化工学报 ›› 2022, Vol. 73 ›› Issue (2): 577-586.DOI: 10.11949/0438-1157.20211050
收稿日期:2021-07-27
修回日期:2021-10-29
出版日期:2022-02-05
发布日期:2022-02-18
通讯作者:
陈伟
作者简介:许昊(1998—),男,硕士研究生,基金资助:Received:2021-07-27
Revised:2021-10-29
Online:2022-02-05
Published:2022-02-18
Contact:
Wei CHEN
摘要:
通过静态法测量了[Li(TX-7)]SCN/H2O溶液在283.15~443.15 K温度条件下的饱和蒸气压,通过差式扫描量热法测量了离子液体[Li(TX-7)]SCN在温度T=73.15~423.15 K条件下的比热容,并建立了经验关联式,通过Wilson模型对[Li(TX-7)]SCN/H2O溶液组分活度系数的关联预测了其过量焓,并通过预测值计算了二元溶液的比焓,建立了[Li(TX-7)]SCN/H2O的热力学模型。基于实验数据与热力学模型对以[Li(TX-7)]SCN/H2O为工质对的第二类热泵的理论循环特性进行了仿真分析,研究了各部件不同工作温度对系统性能参数的影响,并与其他吸收式系统的性能参数进行了比对。[Li(TX-7)]SCN/H2O系统的性能优于LiBr/H2O系统,适用于发生温度和蒸发温度较低的工况。
中图分类号:
许昊, 陈伟, 李邹路. 以[Li(TX-7)]SCN/H2O为工质对的第二类热泵特性研究[J]. 化工学报, 2022, 73(2): 577-586.
Hao XU, Wei CHEN, Zoulu LI. Study on the characteristics of the second type heat pump with [Li(TX-7)]SCN/H2O as the working fluid pair[J]. CIESC Journal, 2022, 73(2): 577-586.
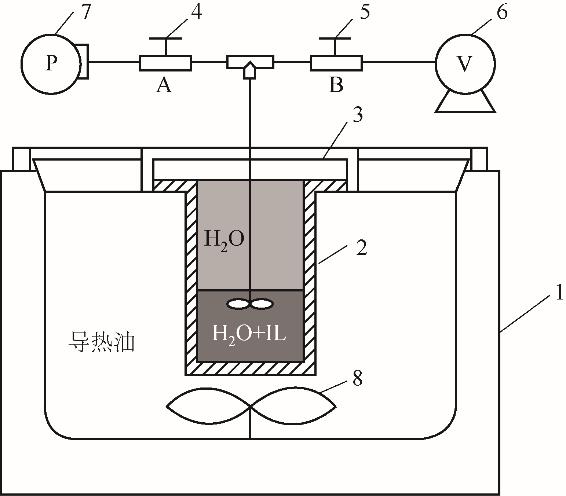
图1 [Li(TX-7)]SCN/H2O气液相平衡实验装置原理图1—恒温油槽;2—高压反应釜釜身;3—釜盖;4—阀门A;5—阀门B;6—真空泵;7—压力表;8—电动叶片搅拌器
Fig.1 Schematic diagram of [Li(TX-7)] SCN/H2O vapor-liquid equilibrium experimental device1—thermostatic oil tank; 2—high pressure reactor body; 3—kettle cover; 4—valve A; 5—valve B; 6—vacuum pump; 7—pressure gauge; 8—electric blade stirrer
| T/K | x1 | pexp/kPa | pcal/kPa | T/K | x1 | pexp/kPa | pcal/kPa |
|---|---|---|---|---|---|---|---|
| 283.15 | 0.797 | 0.0856 | 0.08299 | 363.15 | 0.9438 | 5.57287 | 5.604 |
| 283.15 | 0.8381 | 0.09166 | 0.0875 | 363.15 | 0.962 | 5.96216 | 5.808 |
| 283.15 | 0.8863 | 0.09584 | 0.09303 | 363.15 | 0.9748 | 6.09391 | 6.038 |
| 283.15 | 0.9181 | 0.09602 | 0.09704 | 363.15 | 0.9864 | 6.28673 | 6.516 |
| 283.15 | 0.9441 | 0.10306 | 0.101 | 383.15 | 0.7883 | 8.8827 | 9.254 |
| 283.15 | 0.9622 | 0.10464 | 0.1047 | 383.15 | 0.8327 | 10.09642 | 9.8 |
| 283.15 | 0.9748 | 0.1048 | 0.1089 | 383.15 | 0.8837 | 10.90799 | 10.45 |
| 283.15 | 0.9865 | 0.1201 | 0.1177 | 383.15 | 0.9168 | 10.68407 | 10.92 |
