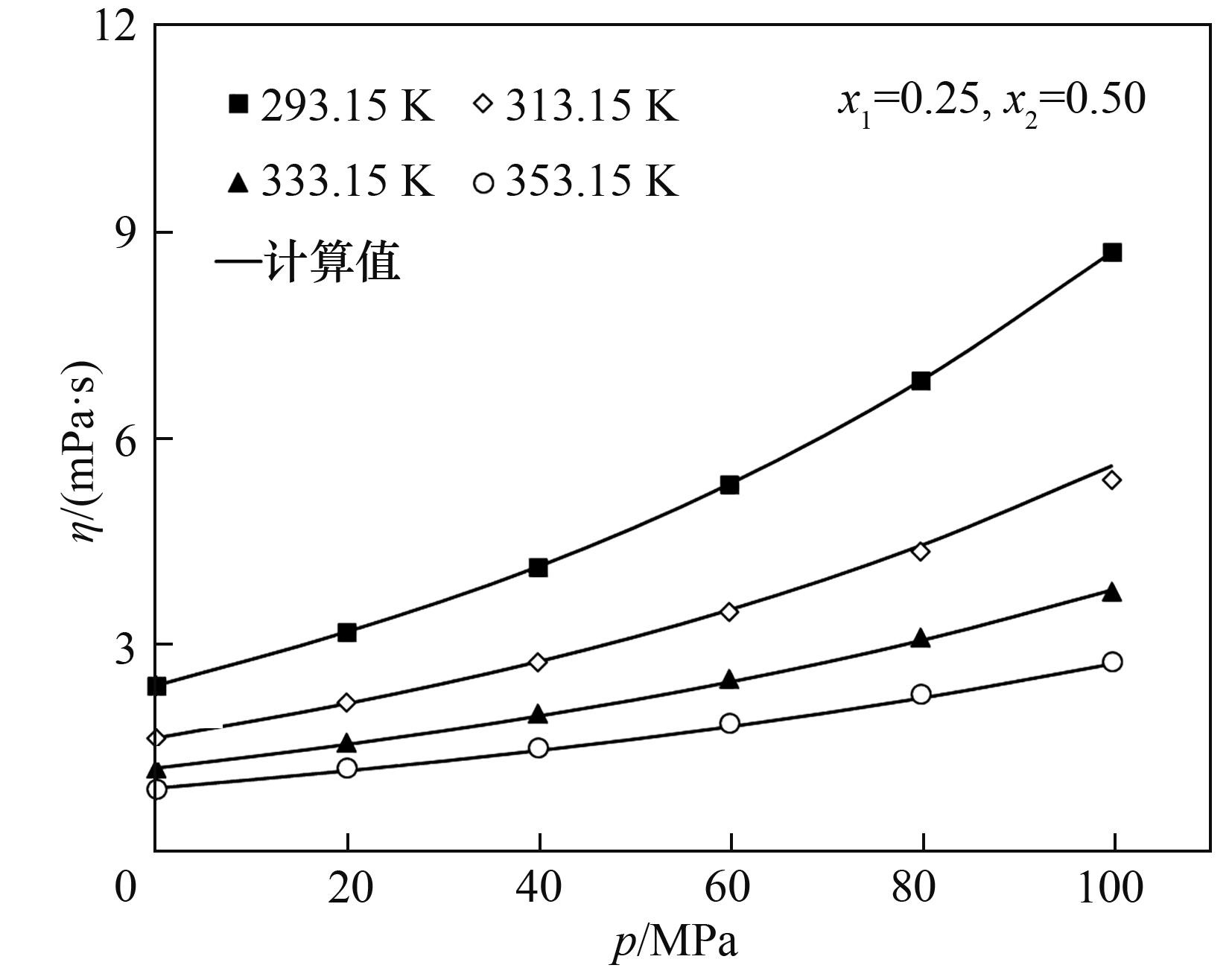
Fig.8 Comparison of viscosity of methylcyclohexane + cis-decaline + heptamethylnonane between results using the absolute rate theory model and experiment
| 1 | Liu H T, Yang F F, Yang Z, et al. Modeling the viscosity of hydrofluorocarbons, hydrofluoroolefins and their binary mixtures using residual entropy scaling and cubic-plus-association equation of state[J]. Journal of Molecular Liquids, 2020, 308: 113027. |
| 2 | 韩光泽, 房增科, 陈明东. Eyring黏度公式的几率修正及基于液体准晶模型分子活化能的计算[J]. 中国科学: 物理学 力学 天文学, 2010, 40(9): 1092-1098. |
| Han G Z, Fang Z K, Chen M D. Probability correction of Eyring viscosity equation and the calculation of activation energy based on the liquid quasicrystal model[J]. Scientia Sinica (Physica, Mechanica & Astronomica), 2010, 40(9): 1092-1098. | |
| 3 | 房升. 基于Eyring反应速率理论的溶液黏度模型[J]. 化学进展, 2010, 22(Z1): 309-314. |
| Fang S. Viscosity model for mixtures based on Eyring's absolute reaction theory[J]. Progress in Chemistry, 2010, 22(Z1): 309-314. | |
| 4 | Quiñones-Cisneros S E, Zéberg-Mikkelsen C K, Stenby E H. The friction theory (f-theory) for viscosity modeling[J]. Fluid Phase Equilibria, 2000, 169(2): 249-276. |
| 5 | Allal A, Boned C, Baylaucq A. Free-volume viscosity model for fluids in the dense and gaseous states[J]. Physical Review E, 2001, 64(1): 011203. |
| 6 | Allal A, Moha-ouchane M, Boned C. A new free volume model for dynamic viscosity and density of dense fluids versus pressure and temperature[J]. Physics and Chemistry of Liquids, 2001, 39(1): 1-30. |
| 7 | Kincaid J F, Eyring H, Stearn A E. The theory of absolute reaction rates and its application to viscosity and diffusion in the liquid state[J]. Chemical Reviews, 1941, 28(2): 301-365. |
| 8 | Lei Q F, Hou Y C, Lin R S. Correlation of viscosities of pure liquids in a wide temperature range[J]. Fluid Phase Equilibria, 1997, 140(1/2): 221-231. |
| 9 | Macías-Salinas R, García-Sánchez F, Hernández-Garduza O. Viscosity model for pure liquids based on Eyring theory and cubic EOS[J]. AIChE Journal, 2003, 49(3): 799-804. |
| 10 | Macías-Salinas R, Garcı́a-Sánchez F, Eliosa-Jiménez G. An equation-of-state-based viscosity model for non-ideal liquid mixtures[J]. Fluid Phase Equilibria, 2003, 210(2): 319-334. |
| 11 | Martins R J, de M Cardoso M J E, Barcia O E. A new model for calculating the viscosity of pure liquids at high pressures[J]. Industrial & Engineering Chemistry Research, 2003, 42(16): 3824-3830. |
| 12 | Martins R J, de M Cardoso M J E, Barcia O E. Excess Gibbs free energy model for calculating the viscosity of binary liquid mixtures[J]. Industrial & Engineering Chemistry Research, 2000, 39(3): 849-854. |
