化工学报 ›› 2020, Vol. 71 ›› Issue (1): 209-215.DOI: 10.11949/0438-1157.20191148
收稿日期:2019-10-09
修回日期:2019-11-09
出版日期:2020-01-05
发布日期:2020-01-05
通讯作者:
邓天龙
作者简介:袁菲(1994—),女,博士研究生,基金资助:
Fei YUAN( ),Jiangtao SONG,Jiayin HU,Yafei GUO,Shiqiang WANG,Tianlong DENG(
),Jiangtao SONG,Jiayin HU,Yafei GUO,Shiqiang WANG,Tianlong DENG( )
)
Received:2019-10-09
Revised:2019-11-09
Online:2020-01-05
Published:2020-01-05
Contact:
Tianlong DENG
摘要:
采用等温溶解平衡法研究了四元体系CaCl2-CaSO4-CaB6O10-H2O在308.15 K下的稳定相平衡。测定了该体系的溶解度及平衡溶液的物化性质(包括折射率、密度和pH)。根据实验数据,分别绘制了该四元体系的干基图、水图以及相应的物化性质–组成图。研究结果表明:该体系在308.15 K时有1个共饱点(CaCl2·4H2O + CaSO4·2H2O + CaB6O10·5H2O),3条单变量溶解度曲线,3个单盐结晶区,分别对应于CaCl2·4H2O、硬石膏(CaSO4·2H2O)和高硼钙石(CaB6O10·5H2O)。其中,硬石膏CaSO4·2H2O结晶区最大、高硼钙石CaB6O10·5H2O结晶区次之,而CaCl2·4H2O结晶区最小,表明硬石膏最易于结晶析出。此外,该四元体系在308.15 K下没有复盐和固溶体生成,属于简单水合物I型。平衡液相的物化性质随着CaCl2浓度的增大呈规律性变化,并在共饱点处发生转折。其中,折射率和密度的变化规律相近,而pH的变化规律则与之相反。对该四元体系的稳定相平衡进行研究,将为综合开发利用油田卤水中的钙、硼等资源提供理论依据。
中图分类号:
袁菲, 宋江涛, 胡佳音, 郭亚飞, 王士强, 邓天龙. 四元体系CaCl2-CaSO4-CaB6O10-H2O 308.15 K相平衡研究[J]. 化工学报, 2020, 71(1): 209-215.
Fei YUAN, Jiangtao SONG, Jiayin HU, Yafei GUO, Shiqiang WANG, Tianlong DENG. Phase equilibria of quaternary system CaCl2-CaSO4-CaB6O10-H2O at 308.15 K[J]. CIESC Journal, 2020, 71(1): 209-215.
| 类型 | CaO, wb/% | B2O3, wb/% | H2O, wb/% | CaO∶B2O3∶H2O (摩尔比) |
|---|---|---|---|---|
| 理论值 | 15.77 | 58.88 | 25.35 | 1∶3∶5 |
| 实验值 | 15.81 | 58.83 | 25.38 | 1∶3∶5 |
表1 CaB6O10·5H2O化学分析数据
Table 1 Chemical analysis data of CaB6O10·5H2O
| 类型 | CaO, wb/% | B2O3, wb/% | H2O, wb/% | CaO∶B2O3∶H2O (摩尔比) |
|---|---|---|---|---|
| 理论值 | 15.77 | 58.88 | 25.35 | 1∶3∶5 |
| 实验值 | 15.81 | 58.83 | 25.38 | 1∶3∶5 |
| No. | Liquid composition, wB/% | Dry-salt composition, ZB/[g·(100 g·S)-1] | Solid phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | CaSO4 | CaB6O10 | H2O | CaCl2 | CaSO4 | CaB6O10 | H2O | ||
| 1, A | 51.98 | 0.23 | 0 | 51.56 | 99.56 | 0.44 | 0 | 91.51 | CaCl2·4H2O + Gyps |
