CIESC Journal ›› 2025, Vol. 76 ›› Issue (2): 686-694.DOI: 10.11949/0438-1157.20241074
• Separation engineering • Previous Articles
Jiaxin CUI( ), Mengfan YIN, Tao ZHENG, Han LIU, Rui ZHANG, Zhichang LIU, Haiyan LIU, Chunming XU, Xianghai MENG(
), Mengfan YIN, Tao ZHENG, Han LIU, Rui ZHANG, Zhichang LIU, Haiyan LIU, Chunming XU, Xianghai MENG( )
)
Received:2024-09-25
Revised:2024-11-04
Online:2025-03-10
Published:2025-03-25
Contact:
Xianghai MENG
崔家馨( ), 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海(
), 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海( )
)
通讯作者:
孟祥海
作者简介:崔家馨(1997—),女,博士研究生,2796867498@qq.com
基金资助:CLC Number:
Jiaxin CUI, Mengfan YIN, Tao ZHENG, Han LIU, Rui ZHANG, Zhichang LIU, Haiyan LIU, Chunming XU, Xianghai MENG. Application of aluminum-copper bimetallic ionic liquids in 1-hexene/n-hexane separation[J]. CIESC Journal, 2025, 76(2): 686-694.
崔家馨, 殷梦凡, 郑涛, 刘晗, 张睿, 刘植昌, 刘海燕, 徐春明, 孟祥海. 铝铜双金属离子液体在1-己烯/正己烷分离中的应用[J]. 化工学报, 2025, 76(2): 686-694.
Add to citation manager EndNote|Ris|BibTeX
| 萃取剂 | S | D | PI |
|---|---|---|---|
| N-甲酰基吗啉 | 2.12 | 0.046 | 0.10 |
| N-甲基吡咯烷酮 | 1.31 | 0.301 | 0.39 |
| 乙二醇 | 2.25 | 0.028 | 0.06 |
| [Bmim]Cl-1.0FeCl3 | 2.40 | 0.041 | 0.10 |
| [Bmim]Cl-1.0AlCl3 | 2.39 | 0.036 | 0.09 |
[Bmim]Cl-1.0CuCl [Bmim]Cl-1.5CuCl [Bmim]Cl-2.0CuCl | 2.96 4.39 6.41 | 0.034 0.034 0.039 | 0.10 0.15 0.25 |
| [Bmim]NTf2 | 1.82 | 0.142 | 0.26 |
| [Bmim]BF4 | 2.45 | 0.032 | 0.08 |
| [Bmim]PF6 | 2.53 | 0.042 | 0.11 |
| [Bmim]Cl-1.0AlCl3+[Bmim]Cl-2.0CuCl | 3.24 | 0.040 | 0.13 |
| [Bmim]Cl-0.6AlCl3-1.0CuCl | 6.33 | 0.073 | 0.46 |
Table 1 Olefin separation performance of extractants
| 萃取剂 | S | D | PI |
|---|---|---|---|
| N-甲酰基吗啉 | 2.12 | 0.046 | 0.10 |
| N-甲基吡咯烷酮 | 1.31 | 0.301 | 0.39 |
| 乙二醇 | 2.25 | 0.028 | 0.06 |
| [Bmim]Cl-1.0FeCl3 | 2.40 | 0.041 | 0.10 |
| [Bmim]Cl-1.0AlCl3 | 2.39 | 0.036 | 0.09 |
[Bmim]Cl-1.0CuCl [Bmim]Cl-1.5CuCl [Bmim]Cl-2.0CuCl | 2.96 4.39 6.41 | 0.034 0.034 0.039 | 0.10 0.15 0.25 |
| [Bmim]NTf2 | 1.82 | 0.142 | 0.26 |
| [Bmim]BF4 | 2.45 | 0.032 | 0.08 |
| [Bmim]PF6 | 2.53 | 0.042 | 0.11 |
| [Bmim]Cl-1.0AlCl3+[Bmim]Cl-2.0CuCl | 3.24 | 0.040 | 0.13 |
| [Bmim]Cl-0.6AlCl3-1.0CuCl | 6.33 | 0.073 | 0.46 |
| Path | N | R/Å | σ2/Å2 |
|---|---|---|---|
| Cu—Cl | 2.00 | 2.21 | 0.013 |
| Cu—Al | 1.45 | 2.93 | 0.024 |
Table 2 Curve-fit parameters for Cu K-edge XAFS