| 303.15 | 0.7962 | 0.79216 | 0.8221 | 383.15 | 0.9435 | 11.22677 | 11.36 |
| 303.15 | 0.8377 | 0.90821 | 0.867 | 383.15 | 0.9619 | 11.52549 | 11.78 |
| 303.15 | 0.8861 | 0.93747 | 0.9221 | 383.15 | 0.9747 | 11.91511 | 12.24 |
| 303.15 | 0.918 | 0.93043 | 0.9619 | 383.15 | 0.9864 | 12.76151 | 13.21 |
| 303.15 | 0.9441 | 1.04611 | 1.001 | 403.15 | 0.7806 | 16.78926 | 17.16 |
| 303.15 | 0.9622 | 1.053 | 1.037 | 403.15 | 0.8279 | 17.59822 | 18.24 |
| 303.15 | 0.9748 | 1.12895 | 1.079 | 403.15 | 0.8814 | 19.86943 | 19.52 |
| 303.15 | 0.9865 | 1.21894 | 1.165 | 403.15 | 0.9156 | 19.49984 | 20.42 |
| 323.15 | 0.795 | 2.14416 | 2.059 | 403.15 | 0.943 | 21.85056 | 21.26 |
| 323.15 | 0.8369 | 2.2736 | 2.173 | 403.15 | 0.9617 | 22.46175 | 22.04 |
| 323.15 | 0.8857 | 2.20496 | 2.312 | 403.15 | 0.9746 | 23.48982 | 22.91 |
| 323.15 | 0.9178 | 2.46411 | 2.412 | 403.15 | 0.9864 | 24.11513 | 24.71 |
| 323.15 | 0.944 | 2.39284 | 2.509 | 423.15 | 0.7672 | 29.66389 | 29.5 |
| 323.15 | 0.9621 | 2.65719 | 2.601 | 423.15 | 0.8199 | 31.38543 | 31.6 |
| 323.15 | 0.9748 | 2.66227 | 2.704 | 423.15 | 0.8777 | 34.91273 | 34.01 |
| 323.15 | 0.9865 | 2.93622 | 2.92 | 423.15 | 0.9138 | 34.21 | 35.64 |
| 343.15 | 0.795 | 2.14416 | 2.059 | 423.15 | 0.9422 | 38.28398 | 37.16 |
| 343.15 | 0.8369 | 2.2736 | 2.173 | 423.15 | 0.9613 | 39.18233 | 38.54 |
| 343.15 | 0.8857 | 2.20496 | 2.312 | 423.15 | 0.9745 | 39.09559 | 40.06 |
| 343.15 | 0.9178 | 2.46411 | 2.412 | 423.15 | 0.9864 | 44.70965 | 43.19 |
| 343.15 | 0.944 | 2.39284 | 2.509 | 443.15 | 0.7439 | 49.42704 | 47.24 |
| 343.15 | 0.9621 | 2.65719 | 2.601 | 443.15 | 0.8063 | 53.51628 | 51.33 |
| 343.15 | 0.9748 | 2.66227 | 2.704 | 443.15 | 0.8717 | 57.19172 | 55.78 |
| 343.15 | 0.9865 | 2.93622 | 2.92 | 443.15 | 0.9109 | 60.59978 | 58.68 |
| 363.15 | 0.7927 | 4.65111 | 4.586 | 443.15 | 0.941 | 59.42647 | 61.28 |
| 363.15 | 0.8354 | 4.84301 | 4.846 | 443.15 | 0.9608 | 61.51926 | 63.6 |