| 2 | 51.65 | 0.19 | 0.15 | 48.01 | 99.35 | 0.37 | 0.29 | 92.34 | CaCl2·4H2O + Gyps |
| 3 | 51.74 | 0.16 | 0.45 | 47.65 | 98.83 | 0.31 | 0.86 | 91.02 | CaCl2·4H2O + Gyps |
| 4 | 51.70 | 0.15 | 0.62 | 47.53 | 98.53 | 0.29 | 1.18 | 90.59 | CaCl2·4H2O + Gyps |
| 5, E | 49.59 | 0.14 | 1.00 | 49.27 | 97.75 | 0.27 | 1.98 | 97.12 | CaCl2·4H2O+Gyps+Gowerite |
| 6, B | 50.01 | 0 | 0.04 | 49.95 | 99.92 | 0 | 0.08 | 99.80 | CaCl2·4H2O + Gowerite |
| 7, C | 0 | 0.63 | 1.55 | 97.82 | 0 | 29.04 | 70.96 | 4705.16 | Gyps + Gowerite |
| 8 | 0.50 | 0.85 | 1.97 | 96.68 | 15.08 | 25.50 | 59.42 | 2916.50 | Gyps + Gowerite |
| 9 | 1.40 | 0.71 | 1.47 | 96.42 | 39.11 | 19.83 | 41.06 | 2693.30 | Gyps + Gowerite |
| 10 | 3.00 | 0.40 | 0.79 | 95.81 | 71.60 | 9.55 | 18.85 | 2286.63 | Gyps + Gowerite |
| 11 | 7.02 | 0.11 | 0.77 | 92.09 | 88.86 | 1.41 | 9.73 | 1164.98 | Gyps + Gowerite |
| 12 | 10.82 | 0.11 | 0.95 | 88.12 | 91.08 | 0.93 | 7.99 | 741.72 | Gyps + Gowerite |
| 13 | 27.19 | 0.48 | 0.80 | 71.53 | 95.50 | 1.69 | 2.81 | 251.25 | Gyps + Gowerite |
| 14 | 42.41 | 0.32 | 1.83 | 55.44 | 95.18 | 0.72 | 4.10 | 124.43 | Gyps + Gowerite |
| 15 | 48.28 | 0.27 | 1.89 | 49.55 | 95.71 | 0.54 | 3.75 | 98.23 | Gyps + Gowerite |
表2 四元体系CaCl2-CaSO4-CaB6O10-H2O在308.15 K下溶解度数据
Table 2 Solubilities of quaternary system (CaCl2-CaSO4-CaB6O10-H2O) at 308.15 K
| No. | Liquid composition, wB/% | Dry-salt composition, ZB/[g·(100 g·S)-1] | Solid phase | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CaCl2 | CaSO4 | CaB6O10 | H2O | CaCl2 | CaSO4 | CaB6O10 | H2O | ||
| 1, A | 51.98 | 0.23 | 0 | 51.56 | 99.56 | 0.44 | 0 | 91.51 | CaCl2·4H2O + Gyps |
| 2 | 51.65 | 0.19 | 0.15 | 48.01 | 99.35 | 0.37 | 0.29 | 92.34 | CaCl2·4H2O + Gyps |
| 3 | 51.74 | 0.16 | 0.45 | 47.65 | 98.83 | 0.31 | 0.86 | 91.02 | CaCl2·4H2O + Gyps |
| 4 | 51.70 | 0.15 | 0.62 | 47.53 | 98.53 | 0.29 | 1.18 | 90.59 | CaCl2·4H2O + Gyps |