| Path | N | R/Å | σ2/Å2 |
|---|---|---|---|
| Cu—Cl | 2.00 | 2.21 | 0.013 |
| Cu—Al | 1.45 | 2.93 | 0.024 |
| 离子液体+1-己烯/正己烷 | ΔE/(kJ/mol) |
|---|---|
| [Bmim][CuCl2]+1-己烯 | -89.79 |
| [Bmim][CuAlCl5]+1-己烯 | -138.08 |
| [Bmim][CuCl2]+正己烷 | -24.89 |
| [Bmim][CuAlCl5]+正己烷 | -19.99 |
Table 3 Comparison of binding energies of ionic liquids with 1-hexene and n-hexane
| 离子液体+1-己烯/正己烷 | ΔE/(kJ/mol) |
|---|---|
| [Bmim][CuCl2]+1-己烯 | -89.79 |
| [Bmim][CuAlCl5]+1-己烯 | -138.08 |
| [Bmim][CuCl2]+正己烷 | -24.89 |
| [Bmim][CuAlCl5]+正己烷 | -19.99 |
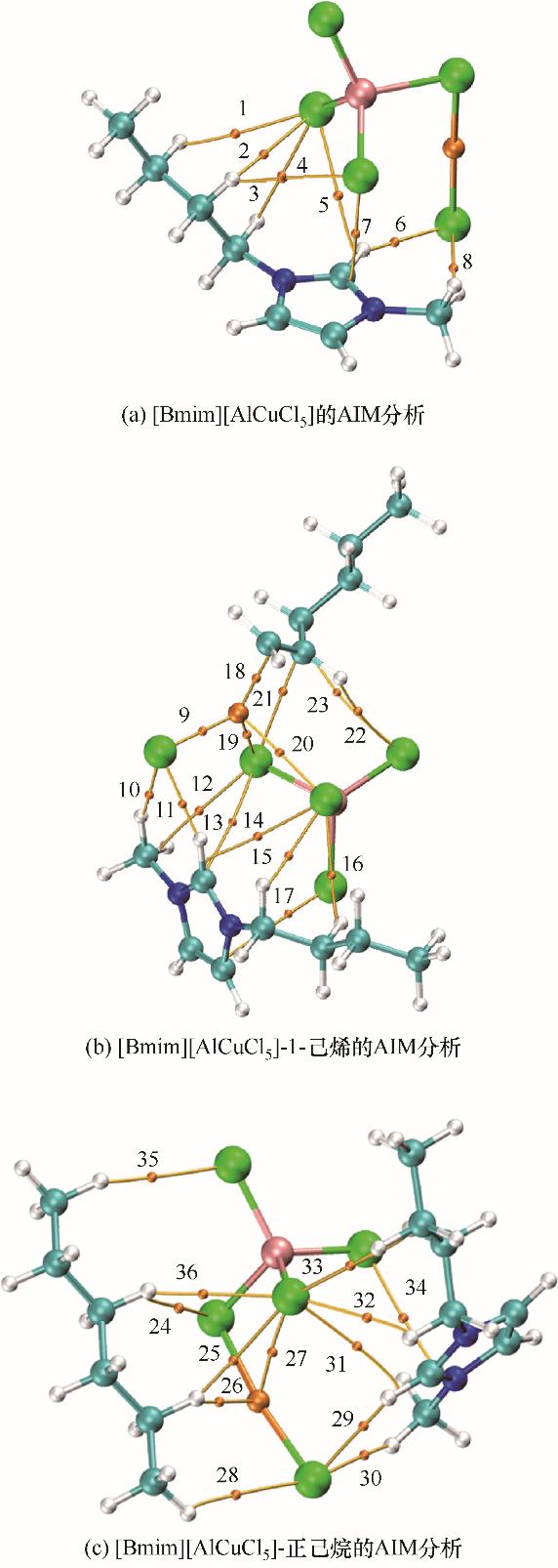
Fig.10 The AIM analysis for [Bmim][AlCuCl5], [Bmim][AlCuCl5]-1-hexene and [Bmim][AlCuCl5]-n-hexane (spheres represent the atoms; N: blue; H: white; C: cyan; Cl: green; Cu: orange; Al: pink)
| 体系 | BCP | 原子标签 | ρ(BCP) | ∇2ρ(BCP) | H(BCP) | |
|---|---|---|---|---|---|---|
| [Bmim][AlCuCl5] | 1 | C—H…Cl | 0.0046 | 0.0159 | 0.0009 | |
| 2 | C—H…Cl | 0.0072 | 0.0241 | 0.0012 | ||
| 3 | C—H…Cl | 0.0077 | 0.0257 | 0.0013 | ||
| 4 | C—H…Cl | 0.0045 | 0.0135 | 0.0007 | ||
| 5 | C—H…Cl | 0.0041 | 0.0142 | 0.0008 | ||
| 6 | C—H…Cl | 0.0174 | 0.0532 | 0.0019 | ||
| 7 | C…Cl | 0.0082 | 0.0279 | 0.0013 | ||
| 8 | C—H…Cl | 0.0131 | 0.0397 | 0.0017 | ||
| [Bmim][AlCuCl5]-1-己烯 | 9 | Cu…Cl | 0.0783 | 0.2614 | 0.0238 | |
| 10 | C—H…Cl | 0.0110 | 0.0328 | 0.0015 | ||
| 11 | C—H…Cl | 0.0217 | 0.0631 | 0.0015 | ||
| 12 | C…Cl | 0.0065 | 0.0239 | 0.0013 | ||
| 13 | C…Cl | 0.0036 | 0.0122 | 0.0008 | ||
| 14 | C…Cl | 0.0050 | 0.0168 | 0.0009 | ||
| 15 | C—H…Cl | 0.0068 | 0.0209 | 0.0010 | ||
| 16 | C—H…Cl | 0.0068 | 0.0232 | 0.0012 | ||
| 17 | C—H…Cl | 0.0077 | 0.0259 | 0.0013 | ||
| 18 | Cu…C | 0.0831 | 0.2393 | -0.0257 | ||
| 19 | Cu…Cl | 0.0463 | 0.1723 | -0.0061 | ||
| 20 | Cu…Cl | 0.0155 | 0.0433 | -0.0001 | ||
| 21 | C—H…Cl | 0.0075 | 0.0268 | 0.0014 | ||
| 22 | C—H…Cl | 0.0047 | 0.0152 | 0.0008 | ||
| 23 | C—H…Cl | 0.0027 | 0.0097 | 0.0006 | ||
| [Bmim][AlCuCl5]-正己烷 | 24 | C—H…Cl | 0.0052 | 0.0156 | 0.0008 | |
| 25 | C—H…Cl | 0.0064 | 0.0190 | 0.0009 | ||
| 26 | Cu…H | 0.0134 | 0.0337 | -0.0004 | ||
| 27 | Cu…Cl | 0.0145 | 0.0354 | -0.0003 | ||
| 28 | C—H…Cl | 0.0034 | 0.0110 | 0.0006 | ||
| 29 | C—H…Cl | 0.0176 | 0.0538 | 0.0019 | ||
| 30 | C—H…Cl | 0.0124 | 0.0376 | 0.0016 | ||
| 31 | C—H…Cl | 0.0049 | 0.0168 | 0.0010 | ||