| 363.15 | 0.885 | 5.34156 | 5.16 | 443.15 | 0.9742 | 66.17082 | 66.13 |
| 363.15 | 0.9174 | 5.13665 | 5.386 | 443.15 | 0.9863 | 69.564 | 71.28 |
表1 H2O (1) + [Li(TX-7)]SCN (2)二元体系压力-温度-摩尔分数(p-T-x)实验数据
Table 1 The p-T-x data of binary system H2O (1) + [Li(TX-7)]SCN (2)
| T/K | x1 | pexp/kPa | pcal/kPa | T/K | x1 | pexp/kPa | pcal/kPa |
|---|---|---|---|---|---|---|---|
| 283.15 | 0.797 | 0.0856 | 0.08299 | 363.15 | 0.9438 | 5.57287 | 5.604 |
| 283.15 | 0.8381 | 0.09166 | 0.0875 | 363.15 | 0.962 | 5.96216 | 5.808 |
| 283.15 | 0.8863 | 0.09584 | 0.09303 | 363.15 | 0.9748 | 6.09391 | 6.038 |
| 283.15 | 0.9181 | 0.09602 | 0.09704 | 363.15 | 0.9864 | 6.28673 | 6.516 |
| 283.15 | 0.9441 | 0.10306 | 0.101 | 383.15 | 0.7883 | 8.8827 | 9.254 |
| 283.15 | 0.9622 | 0.10464 | 0.1047 | 383.15 | 0.8327 | 10.09642 | 9.8 |
| 283.15 | 0.9748 | 0.1048 | 0.1089 | 383.15 | 0.8837 | 10.90799 | 10.45 |
| 283.15 | 0.9865 | 0.1201 | 0.1177 | 383.15 | 0.9168 | 10.68407 | 10.92 |
| 303.15 | 0.7962 | 0.79216 | 0.8221 | 383.15 | 0.9435 | 11.22677 | 11.36 |
| 303.15 | 0.8377 | 0.90821 | 0.867 | 383.15 | 0.9619 | 11.52549 | 11.78 |
| 303.15 | 0.8861 | 0.93747 | 0.9221 | 383.15 | 0.9747 | 11.91511 | 12.24 |
| 303.15 | 0.918 | 0.93043 | 0.9619 | 383.15 | 0.9864 | 12.76151 | 13.21 |
| 303.15 | 0.9441 | 1.04611 | 1.001 | 403.15 | 0.7806 | 16.78926 | 17.16 |
| 303.15 | 0.9622 | 1.053 | 1.037 | 403.15 | 0.8279 | 17.59822 | 18.24 |
| 303.15 | 0.9748 | 1.12895 | 1.079 | 403.15 | 0.8814 | 19.86943 | 19.52 |
| 303.15 | 0.9865 | 1.21894 | 1.165 | 403.15 | 0.9156 | 19.49984 | 20.42 |
| 323.15 | 0.795 | 2.14416 | 2.059 | 403.15 | 0.943 | 21.85056 | 21.26 |
| 323.15 | 0.8369 | 2.2736 | 2.173 | 403.15 | 0.9617 | 22.46175 | 22.04 |
| 323.15 | 0.8857 | 2.20496 | 2.312 | 403.15 | 0.9746 | 23.48982 | 22.91 |
| 323.15 | 0.9178 | 2.46411 | 2.412 | 403.15 | 0.9864 | 24.11513 | 24.71 |
| 323.15 | 0.944 | 2.39284 | 2.509 | 423.15 | 0.7672 | 29.66389 | 29.5 |