| 5, E | 49.59 | 0.14 | 1.00 | 49.27 | 97.75 | 0.27 | 1.98 | 97.12 | CaCl2·4H2O+Gyps+Gowerite |
| 6, B | 50.01 | 0 | 0.04 | 49.95 | 99.92 | 0 | 0.08 | 99.80 | CaCl2·4H2O + Gowerite |
| 7, C | 0 | 0.63 | 1.55 | 97.82 | 0 | 29.04 | 70.96 | 4705.16 | Gyps + Gowerite |
| 8 | 0.50 | 0.85 | 1.97 | 96.68 | 15.08 | 25.50 | 59.42 | 2916.50 | Gyps + Gowerite |
| 9 | 1.40 | 0.71 | 1.47 | 96.42 | 39.11 | 19.83 | 41.06 | 2693.30 | Gyps + Gowerite |
| 10 | 3.00 | 0.40 | 0.79 | 95.81 | 71.60 | 9.55 | 18.85 | 2286.63 | Gyps + Gowerite |
| 11 | 7.02 | 0.11 | 0.77 | 92.09 | 88.86 | 1.41 | 9.73 | 1164.98 | Gyps + Gowerite |
| 12 | 10.82 | 0.11 | 0.95 | 88.12 | 91.08 | 0.93 | 7.99 | 741.72 | Gyps + Gowerite |
| 13 | 27.19 | 0.48 | 0.80 | 71.53 | 95.50 | 1.69 | 2.81 | 251.25 | Gyps + Gowerite |
| 14 | 42.41 | 0.32 | 1.83 | 55.44 | 95.18 | 0.72 | 4.10 | 124.43 | Gyps + Gowerite |
| 15 | 48.28 | 0.27 | 1.89 | 49.55 | 95.71 | 0.54 | 3.75 | 98.23 | Gyps + Gowerite |
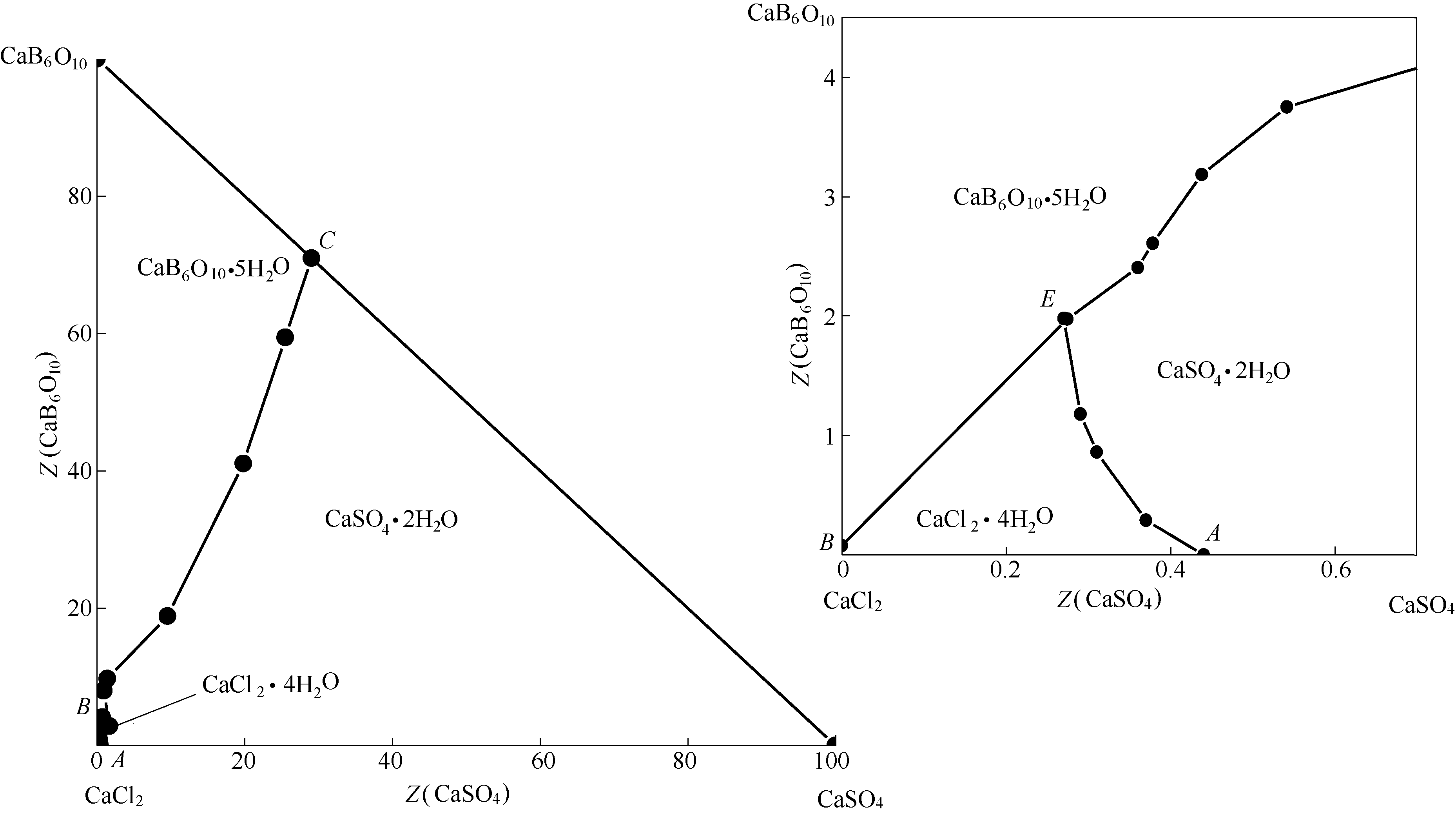
图2 四元体系CaCl2-CaSO4-CaB6O10-H2O在308.15 K下的干基图及局部放大图
Fig.2 Dry-salt phase diagram and part enlargement of phase diagram for quaternary system (CaCl2-CaSO4-CaB6O10-H2O) at 308.15 K
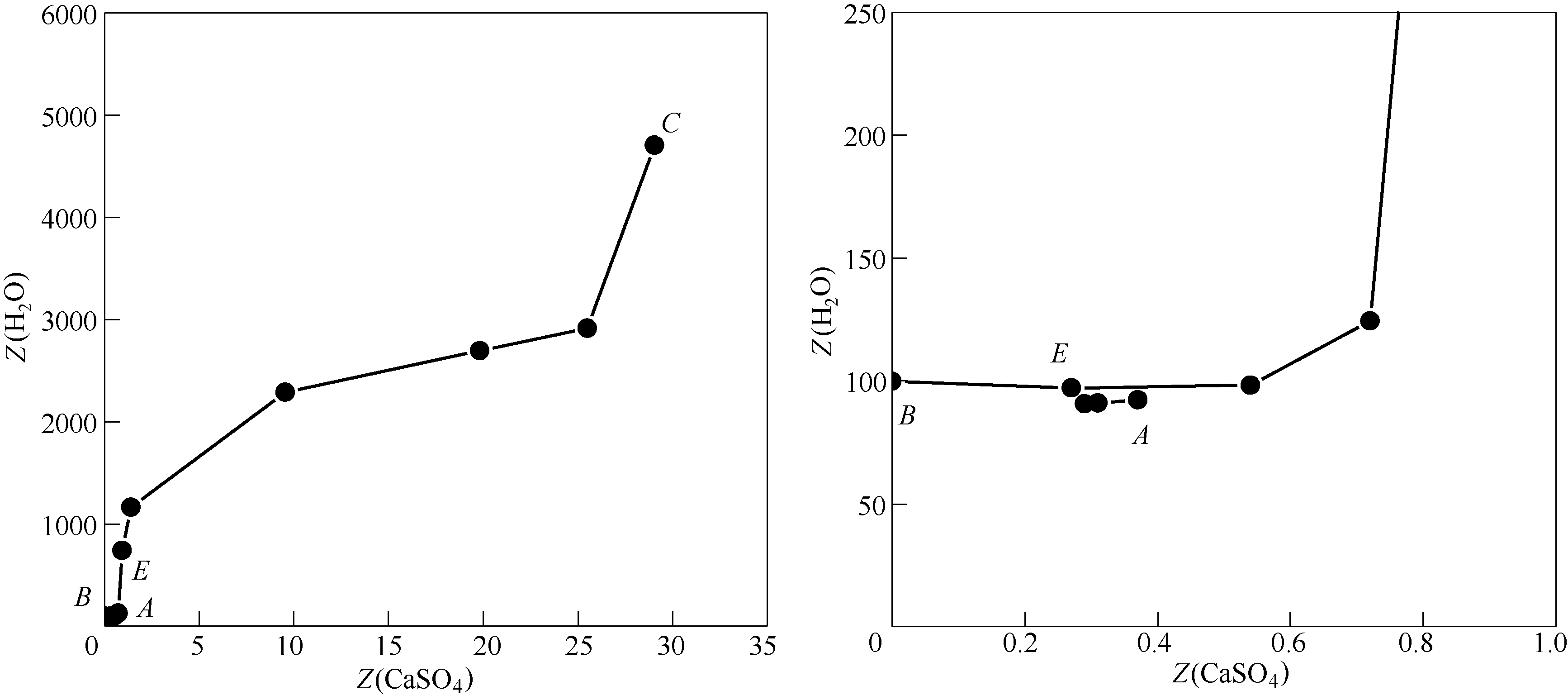
图3 四元体系CaCl2-CaSO4-CaB6O10-H2O在308.15 K下的水图及局部放大图
Fig.3 Water-phase diagram and part enlargement of water-phase diagram for quaternary system (CaCl2-CaSO4-CaB6O10-H2O) at 308.15 K
| No. | Liquid phase composition CaCl2, wb/% | Physicochemical properties | ||
|---|---|---|---|---|
| Refractive index, nD | Density, ρ/(g·cm-3) | pH | ||
| 1, A | 51.98 | 1.466014 | 1.50209 | 4.87 |
| 2 | 51.65 | 1.466722 | 1.51007 | 4.69 |
| 3 | 51.74 | 1.467257 | 1.51089 | 3.84 |
| 4 | 51.70 | 1.467872 | 1.51525 | 3.34 |
| 5, E | 49.59 | 1.470097 | 1.51522 | 2.80 |
| 6, B | 50.01 | 1.473100 | 1.52760 | 2.01 |
| 7, C | 0 | 1.334924 | 1.00666 | 7.77 |
| 8 | 0.50 | 1.336978 | 1.01205 | 7.70 |