| 32 | C—H…Cl | 0.0076 | 0.0257 | 0.0013 | ||
| 33 | C—H…Cl | 0.0041 | 0.0148 | 0.0009 | ||
| 34 | C—N…Cl | 0.0077 | 0.0269 | 0.0013 | ||
| 35 | C—H…Cl | 0.0038 | 0.0116 | 0.0006 | ||
| 36 | C—H…Cl | 0.0041 | 0.0143 | 0.0009 | ||
Table 4 Topological properties of BCPs
| 体系 | BCP | 原子标签 | ρ(BCP) | ∇2ρ(BCP) | H(BCP) | |
|---|---|---|---|---|---|---|
| [Bmim][AlCuCl5] | 1 | C—H…Cl | 0.0046 | 0.0159 | 0.0009 | |
| 2 | C—H…Cl | 0.0072 | 0.0241 | 0.0012 | ||
| 3 | C—H…Cl | 0.0077 | 0.0257 | 0.0013 | ||
| 4 | C—H…Cl | 0.0045 | 0.0135 | 0.0007 | ||
| 5 | C—H…Cl | 0.0041 | 0.0142 | 0.0008 | ||
| 6 | C—H…Cl | 0.0174 | 0.0532 | 0.0019 | ||
| 7 | C…Cl | 0.0082 | 0.0279 | 0.0013 | ||
| 8 | C—H…Cl | 0.0131 | 0.0397 | 0.0017 | ||
| [Bmim][AlCuCl5]-1-己烯 | 9 | Cu…Cl | 0.0783 | 0.2614 | 0.0238 | |
| 10 | C—H…Cl | 0.0110 | 0.0328 | 0.0015 | ||
| 11 | C—H…Cl | 0.0217 | 0.0631 | 0.0015 | ||
| 12 | C…Cl | 0.0065 | 0.0239 | 0.0013 | ||
| 13 | C…Cl | 0.0036 | 0.0122 | 0.0008 | ||
| 14 | C…Cl | 0.0050 | 0.0168 | 0.0009 | ||
| 15 | C—H…Cl | 0.0068 | 0.0209 | 0.0010 | ||
| 16 | C—H…Cl | 0.0068 | 0.0232 | 0.0012 | ||
| 17 | C—H…Cl | 0.0077 | 0.0259 | 0.0013 | ||
| 18 | Cu…C | 0.0831 | 0.2393 | -0.0257 | ||
| 19 | Cu…Cl | 0.0463 | 0.1723 | -0.0061 | ||
| 20 | Cu…Cl | 0.0155 | 0.0433 | -0.0001 | ||
| 21 | C—H…Cl | 0.0075 | 0.0268 | 0.0014 | ||
| 22 | C—H…Cl | 0.0047 | 0.0152 | 0.0008 | ||
| 23 | C—H…Cl | 0.0027 | 0.0097 | 0.0006 | ||
| [Bmim][AlCuCl5]-正己烷 | 24 | C—H…Cl | 0.0052 | 0.0156 | 0.0008 | |
| 25 | C—H…Cl | 0.0064 | 0.0190 | 0.0009 | ||
| 26 | Cu…H | 0.0134 | 0.0337 | -0.0004 | ||
| 27 | Cu…Cl | 0.0145 | 0.0354 | -0.0003 | ||
| 28 | C—H…Cl | 0.0034 | 0.0110 | 0.0006 | ||
| 29 | C—H…Cl | 0.0176 | 0.0538 | 0.0019 | ||
| 30 | C—H…Cl | 0.0124 | 0.0376 | 0.0016 | ||
| 31 | C—H…Cl | 0.0049 | 0.0168 | 0.0010 | ||
| 32 | C—H…Cl | 0.0076 | 0.0257 | 0.0013 | ||
| 33 | C—H…Cl | 0.0041 | 0.0148 | 0.0009 | ||
| 34 | C—N…Cl | 0.0077 | 0.0269 | 0.0013 | ||
| 35 | C—H…Cl | 0.0038 | 0.0116 | 0.0006 | ||
| 36 | C—H…Cl | 0.0041 | 0.0143 | 0.0009 | ||
| 1 | 石博文, 朱楠, 海红莲, 等. 煤制油费托α-烯烃增值利用及发展展望[J]. 合成材料老化与应用, 2023, 52(5): 113-116. |
| Shi B W, Zhu N, Hai H L, et al. Value-added utilization and development prospect of Fischer-α-olefin from coal to oil[J]. Synthetic Materials Aging and Application, 2023, 52(5): 113-116. | |
| 2 | 白玫. ACO技术制备烯烃工艺研究及展望[J]. 化工与医药工程, 2017, 38(3): 18-23. |