| 323.15 | 0.9621 | 2.65719 | 2.601 | 423.15 | 0.8199 | 31.38543 | 31.6 |
| 323.15 | 0.9748 | 2.66227 | 2.704 | 423.15 | 0.8777 | 34.91273 | 34.01 |
| 323.15 | 0.9865 | 2.93622 | 2.92 | 423.15 | 0.9138 | 34.21 | 35.64 |
| 343.15 | 0.795 | 2.14416 | 2.059 | 423.15 | 0.9422 | 38.28398 | 37.16 |
| 343.15 | 0.8369 | 2.2736 | 2.173 | 423.15 | 0.9613 | 39.18233 | 38.54 |
| 343.15 | 0.8857 | 2.20496 | 2.312 | 423.15 | 0.9745 | 39.09559 | 40.06 |
| 343.15 | 0.9178 | 2.46411 | 2.412 | 423.15 | 0.9864 | 44.70965 | 43.19 |
| 343.15 | 0.944 | 2.39284 | 2.509 | 443.15 | 0.7439 | 49.42704 | 47.24 |
| 343.15 | 0.9621 | 2.65719 | 2.601 | 443.15 | 0.8063 | 53.51628 | 51.33 |
| 343.15 | 0.9748 | 2.66227 | 2.704 | 443.15 | 0.8717 | 57.19172 | 55.78 |
| 343.15 | 0.9865 | 2.93622 | 2.92 | 443.15 | 0.9109 | 60.59978 | 58.68 |
| 363.15 | 0.7927 | 4.65111 | 4.586 | 443.15 | 0.941 | 59.42647 | 61.28 |
| 363.15 | 0.8354 | 4.84301 | 4.846 | 443.15 | 0.9608 | 61.51926 | 63.6 |
| 363.15 | 0.885 | 5.34156 | 5.16 | 443.15 | 0.9742 | 66.17082 | 66.13 |
| 363.15 | 0.9174 | 5.13665 | 5.386 | 443.15 | 0.9863 | 69.564 | 71.28 |
| a1 | b1 | c1 | a2 | b2 | c2 | α12 | ARD |
|---|---|---|---|---|---|---|---|
| 9.814 | -0.08357 | 0.5993 | -0.06598 | -19.87 | -0.002621 | 1.657 | 0.027 |
表2 NRLT模型可调参数及平均相对偏差
Table 2 Adjustable parameters and ARD for the NRTL model
| a1 | b1 | c1 | a2 | b2 | c2 | α12 | ARD |
|---|---|---|---|---|---|---|---|
| 9.814 | -0.08357 | 0.5993 | -0.06598 | -19.87 | -0.002621 | 1.657 | 0.027 |

图3 [Li(TX-7)]SCN/H2O二元体系实验值与预测值的绝对偏差和相对偏差
Fig.3 The absolute deviation and relative deviation of the experimental and predicted values of the [Li(TX-7)]SCN/H2O binary system
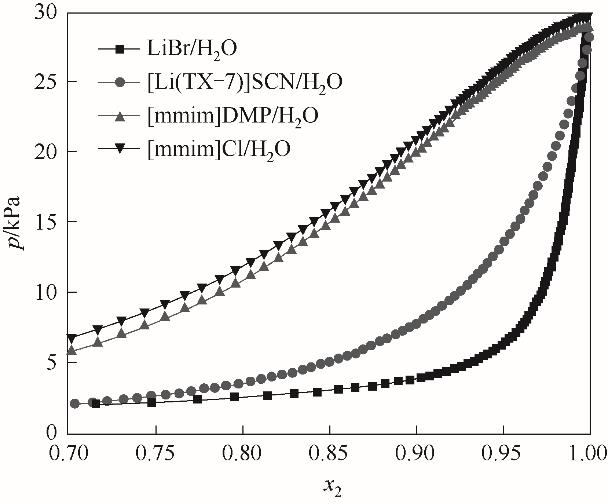
图4 [Li(TX-7)]SCN/H2O溶液饱和蒸气压与其他离子液体/水饱和蒸气压的对比
Fig.4 Comparison of saturated vapor pressure of [Li(TX-7)]SCN/H2O solution and saturated vapor pressure of water/other ionic liquids
| t/℃ | cp/(kJ/(kg·K)) | t/℃ | cp/(kJ/(kg·K)) | t/℃ | cp/(kJ/(kg·K)) |
|---|---|---|---|---|---|