| 9 | 1.40 | 1.335609 | 1.00898 | 7.75 |
| 10 | 3.00 | 1.337875 | 1.01887 | 7.69 |
| 11 | 7.02 | 1.349667 | 1.05984 | 7.25 |
| 12 | 10.82 | 1.360155 | 1.09354 | 6.84 |
| 13 | 27.29 | 1.401389 | 1.26400 | 5.50 |
| 14 | 42.41 | 1.443401 | 1.40641 | 4.24 |
| 15 | 48.28 | 1.457317 | 1.48112 | 3.42 |
表3 四元体系CaCl2-CaSO4-CaB6O10-H2O在308.15 K的物化性质
Table 3 Physicochemical properties of quaternary system (CaCl2-CaSO4-CaB6O10-H2O) at 308.15 K
| No. | Liquid phase composition CaCl2, wb/% | Physicochemical properties | ||
|---|---|---|---|---|
| Refractive index, nD | Density, ρ/(g·cm-3) | pH | ||
| 1, A | 51.98 | 1.466014 | 1.50209 | 4.87 |
| 2 | 51.65 | 1.466722 | 1.51007 | 4.69 |
| 3 | 51.74 | 1.467257 | 1.51089 | 3.84 |
| 4 | 51.70 | 1.467872 | 1.51525 | 3.34 |
| 5, E | 49.59 | 1.470097 | 1.51522 | 2.80 |
| 6, B | 50.01 | 1.473100 | 1.52760 | 2.01 |
| 7, C | 0 | 1.334924 | 1.00666 | 7.77 |
| 8 | 0.50 | 1.336978 | 1.01205 | 7.70 |
| 9 | 1.40 | 1.335609 | 1.00898 | 7.75 |
| 10 | 3.00 | 1.337875 | 1.01887 | 7.69 |
| 11 | 7.02 | 1.349667 | 1.05984 | 7.25 |
| 12 | 10.82 | 1.360155 | 1.09354 | 6.84 |
| 13 | 27.29 | 1.401389 | 1.26400 | 5.50 |
| 14 | 42.41 | 1.443401 | 1.40641 | 4.24 |
| 15 | 48.28 | 1.457317 | 1.48112 | 3.42 |
| 1 | 付建龙, 于升松, 李世金, 等. 柴达木盆地西部第三系油田卤水资源可利用性分析[J]. 盐湖研究, 2005, 13(3): 17-21. |
| Fu J L, Yu S S, Li S J, et al. Development availability for tertiary oil-field brine resourc: es in the west of Qaidam basin[J]. Journal of Salt Lake Research, 2005, 13(3): 17-21. | |
| 2 | 李青海, 顾同欣, 于升松, 等. 南翼山油田卤水低温结晶过程研究[J]. 物理化学学报, 2011, 27(8): 1803-1808. |
| Li Q H, Gu T X, Yu S S, et al. Study on the precipitation pathway of Nanyishan oilfield brine at subzero temperatures[J]. Acta Physico-Chimica Sinica, 2011, 27(8): 1803-1808. | |
| 3 | 张永兴, 谭秀民, 张利珍, 等. 萃取法从油田卤水中提硼的试验研究[J]. 化工矿物与加工, 2016, 45(10): 25-28. |