| Bai M. Research of ACO technique used in preparation of olefins and expectation[J]. Chemical and Pharmaceutical Engineering, 2017, 38(3): 18-23. | |
| 3 | Lin Y H, Yu L, Ullah S, et al. Temperature-programmed separation of hexane isomers by a porous calcium chloranilate metal-organic framework[J]. Angewandte Chemie International Edition, 2022, 61(50): e202214060. |
| 4 | Sholl D S, Lively R P. Seven chemical separations to change the world[J]. Nature, 2016, 532(7600): 435-437. |
| 5 | Zhu L, Li F F, Zhu J Q, et al. Liquid-liquid equilibria of ternary systems of 1-hexene/hexane and extraction solvents[J]. Chemical Papers, 2016, 70(5): 585-593. |
| 6 | 容凡丁, 丁泽相, 曹义风, 等. 离子液体强化不饱和键差异化合物分离的研究进展[J]. 化工进展, 2024, 43(1): 198-214. |
| Rong F D, Ding Z X, Cao Y F, et al. Progress in enhanced separation of compounds differing in unsaturated bonds by ionic liquids[J]. Chemical Industry and Engineering Progress, 2024, 43(1): 198-214. | |
| 7 | 吴沛文, 荀苏杭, 蒋伟, 等. 离子液体反应型萃取燃油脱硫研究进展[J]. 化工学报, 2021, 72(1): 276-291. |
| Wu P W, Xun S H, Jiang W, et al. Recent progress on extractive desulfurization of fuel oils through reactions based on ionic liquids as solvents and catalysts[J]. CIESC Journal, 2021, 72(1): 276-291. | |
| 8 | 吕玉苗, 陈伟, 王艳磊, 等. 离子液体二维结构制备及其特性研究进展[J]. 化学学报, 2021, 79(4): 443-458. |
| Lyu Y M, Chen W, Wang Y L, et al. Research progress on the preparation and properties of two dimensional structure of ionic liquids[J]. Acta Chimica Sinica, 2021, 79(4): 443-458. | |
| 9 | 李瑞, 崔现宝, 吴添, 等. 基于COSMO-SAC模型的离子液体萃取剂的选择[J]. 化工学报, 2013, 64(2): 452-469. |
| Li R, Cui X B, Wu T, et al. Selection of ionic liquid solvent for liquid-liquid extraction based on COSMO-SAC model[J]. CIESC Journal, 2013, 64(2): 452-469. | |
| 10 | Xing H B, Zhao X, Li R L, et al. Improved efficiency of ethylene/ethane separation using a symmetrical dual nitrile-functionalized ionic liquid[J]. ACS Sustainable Chemistry & Engineering, 2013, 1(11): 1357-1363. |
| 11 | Wang Y, Hao W Y, Jacquemin J, et al. Enhancing liquid-phase olefin-paraffin separations using novel silver-based ionic liquids[J]. Journal of Chemical & Engineering Data, 2015, 60(1): 28-36. |
| 12 | Li H, Zhang Z S, Sun G L, et al. Performance and mechanism of the separation of C8 α-olefin from F-T synthesis products using novel Ag-DES[J]. AIChE Journal, 2021, 67(8): e17252. |