| 0 | 2.283 | 55 | 2.568 | 115 | 2.784 |
| 10 | 2.303 | 70 | 2.603 | 130 | 2.803 |
| 25 | 2.354 | 85 | 2.687 | 140 | 2.829 |
| 40 | 2.46 | 100 | 2.722 | 150 | 2.858 |
表3 离子液体[Li(TX-7)]SCN比热容测量结果
Table 3 Experimental results of specific heat capacity of ionic liquid [Li(TX-7)]SCN
| t/℃ | cp/(kJ/(kg·K)) | t/℃ | cp/(kJ/(kg·K)) | t/℃ | cp/(kJ/(kg·K)) |
|---|---|---|---|---|---|
| 0 | 2.283 | 55 | 2.568 | 115 | 2.784 |
| 10 | 2.303 | 70 | 2.603 | 130 | 2.803 |
| 25 | 2.354 | 85 | 2.687 | 140 | 2.829 |
| 40 | 2.46 | 100 | 2.722 | 150 | 2.858 |
| 状态点 | T/K | x1 | p/kPa | h/(kJ/kg) |
|---|---|---|---|---|
| 1 | 353.15 | 1.000 | 3.972 | 1758.09 |
| 2 | 371.28 | 0.971 | 17.679 | 147.64 |
| 3 | 353.15 | 0.806 | 3.972 | 168.39 |
| 4 | 303.15 | 1.000 | 3.972 | 125.49 |
| 5 | 303.15 | 1.000 | 44.591 | 125.49 |
| 6 | 353.15 | 1.000 | 44.591 | 156.01 |
| 7 | 403.15 | 0.971 | 44.591 | 187.92 |
| 8 | 377.24 | 0.806 | 9.716 | 236.14 |
| 9 | 383.15 | 0.971 | 29.679 | 187.92 |
| 10 | 377.24 | 0.806 | 9.716 | 236.14 |
表4 第二类热泵系统各状态点的工作参数
Table 4 Operating parameters of each status points for AHT system
| 状态点 | T/K | x1 | p/kPa | h/(kJ/kg) |
|---|---|---|---|---|
| 1 | 353.15 | 1.000 | 3.972 | 1758.09 |
| 2 | 371.28 | 0.971 | 17.679 | 147.64 |
| 3 | 353.15 | 0.806 | 3.972 | 168.39 |
| 4 | 303.15 | 1.000 | 3.972 | 125.49 |
| 5 | 303.15 | 1.000 | 44.591 | 125.49 |
| 6 | 353.15 | 1.000 | 44.591 | 156.01 |
| 7 | 403.15 | 0.971 | 44.591 | 187.92 |
| 8 | 377.24 | 0.806 | 9.716 | 236.14 |
| 9 | 383.15 | 0.971 | 29.679 | 187.92 |
| 10 | 377.24 | 0.806 | 9.716 | 236.14 |
| 工质对 | ω1,A/%(质量) | ω1,G/%(质量) | f | COP |
|---|---|---|---|---|
| [Li(TX-7)]SCN/H2O | 46.5 | 32.3 | 4.78 | 0.469 |
| LiBr/H2O | 49.6 | 37.2 | 5.08 | 0.456 |
| [mmim]DMP/CH3OH | 31.2 | 17.2 | 5.90 | 0.436 |
| [mmim]DMP/H2O | 35.3 | 23.2 | 6.35 | 0.423 |
| NaSCN/NH3 | 53.1 | 43.2 | 5.73 | 0.428 |
表5 [Li(TX-7)]SCN/H2O与其他工质对的第二类热泵系统的性能比较