| 13 | Liu X Y, Zhu C Y, He M G, et al. Correlation for viscosities of pure liquids at high pressures[J]. Journal of Molecular Liquids, 2017, 231: 404-410. |
| 14 | Han G Z, Fang Z K, Chen M D. Modified Eyring viscosity equation and calculation of activation energy based on the liquid quasi-lattice model[J]. Science China Physics, Mechanics and Astronomy, 2010, 53(10): 1853-1860. |
| 15 | Tochigi K, Okamura T, Rattan V K. Prediction of high-pressure viscosities for binary liquid mixtures using the EOS-GE mixing rule with low-pressure viscosity data[J]. Fluid Phase Equilibria, 2007, 257(2): 228-232. |
| 16 | Wang Y G, Chen D X, Ouyang X K. Viscosity calculations for ionic liquid-cosolvent mixtures based on Eyring's absolute rate theory and activity coefficient models[J]. Journal of Chemical & Engineering Data, 2010, 55(11): 4878-4884. |
| 17 | He Y C, Xu X J, Yang L J, et al. Viscosity modeling for ionic liquid solutions by Eyring-Wilson equation[J]. Chemical Industry and Chemical Engineering Quarterly, 2012, 18(3): 441-447. |
| 18 | Atashrouz S, Zarghampour M, Abdolrahimi S, et al. Estimation of the viscosity of ionic liquids containing binary mixtures based on the Eyring's theory and a modified Gibbs energy model[J]. Journal of Chemical & Engineering Data, 2014, 59(11): 3691-3704. |
| 19 | Xu Y J, Tang X C, Li J H, et al. Viscosity estimation of ternary mixtures containing ionic liquid from their binary subsystems: a comparison of three viscosity equations[J]. Fluid Phase Equilibria, 2016, 427: 166-174. |
| 20 | 朱晨阳, 刘向阳, 何茂刚. 基于绝对速率理论的高压液体黏度模型[J]. 工程热物理学报, 2018, 39(7): 1407-1411. |
| Zhu C Y, Liu X Y, He M G. A viscosity model of liquid based on absolute rate theory at high pressures[J]. Journal of Engineering Thermophysics, 2018, 39(7): 1407-1411. | |
| 21 | He M G, Zhu C Y, Liu X Y. Estimating the viscosity of ionic liquid at high pressure using Eyring's absolute rate theory[J]. Fluid Phase Equilibria, 2018, 458: 170-176. |
| 22 | Zhu C Y, Yang F, Liu X Y, et al. Viscosity of oxygenated fuel: a model based on Eyring's absolute rate theory[J]. Fuel, 2019, 241: 218-226. |
| 23 | 刘国杰, 胡英. 液体的黏度与内压[J]. 化学学报, 1991, 49(7): 649-655. |
| Liu G J, Hu Y. Viscosity and internal pressure for liquids[J]. Acta Chimica Sinica, 1991, 49(7): 649-655. | |
| 24 | Poling B E, Prausnitz J M, O'connell J P. Properties of Gases and Liquids[M]. 5th ed. New York: McGraw-Hill Education, 2001: A.5-A.19. |
| 25 | Grunberg L, Nissan A H. Mixture law for viscosity[J]. Nature, 1949, 164(4175): 799-800. |
| 26 | Yaws C L, Narasimhan P K. Critical properties and acentric factor—organic compounds[M]//Thermophysical Properties of Chemicals and Hydrocarbons. New York: William Andrew Publishing, 2009: 1-95. |