| 13 | Yu G R, Deng L Y, Abdeltawab A A, et al. Functional solution composed of Cu(Ⅰ) salt and ionic liquids to separate propylene from propane[J]. Industrial & Engineering Chemistry Research, 2014, 53(34): 13430-13435. |
| 14 | Yu G R, Zhang L, Alhumaydhi I A, et al. Separation of propylene and propane by alkylimidazolium thiocyanate ionic liquids with Cu+ salt[J]. Separation and Purification Technology, 2015, 156: 356-362. |
| 15 | 张睿, 董淑媛, 伍洛, 等. 小分子烷烃与烯烃在离子液体中的溶解性能[J]. 化工学报, 2020, 71(10): 4674-4687. |
| Zhang R, Dong S Y, Wu L, et al. Solubility of light alkanes and alkenes in ionic liquids[J]. CIESC Journal, 2020, 71(10): 4674-4687. | |
| 16 | Chen X C, Ming S M, Wu X Y, et al. Cu(Ⅰ)-based ionic liquids as potential absorbents to separate propylene and propane[J]. Separation Science and Technology, 2013, 48(15): 2317-2323. |
| 17 | Wentink A E, Kockmann D, Kuipers N J M, et al. Effect of C6-olefin isomers on π-complexation for purification of 1-hexene by reactive extractive distillation[J]. Separation and Purification Technology, 2005, 43(2): 149-162. |
| 18 | Capracotta M D, Sullivan R M, Martin J D. Sorptive reconstruction of CuMCl4 (M = Al and Ga) upon small-molecule binding and the competitive binding of CO and ethylene[J]. Journal of the American Chemical Society, 2006, 128(41): 13463-13473. |
| 19 | Anantharaj R, Banerjee T. COSMO-RS-based screening of ionic liquids as green solvents in denitrification studies[J]. Industrial & Engineering Chemistry Research, 2010, 49(18): 8705-8725. |
| 20 | Adamo C, Barone V. Toward reliable density functional methods without adjustable parameters: the PBE0 model[J]. Journal of Chemical Physics, 1999, 110(13): 6158-6170. |
| 21 | Neese F. Software update: the ORCA program system, version 4.0[J]. WIREs Computational Molecular Science, 2018, 8(1): e1327. |
| 22 | Grimme S, Ehrlich S, Goerigk L. Effect of the damping function in dispersion corrected density functional theory[J]. Journal of Computational Chemistry, 2011, 32(7): 1456-1465. |
| 23 | Grimme S, Antony J, Ehrlich S, et al. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu[J]. The Journal of Chemical Physics, 2010, 132(15): 154104. |
| 24 | 倪清, 来锦波, 彭东岳, 等. 离子液体萃取分离烃类化合物的研究进展[J]. 化工进展, 2022, 41(2): 619-627. |