| Zhang Y X, Tan X M, Zhang L Z, et al. Research on extracting boron from brine in oil field with extraction method[J]. Industrial Minerals & Processing, 2016, 45(10): 25-28. | |
| 4 | 李洪普. 柴达木西部南翼山构造富钾深层卤水矿的控制因素及水化学特征[J]. 地球学报, 2015, 36(1): 41-50. |
| Li H P. Control factors and water chemical characteristics of potassium-rich deep brine in Nanyishan structure of western Qaidam Basin[J]. Acta Geoscientica Sinica, 2015, 36(1): 41-50. | |
| 5 | Chen S Q, Cui W J, Hu J Y, et al. Phase equilibria and phase diagrams for the aqueous ternary system containing sodium, sulfate, and metaborate ions at 288.15 and 308.15 K and 101.325 kPa[J]. Journal of Chemical & Engineering Data, 2019, 64(6): 2809-2815. |
| 6 | Chen S Q, Wang M X, Hu J Y, et al. Phase equilibria in the aqueous ternary systems (NaCl + NaBO2 + H2O) and (Na2SO4 + NaBO2 + H2O) at 298.15 K and 0.1 MPa[J]. Journal of Chemical & Engineering Data, 2018, 63(12): 4662-4668. |
| 7 | 任永胜, 何婷婷, 谢娟, 等. 333.15 K K+, NH4+//Cl-, SO42--H2O和K+, NH4+//Cl-, SO42--(CH2OH)2-H2O体系固液相平衡[J]. 化工学报, 2018, 69 (7): 2838-2850. |
| Ren Y S, He T T, Xie J, et al. Phase equilibria in systems K+, NH4+//Cl-, SO42--H2O and K+, NH4+//Cl-, SO42--(CH2OH)2-H2O at 313.15 K[J]. CIESC Journal, 2018, 69(7): 2838-2850. | |
| 8 | 宋彭生. 水盐体系相图与盐湖资源开发利用[J]. 盐湖研究, 2016, 24(3): 35-49. |
| Song P S. The phase diagram of salt-water systems and utilization of salt lake resources[J]. Journal of Salt Lake Research, 2016, 24(3): 35-49. | |
| 9 | 桑世华, 张婷婷, 傅超, 等. 四元体系Li+, K+, Mg2+//B4O72–-H2O 273 K相平衡[J]. 化工学报, 2017, 68(9): 3343-3349. |
| Sang S H, Zhang T T, Fu C, et al. Phase equilibria in quaternary system Li+, K+, Mg2+//B4O72–-H2O at 273 K[J]. CIESC Journal, 2017, 68(9): 3343-3349. | |
| 10 | Lei L Y, Li L, Cao L N, et al. Solid–liquid phase equilibria of the aqueous ternary system (CaCl2-CaB6O10-H2O) at 308.15, 323.15 K and 0.1 MPa[J]. Journal of Chemical Engineering of Japan, 2017, 50(4): 1-5. |