| 27 | Design Institute for Physical Property Data. DIPPR 801 database[DB]. New York: AIChE, 1998. |
| 28 | Comuñas M J P, Baylaucq A, Plantier F, et al. Influence of the number of CH2-CH2-O groups on the viscosity of polyethylene glycol dimethyl ethers at high pressure[J]. Fluid Phase Equilibria, 2004, 222/223: 331-338. |
| 29 | Estrada-Baltazar A, Iglesias-Silva G A, Barrufet M A. Liquid viscosities of pentane and pentane + decane from 298.15 K to 373.15 K and up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1998, 43(4): 601-604. |
| 30 | Dymond J H, Young K J, Isdale J D. Transport properties of nonelectrolyte liquid mixtures (Ⅱ): Viscosity coefficients for the n-hexane + n-hexadecane system at temperatures from 25 to 100℃ at pressures up to the freezing pressure or 500 MPa[J]. International Journal of Thermophysics, 1980, 1(4): 345-373. |
| 31 | Kumagai A, Tomida D, Yokoyama C. Measurements of the liquid viscosities of mixtures of n-butane, n-hexane, and n-octane with squalane to 30 MPa[J]. International Journal of Thermophysics, 2006, 27(2): 376-393. |
| 32 | Baylaucq A, Boned C, Dauge P, et al. Measurements of the viscosity and density of three hydrocarbons and the three associated binary mixtures versus pressure and temperature[J]. International Journal of Thermophysics, 1997, 18(1): 3-23. |
| 33 | Abdulagatov I M, Azizov N D. (p, ρ, T, x) and viscosity measurements of {x1 n-heptane + (1-x1)n-octane} mixtures at high temperatures and high pressures[J]. The Journal of Chemical Thermodynamics, 2006, 38(11): 1402-1415. |
| 34 | Barrufet M A, Hall K R, Estrada-Baltazar A, et al. Liquid viscosity of octane and pentane + octane mixtures from 298.15 K to 373.15 K up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1999, 44(6): 1310-1314. |
| 35 | Dymond J H, Robertson J, Isdale J D. Transport properties of nonelectrolyte liquid mixtures (Ⅲ): Viscosity coefficients for n-octane, n-dodecane, and equimolar mixtures of n-octane + n-dodecane and n-hexane + n-dodecane from 25 to 100℃ at pressures up to the freezing pressure or 500 MPa[J]. International Journal of Thermophysics, 1981, 2(2): 133-154. |
| 36 | Tanaka Y, Hosokawa H, Kubota H, et al. Viscosity and density of binary mixtures of cyclohexane with n-octane, n-dodecane, and n-hexadecane under high pressures[J]. International Journal of Thermophysics, 1991, 12(2): 245-264. |
| 37 | Stephan K. Numerical data on viscosity[M]//Viscosity of Dense Fluids. Boston: Springer, 1979: 188-190. |
| 38 | Estrada-Baltazar A, Alvarado J F J, Iglesias-Silva G A, et al. Experimental liquid viscosities of decane and octane + decane from 298.15 K to 373.15 K and up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1998, 43(3): 441-446. |