Table 5 The performance comparison of AHT systems using [Li(TX-7)]SCN/H2O and other working fluids
| 工质对 | ω1,A/%(质量) | ω1,G/%(质量) | f | COP |
|---|---|---|---|---|
| [Li(TX-7)]SCN/H2O | 46.5 | 32.3 | 4.78 | 0.469 |
| LiBr/H2O | 49.6 | 37.2 | 5.08 | 0.456 |
| [mmim]DMP/CH3OH | 31.2 | 17.2 | 5.90 | 0.436 |
| [mmim]DMP/H2O | 35.3 | 23.2 | 6.35 | 0.423 |
| NaSCN/NH3 | 53.1 | 43.2 | 5.73 | 0.428 |
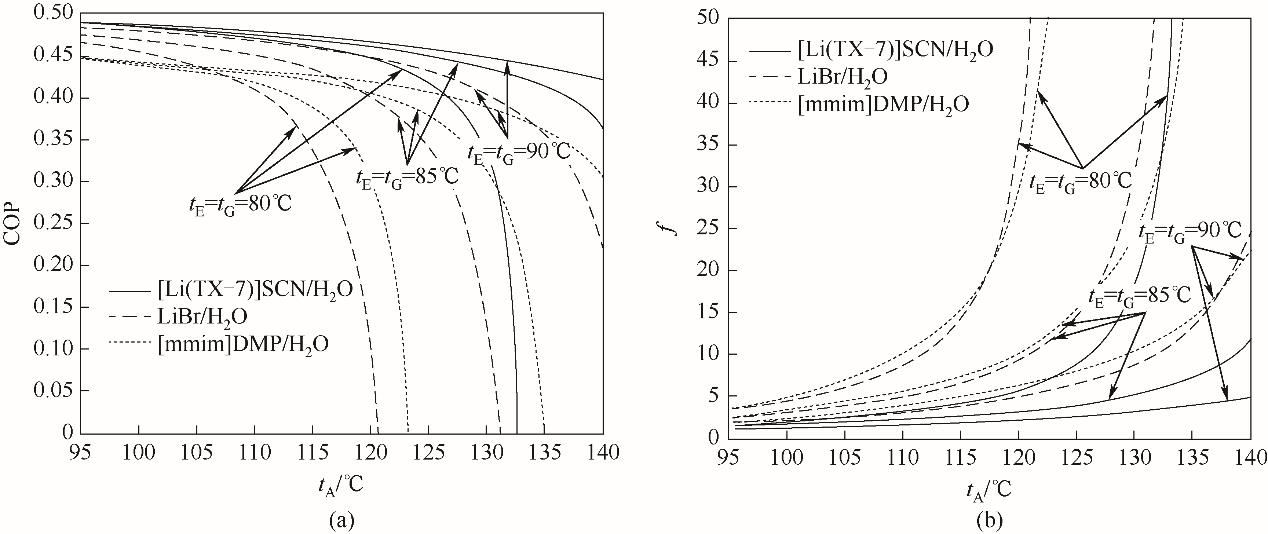
图9 tA对[Li(TX-7)]SCN/H2O、LiBr/H2O、[mmim]DMP/H2O第二类热泵系统热力学性能的影响
Fig.9 Effects of tA on the thermodynamic performances of [Li(TX-7)]SCN/H2O, LiBr/H2O, [mmim]DMP/H2O absorption heat transformer system
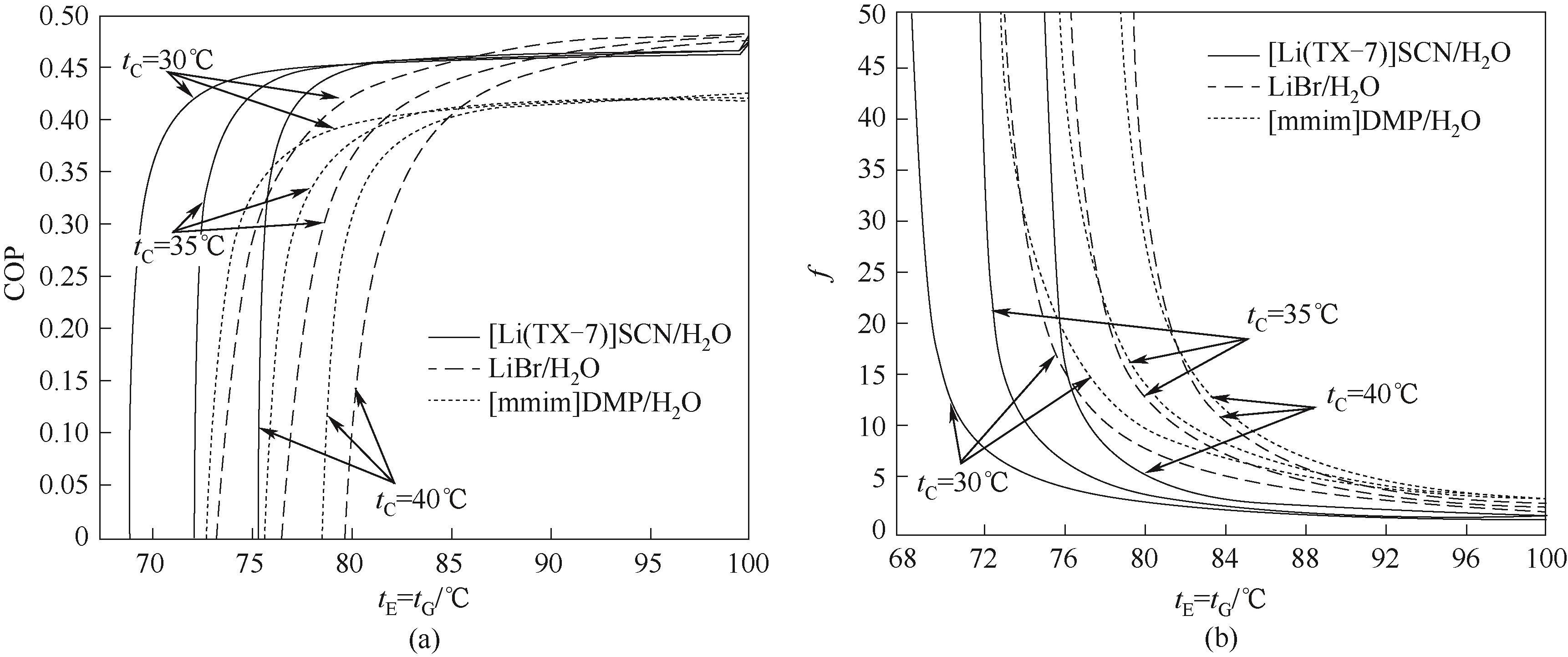
图10 tE和tG对[Li(TX-7)]SCN/H2O、LiBr/H2O、[mmim]DMP/H2O第二类热泵系统热力学性能的影响
Fig.10 Effects of tE and tG on the thermodynamic performances of [Li(TX-7)]SCN/H2O, LiBr/H2O, [mmim]DMP/H2O absorption heat transformer system
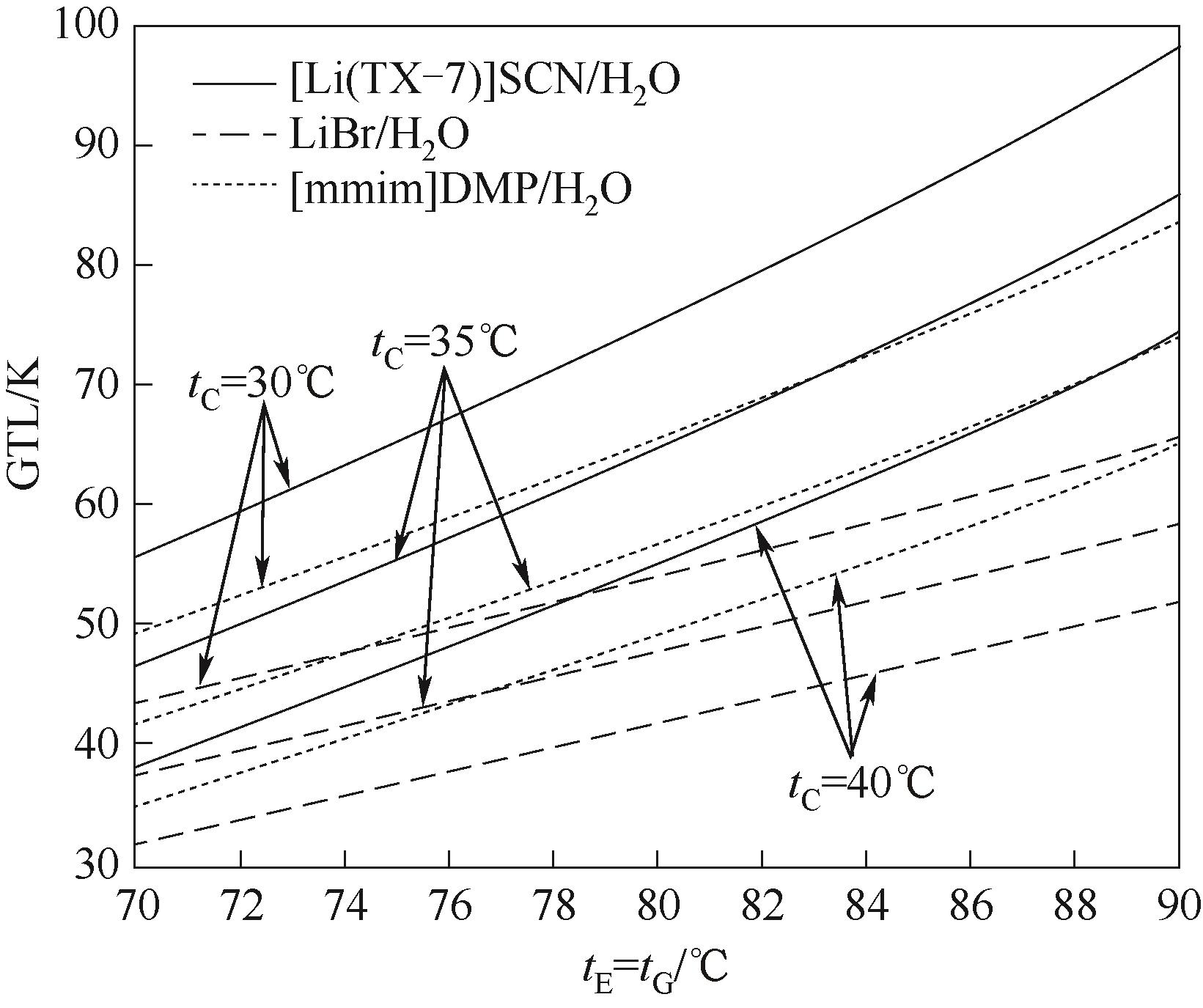
图11 tE和tG对[Li(TX-7)]SCN/H2O、LiBr/H2O、[mmim]DMP/H2O第二类热泵系统GTL的影响
Fig.11 Effects of tEand tG on the GTL of [Li(TX-7)]SCN/H2O, LiBr/H2O and [mmim]DMP/H2O absorption heat transformer system
| 1 | Ma X H, Chen J B, Li S P, et al. Application of absorption heat transformer to recover waste heat from a synthetic rubber plant[J]. Applied Thermal Engineering, 2003, 23(7): 797-806. |
| 2 | 洪文鹏, 何建军. 回收电厂余热的新型吸收式热泵系统[J]. 东北电力大学学报, 2019, 39(3): 67-73. |
| Hong W P, He J J. The new absorption heat pump system to reclam waste heat in power plant[J]. Journal of Northeast Electric Power University, 2019, 39(3): 67-73. | |
| 3 | 方书起, 骆萍梅. 第二类吸收式热泵的研究及应用[J]. 应用能源技术, 2008(10): 36-39. |
| Fang S Q, Luo P M. The research and application of the absorption heat transformer[J]. Applied Energy Technology, 2008(10): 36-39. | |
| 4 | Arora A, Kaushik S C. Theoretical analysis of LiBr/H2O absorption refrigeration systems[J]. International Journal of Energy Research, 2009, 33(15): 1321-1340. |