| 11 | Deng T L, Li D C, Wang S Q. Metastable phase equilibrium in the aqueous ternary system (KCl + CaCl2 + H2O) at T = 288.15 and 308.15 K[J]. Journal of Chemical & Engineering Data, 2008, 53(4): 1007–1011. |
| 12 | 孟令宗, 李丹, 邓天龙, 等. 三元体系Mg2+//Cl-, borate-H2O在308.15 K时稳定相平衡研究[J]. 世界科技研究与发展, 2011, 33(5): 784-786. |
| Meng L Z, Li D, Deng T L, et al. Phase equilibrium of ternary system Mg2+//Cl-, borate-H2O at 308.15 K[J]. World Sci-Tech R&D, 2011, 33(5): 784-786. | |
| 13 | 罗炳威, 邓天龙, 李丹. 三元体系Mg2+//Cl-, borate-H2O在323.15 K时相平衡研究[J]. 无机盐工业, 2011, 43(1): 15-18. |
| Luo B W, Deng T L, Li D. Phase equilibrium in ternary system (Mg2+//Cl-, borate-H2O) at 323.15 K[J]. Inorganic Chemicals Industry, 2011, 43(1): 15-18. | |
| 14 | Wang M X, Lei L Y, Guo Y F, et al. Phase equilibria of the reciprocal quaternary system (Na+, Ca2+//Cl-, Borate-H2O) at 288.15 K and 0.1 MPa[J]. Journal of Chemical & Engineering Data, 2018, 63(11): 4005-4011. |
| 15 | 桑世华, 彭江, 魏丽娜. Mg2+, K+//Cl-, B4O72--H2O四元体系288 K固液相平衡[J]. 物理化学学报, 2009, 25(2): 331-335. |
| Sang S H, Peng J, Wei L N. Solid-liquid phase equilibrium of the quaternary system Mg2+, K+//Cl-, B4O72--H2O at 288 K[J]. Acta Physico-Chimica Sinica, 2009, 25(2): 331-335. | |
| 16 | 桑世华, 唐明林, 殷辉安, 等. Na2B4O7-K2B4O7-H2O三元体系288 K相平衡研究[J]. 盐科学与化工, 2002, 31(1): 16-18. |
| Sang S H, Tang M L, Yin H A, et al. Phase equilibrium in ternary system Na2B4O7-K2B4O7-H2O at 288 K[J]. Journal of Salt Science and Chemical Industry, 2002, 31(1): 16-18. | |
| 17 | 曾英, 王瑞林, 林晓峰, 等. Na+, K+//Cl-, B4O72--H2O四元体系273 K介稳相平衡[J]. 物理化学学报, 2008, 24(3): 471-474. |
| Zeng Y, Wang R L, Lin X F, et al. Metastable phase equilibria of the quaternary system Na+, K+//Cl-, B4O72--H2O at 273 K[J]. Acta Physico-Chimica Sinica, 2008, 24(3): 471-474. | |
| 18 | Kakiage M, Shiomi S, Yanase I, et al. Low-temperature synthesis of calcium hexaboride powder via transient boron carbide formation[J]. Journal of the American Ceramic Society. 2015, 98(9): 2724-2727. |