| 39 | Ducoulombier D, Zhou H, Boned C, et al. Pressure (1—1000 bars) and temperature (20—100℃) dependence of the viscosity of liquid hydrocarbons[J]. The Journal of Physical Chemistry, 1986, 90(8): 1692-1700. |
| 40 | Zambrano J R, Sobrino M, Martín M C, et al. Contributing to accurate high pressure viscosity measurements: vibrating wire viscometer and falling body viscometer techniques[J]. The Journal of Chemical Thermodynamics, 2016, 96: 104-116. |
| 41 | Daugé P, Canet X, Baylaucq A, et al. Measurements of the density and viscosity of the tridecane + 2,2,4,4,6,8,8-heptamethylnonane mixtures in the temperature range 293.15—353.15 K at pressures up to 100 MPa[J]. High Temperatures-High Pressures, 2001, 33(2): 213-230. |
| 42 | Hernández-Galván M A, García-Sánchez F, Macías-Salinas R. Liquid viscosities of benzene, n-tetradecane, and benzene + n-tetradecane from 313 to 393 K and pressures up to 60 MPa: experiment and modeling[J]. Fluid Phase Equilibria, 2007, 262(1/2): 51-60. |
| 43 | Rajagopal K, Andrade L L P R, Paredes M L L. High-pressure viscosity measurements for the binary system cyclohexane + n-hexadecane in the temperature range of (318.15 to 413.15) K[J]. Journal of Chemical & Engineering Data, 2009, 54(10): 2967-2970. |
| 44 | Hernández-Galván M A, García-Sánchez F, García-Flores B E, et al. Liquid viscosities of cyclohexane, cyclohexane + tetradecane, and cyclohexane + benzene from (313 to 393) K and pressures up to 60 MPa[J]. Journal of Chemical & Engineering Data, 2009, 54(10): 2831-2838. |
| 45 | Barrouhou M, Zéberg-Mikkelsen C K, Baylaucq A, et al. High-pressure viscosity and density measurements for the asymmetric binary system cis-decalin + 2,2,4,4,6,8,8-heptamethylnonane[J]. International Journal of Thermophysics, 2003, 24(4): 937-952. |
| 46 | Zéberg-Mikkelsen C K, Barrouhou M, Baylaucq A, et al. Viscosity and density measurements of binary mixtures composed of methylcyclohexane + cis-decalin versus temperature and pressure[J]. International Journal of Thermophysics, 2003, 24(2): 361-374. |
| 47 | Vieira dos Santos F J, Nieto de Castro C A. Viscosity of toluene and benzene under high pressure[J]. International Journal of Thermophysics, 1997, 18(2): 367-378. |
| 48 | Dymond J H, Awan M A, Glen N F, et al. Transport properties of nonelectrolyte liquid mixtures (Ⅷ): Viscosity coefficients for toluene and for three mixtures of toluene + hexane from 25 to 100℃ at pressures up to 500 MPa[J]. International Journal of Thermophysics, 1991, 12(2): 275-287. |