| 5 | Kamali M, Parham K, Assadi M. Performance analysis of a single stage absorption heat transformer-based desalination system employing a new working pair of (EMIM) (DMP)/H2O[J]. International Journal of Energy Research, 2018, 42(15): 4790-4804. |
| 6 | Song J Y, Park J H, Kang Y T. Heat transfer and frictional pressure drop characteristics of H2O/LiBr solution in plate heat exchangers for triple-effect absorption application[J]. Applied Thermal Engineering, 2021, 189: 116730. |
| 7 | Seddon K R. Ionic liquids for clean technology[J]. Journal of Chemical Technology & Biotechnology, 1997, 68(4): 351-356. |
| 8 | Horwitz G, Steinberg P Y, Corti H R. Volumetric and viscosity properties of water-in-salt lithium electrolytes: a comparison with ionic liquids and hydrated molten salts[J]. The Journal of Chemical Thermodynamics, 2021, 158: 106457. |
| 9 | Abumandour E S, Mutelet F, Alonso D. Performance of an absorption heat transformer using new working binary systems composed of {ionic liquid and water}[J]. Applied Thermal Engineering, 2016, 94: 579-589. |
| 10 | 周忠迎. 离子液体[EMIM]AC+水/醇新工质的热力学性质研究[D]. 大连: 大连理工大学, 2014. |
| Zhou Z Y. Study on the thermodynamic properties research of new working pairs of ionic liquid [EMIM] AC and water/alcohol[D]. Dalian: Dalian University of Technology, 2014. | |
| 11 | Ayou D S, Currás M R, Salavera D, et al. Performance analysis of absorption heat transformer cycles using ionic liquids based on imidazolium cation as absorbents with 2, 2, 2-trifluoroethanol as refrigerant[J]. Energy Conversion and Management, 2014, 84: 512-523. |
| 12 | Sujatha I, Venkatarathnam G. Performance of a vapour absorption heat transformer operating with ionic liquids and ammonia[J]. Energy, 2017, 141: 924-936. |
| 13 | Merkel N, Bücherl M, Zimmermann M, et al. Operation of an absorption heat transformer using water/ionic liquid as working fluid[J]. Applied Thermal Engineering, 2018, 131: 370-380. |
| 14 | Merkel N, Weber C, Faust M, et al. Influence of anion and cation on the vapor pressure of binary mixtures of water + ionic liquid and on the thermal stability of the ionic liquid[J]. Fluid Phase Equilibria, 2015, 394: 29-37. |