| Ni Q, Lai J B, Peng D Y, et al. Progress in extraction separation of hydrocarbons by ionic liquids[J]. Chemical Industry and Engineering Progress, 2022, 41(2): 619-627. | |
| 25 | Sasaki T, Tada M, Zhong C M, et al. Immobilized metal ion-containing ionic liquids: preparation, structure and catalytic performances in kharasch addition reaction and suzuki cross-coupling reactions[J]. Journal of Molecular Catalysis A: Chemical, 2008, 279(2): 200-209. |
| 26 | Fulton J L, Hoffmann M M, Darab J G. An X-ray absorption fine structure study of copper(Ⅰ) chloride coordination structure in water up to 325℃[J]. Chemical Physics Letters, 2000, 330(3/4): 300-308. |
| 27 | Schäafer H, Binnewies M, Laumanns R, et al. CuAl2Cl8. Darstellung und kristallstruktur[J]. Zeitschrift Für Anorganische und Allgemeine Chemie, 1980, 461(1): 31-34. |
| 28 | Safarik D J, Eldridge R B. Olefin/paraffin separations by reactive absorption: a review[J]. Industrial & Engineering Chemistry Research, 1998, 37(7): 2571-2581. |
| 29 | Bader R F W. Atoms in molecules[J]. Accounts of Chemical Research, 1985, 18(1): 9-15. |
| 30 | Lipkowski P, Grabowski S J, Robinson T L, et al. Properties of the C—H…H dihydrogen bond: an ab initio and topological analysis[J]. The Journal of Physical Chemistry A, 2004, 108(49): 10865-10872. |
| 31 | Cremer D, Kraka E. Chemical bonds without bonding electron density—Does the difference electron-density analysis suffice for a description of the chemical bond?[J]. Angewandte Chemie International Edition in English, 1984, 23(8): 627-628. |
| [1] | Haijun FENG, Bingxuan ZHANG, Jian ZHOU. Predicting and interpreting the toxicity of ionic liquids using graph neural network [J]. CIESC Journal, 2025, 76(1): 93-106. |
| [2] | Kuangxi LI, Peiqian YU, Jiangyun WANG, Haoran WEI, Zhigang ZHENG, Liuhai FENG. Flow analysis and structure optimization of micro-bubble swirling air flotation device [J]. CIESC Journal, 2024, 75(S1): 223-234. |
| [3] | Huihui XIE, Jiaxin JIANG, Xin WANG, Zheng LI, Xin GUO, Xinran LYU, Lingyun WANG, Yang LIU. Study on transport separation of platinum and palladium by deep eutectic solvent polymer inclusion membrane [J]. CIESC Journal, 2024, 75(S1): 235-243. |
| [4] | Zhi QIU, Ming TAN. Preparation of polyionic liquid membrane and its application in low-sodium and high-potassium healthy soy sauce [J]. CIESC Journal, 2024, 75(S1): 244-250. |