| 19 | Yilmaz D, Koc N, Turan S. Synthesis of calcium hexaboride powder via boro/carbothermal reduction with a gel precursor[J]. J. Ceram. Sci. Tech., 2016, 356: 7-349. |
| 20 | Yilmaz D, Savaci U, Koc N, et al. Carbothermic reduction synthesis of calcium hexaboride using PVA-calcium hexaborate mixed gels[J]. Ceramics Int., 2018, 44: 2976-2981. |
| 21 | 丁士文, 李岩. 水热法制备纳米CaB6O10润滑油添加剂[J]. 河北大学学报(自然科学版), 2014, 34(2): 154-159. |
| Ding S W, Li Y. Hydrothermal synthesis of nano-CaB6O10 used as lubricant additives[J]. Journal of Hebei University (Natural Science Edition), 2014, 34(2): 154-159. | |
| 22 | 李强. 硼酸镁与硼酸钙基质发光材料的制备及发光性能研究[D]. 呼和浩特: 内蒙古大学, 2016. |
| Li Q. Research of preparation and lumin-escent properties about mangesium and calcium bornate substrate materials[D]. Hohhot: Inner Mongolia University, 2016. | |
| 23 | 李廷伟, 谭红兵, 樊启顺. 柴达木盆地西部地下卤水水化学特征及成因分析[J]. 盐湖研究, 2006, 14(4): 26-32. |
| Li T W, Tan H B, Fan Q S. Hydrochemical characteristics and origin analysis of the underground brines in west Qaidam Basin[J]. Journal of Salt Lake Research, 2006, 14(4): 26-32. | |
| 24 | 李武, 董亚萍, 宋彭生. 盐湖卤水资源开发利用[M]. 北京: 化学工业出版社, 2012. |
| Li W, Dong Y P, Song P S. Exploitation and Utilization of Salt Lake Brine Resources[M]. Beijing: Chemical Industry Press, 2012. | |
| 25 | 王文侠, 李洪岭. 硼酸钙的合成方法探析[J]. 河南化工, 2001, (5): 4-6. |
| Wang W X, Li H L. Analysis of the synthesis method of calcium borate[J]. Henan Chemical Industry, 2001, (5): 4-6. | |
| 26 | 王文侠. 四水合六硼酸钙的合成研究[J]. 天津化工, 2002, (4): 23-24. |
| Wang W X. Synthesis of calcium hexaborate tetrahydrate[J]. Tianjin Chemical Industry, 2002, (4): 23-24. | |
| 27 | 邓天龙, 周桓, 陈侠. 水盐体系相图及应用[M]. 北京: 化学工业出版社, 2013. |
| Deng T L, Zhou H, Chen X. Salt-water System Phase Diagrams and Applications[M]. Beijing: Chemical Industry Press, 2013. | |
| 28 | 牛自得, 程芳琴. 水盐体系相图及其应用[M]. 天津: 天津大学出版社, 2002: 172-182. |