| 49 | Zéberg-Mikkelsen C K, Baylaucq A, Watson G, et al. High-pressure viscosity measurements for the ethanol + toluene binary system[J]. International Journal of Thermophysics, 2005, 26(5): 1289-1302. |
| 50 | Kanti M, Lagourette B, Alliez J, et al. Viscosity of binary heptane-nonylbenzene as a function of pressure and temperature: application of Flory's theory[J]. Fluid Phase Equilibria, 1991, 65: 291-304. |
| 51 | Canet X, Daugé P, Baylaucq A, et al. Density and viscosity of the 1-methylnaphthalene + 2,2,4,4,6,8,8-heptamethylnonane system from 293.15 to 353.15 K at pressures up to 100 MPa[J]. International Journal of Thermophysics, 2001, 22(6): 1669-1689. |
| 52 | Sheu Y W, Tu C H. Densities, viscosities, refractive indices, and surface tensions for 12 flavor esters from T= 288.15 K to T= 358.15 K[J]. Journal of Chemical & Engineering Data, 2005, 50(5): 1706-1710. |
| 53 | Liu X Y, Lai T W, Guo X D, et al. Densities and viscosities of ethyl heptanoate and ethyl octanoate at temperatures from 303 to 353 K and at pressures up to 15 MPa[J]. Journal of Chemical & Engineering Data, 2017, 62(8): 2454-2460. |
| 54 | Wang X J, Du W B, Wang X P. Liquid viscosities of ethyl caprylate and ethyl caprate at elevated temperatures and pressures[J]. Journal of Molecular Liquids, 2020, 309: 113203. |
| 55 | Pratas M J, Freitas S, Oliveira M B, et al. Densities and viscosities of fatty acid methyl and ethyl esters[J]. Journal of Chemical & Engineering Data, 2010, 55(9): 3983-3990. |
| 56 | He M G, Lai T W, Liu X Y. Measurement and correlation of viscosities and densities of methyl dodecanoate and ethyl dodecanoate at elevated pressures[J]. Thermochimica Acta, 2018, 663: 85-92. |
| 57 | Habrioux M, Nasri D, Daridon J L. Measurement of speed of sound, density compressibility and viscosity in liquid methyl laurate and ethyl laurate up to 200 MPa by using acoustic wave sensors[J]. The Journal of Chemical Thermodynamics, 2018, 120: 1-12. |
| 58 | Assael M J, Polimatidou S K. Measurements of the viscosity of alcohols in the temperature range 290—340 K at pressures up to 30 MPa[J]. International Journal of Thermophysics, 1994, 15(1): 95-107. |
| 59 | Tanaka Y, Matsuda Y, Fujiwara H, et al. Viscosity of (water + alcohol) mixtures under high pressure[J]. International Journal of Thermophysics, 1987, 8(2): 147-163. |
| 60 | Zambrano J, Martín M C, Martín Á, et al. Viscosities of binary mixtures containing 1-butanol + 2,2,4-trimethylpentane or + 1,2,4-trimethylbenzene at high pressures for the thermophysical characterization of biofuels[J]. The Journal of Chemical Thermodynamics, 2016, 102: 140-146. |
| 61 | Moha-Ouchane M, Boned C, Allal A, et al. Viscosity and excess volume at high pressures in associative binaries[J]. International Journal of Thermophysics, 1998, 19(1): 161-189. |