| [5] | Lü LIU, Jieru LIU, Liangliang FAN, Liang ZHAO. Study on passive microfluidic method for particle separation based on laminar effect [J]. CIESC Journal, 2024, 75(S1): 67-75. |
| [6] | Jian HU, Jinghua JIANG, Shengjun FAN, Jianhao LIU, Haijiang ZOU, Wanlong CAI, Fenghao WANG. Research on heat extraction performance of deep U-type borehole heat exchanger [J]. CIESC Journal, 2024, 75(S1): 76-84. |
| [7] | Xiaoqiao QIN, Hongbo TAN, Na WEN. Thermodynamic and economic analysis of air separation unit with energy storage and generation [J]. CIESC Journal, 2024, 75(7): 2409-2421. |
| [8] | Haiyan DU, Kai ZHU, Feng YOU, Jinfeng WANG, Yifan ZHAO, Nan ZHANG, Ying LI. Self-healing anti-freezing ionic hydrogel for strain sensors [J]. CIESC Journal, 2024, 75(7): 2709-2722. |
| [9] | Wenxuan ZHOU, Zhen LIU, Fujian ZHANG, Zhongqiang ZHANG. Mechanism of water treatment by high permeability-selectivity time dimension membrane method [J]. CIESC Journal, 2024, 75(7): 2583-2593. |
| [10] | Xiaoping LUO, Yuntian HOU, Yijie FAN. Flow boiling heat transfer and temperature uniformity in micro-channel with countercurrent phase separation structure [J]. CIESC Journal, 2024, 75(7): 2474-2485. |
| [11] | Songhong ZHANG, Xinyi ZHAO, Xiaoling LOU, Shaochuan SHEN, Junxian YUN. Separation of lactoperoxidase using cation exchange nano-cryogels [J]. CIESC Journal, 2024, 75(7): 2574-2582. |
| [12] | Guangyu ZHANG, Ranfei FU, Bing SUN, Juncong YUAN, Xiang FENG, Chaohe YANG, Wei XU. Synthesis of propylene carbonate from CO2 and propylene oxide: hydrogen bond activation strategy [J]. CIESC Journal, 2024, 75(6): 2243-2251. |
| [13] | Zongwei HUO, Yabin NIU, Yanqiu PAN. Behavior of high viscosity oil droplets in oil-water membrane separation and its influencing factors [J]. CIESC Journal, 2024, 75(6): 2262-2273. |
| [14] | Yiqi ZHANG, Xuesong TAN, Wuhuan LI, Quan ZHANG, Changlin MIAO, Xinshu ZHUANG. Efficient fractionation of sugarcane bagasse with phenoxyethanol under mild condition [J]. CIESC Journal, 2024, 75(6): 2274-2282. |
| [15] | Rufeng XU, Yucheng CHEN, Dan GAO, Jingyu JIAO, Dong GAO, Haibin WANG, Shanjing YAO, Dongqiang LIN. Model-assisted process optimization of ion-exchange chromatography for monoclonal antibody charge variant separation [J]. CIESC Journal, 2024, 75(5): 1903-1911. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||