| Niu Z D, Cheng F Q. The Phase Diagrams of Salt-Water Systems and Its Applications[M]. Tianjin: Tianjin University Press, 2002: 172-182. | |
| 29 | Meng L Z, Li D, Guo Y F, et al. Stable phase equilibrium of the aqueous quaternary system (MgCl2-MgSO4-MgB6O10-H2O) at 323.15 K[J]. Journal of Chemical & Engineering Data, 2011, 56(12): 5060-5065. |
| 30 | 中国科学院青海盐湖研究所分析室. 盐湖卤水分析[M]. 2版. 北京: 科学出版社, 1988. |
| Qinghai Institute of Salt Lakes of CAS. Analytical Methods of Brines and Salts[M]. 2nd ed. Beijing: Science Press, 1988. | |
| 31 | Wang X, Zhao K Y, Guo Y F, et al. Experimental determination and thermodynamic model of solid-liquid equilibria in the ternary system (LiCl + CaCl2 + H2O) at 273.15 K[J]. Journal of Chemical & Engineering Data, 2019, 64(1): 249-254. |
| [1] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [2] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [3] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [4] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [5] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [6] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [7] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [8] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [9] | 吴子睿, 孙瑞, 石凌峰, 田华, 王轩, 舒歌群. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| [10] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| [11] | 孙裕坤, 杨焘, 吴江涛. R32+R1234yf+R1234ze(E)混合制冷剂气液相平衡实验研究[J]. 化工学报, 2022, 73(3): 1063-1071. |
| [12] | 许昊, 陈伟, 李邹路. 以[Li(TX-7)]SCN/H2O为工质对的第二类热泵特性研究[J]. 化工学报, 2022, 73(2): 577-586. |
| [13] | 高腾飞, 李国选, 雷志刚. 从催化裂化柴油中分离联苯的溶剂筛选:实验和计算热力学[J]. 化工学报, 2022, 73(12): 5314-5323. |
| [14] | 彭昌炜, 桑世华, 崔瑞芝, 任红保. 五元体系NaBr-KBr-MgBr2-CaBr2-H2O在298.15 K下的空间立体相图研究[J]. 化工学报, 2022, 73(11): 4850-4858. |
| [15] | 刘潜, 张香兰, 李志平, 栗卓琦, 喻红. 油酚分离过程离子液体萃取溶剂的多尺度筛选[J]. 化工学报, 2022, 73(11): 5011-5024. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号