| 62 | Assael M J, Charitidou E, Dymond J H, et al. Viscosity and thermal conductivity of binary n-heptane + n-alkane mixtures[J]. International Journal of Thermophysics, 1992, 13(2): 237-249. |
| 63 | Zéberg-Mikkelsen C K, Barrouhou M, Baylaucq A, et al. Measurements of the viscosity and density versus temperature and pressure for the binary system methylcyclohexane + 2,2,4,4,6,8,8-heptamethylnonane[J]. High Temperatures-High Pressures, 2002, 34(5): 591-601. |
| 64 | Wang X P, Wen K, Wang X J. High-pressure liquid viscosity of n-hexadecane/ethyl octanoate mixtures[J]. Journal of Chemical & Engineering Data, 2021, 66(2): 1185-1190. |
| 65 | Liu X Y, Yang F, Lai T W, et al. Densities and viscosities of mixtures of methyl dodecanoate + ethyl octanoate at pressures up to 15 MPa[J]. Journal of Chemical & Engineering Data, 2018, 63(11): 4085-4094. |
| 66 | Zhu C Y, Zhang Z W, Xue S, et al. Association effect on the density, viscosity and excess properties of fatty acid ester+alcohol mixtures: experiment and modeling[J]. Fuel, 2022, 316: 123425. |
| 67 | Soave G. Equilibrium constants from a modified Redlich-Kwong equation of state[J]. Chemical Engineering Science, 1972, 27(6): 1197-1203. |
| 68 | Iglesias-Silva G A, Estrada-Baltazar A, Hall K R, et al. Experimental liquid viscosity of pentane+octane+decane mixtures from 298.15 to 373.15 K up to 25 MPa[J]. Journal of Chemical & Engineering Data, 1999, 44(6): 1304-1309. |
| 69 | Baylaucq A, Daugé P, Boned C. Viscosity and density of the ternary mixture heptane + methylcyclohexane + 1-methylnaphthalene[J]. International Journal of Thermophysics, 1997, 18(5): 1089-1107. |
| 70 | Zéberg-Mikkelsen C K, Barrouhou M, Baylaucq A, et al. High-pressure viscosity and density measurements of the ternary system methylcyclohexane+cis-decalin+2,2,4,4,6,8,8-heptamethylnonane[J]. Journal of Chemical & Engineering Data, 2003, 48(6): 1387-1392. |
| 71 | Khemka Y, Sisco C J, Abutaqiya M I L, et al. One-parameter friction theory viscosity model for the cubic-plus-chain equation of state[J]. Fluid Phase Equilibria, 2021, 530: 112896. |
| 72 | Khosharay S, Karimi R, Khosharay K. Modeling the viscosity for (nC5+nC8), (nC5+nC10), (nC8+nC10) and (nC5+nC8+nC10) systems with Peng-Robinson viscosity equation of state[J]. Periodica Polytechnica Chemical Engineering, 2016, 60(4): 259-265. |
| 73 | Cain N, Roberts G, Kiserow D, et al. Modeling the thermodynamic and transport properties of decahydronaphthalene/propane mixtures: phase equilibria, density, and viscosity[J]. Fluid Phase Equilibria, 2011, 305(1): 25-33. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||