| 15 | 罗春欢, 张渊, 苏庆泉. LiBr-[BMIM]Cl/H2O工质对的饱和蒸气压、结晶温度和腐蚀性[J]. 化工学报, 2016, 67(4): 1110-1116. |
| Luo C H, Zhang Y, Su Q Q. Saturated vapor pressure, crystallization temperature and corrosivity of LiBr-[BMIM]Cl/H2O working pair[J]. CIESC Journal, 2016, 67(4): 1110-1116. | |
| 16 | 陈伟. 离子液体吸收式制冷工质对基础物性与循环特性研究[D]. 北京: 中国科学院研究生院(工程热物理研究所), 2014. |
| Chen W. Researches on fundamental physicochemical properties and cycle characteristics of novel absorption refrigeration working pairs containing ionic liquid[D]. Beijing: The University of Chinese Academy of Sciences(Institute of Engineering Thermophysics), 2014. | |
| 17 | Chen W, Liang S Q. Thermodynamic analysis of absorption heat transformers using [mmim]DMP/H2O and [mmim]DMP/CH3OH as working fluids[J]. Applied Thermal Engineering, 2016, 99: 846-856. |
| 18 | Zhang X D, Hu D P, Zhao Z C. Thermodynamic performance of absorption heat transformer using a new working pair: water+ionic liquid 1, 3-dimethylimidazolium dimethylphosphate[J]. Advanced Materials Research, 2012, 512/513/514/515: 1258-1262. |
| 19 | Ding F, Zheng J J, Chen Y Q, et al. Highly efficient and reversible SO2 capture by surfactant-derived dual functionalized ionic liquids with metal chelate cations[J]. Industrial & Engineering Chemistry Research, 2014, 53(48): 18568-18574. |
| 20 | Islam A W, Rahman M H. A review of Barker's activity coefficient method and VLE data reduction[J]. The Journal of Chemical Thermodynamics, 2012, 44(1): 31-37. |
| 21 | 王党生, 韩斌, 王彭, 等. 静态法测定液体饱和蒸气压实验的研究[J]. 实验技术与管理, 2009, 26(7): 41-43. |
| Wang D S, Han B, Wang P, et al. Study on measuring the saturated vapor pressure of liquid[J]. Experimental Technology and Management, 2009, 26(7): 41-43. | |
| 22 | Que H L, Chen C C. Thermodynamic modeling of the NH3-CO2-H2O system with electrolyte NRTL model[J]. Industrial & Engineering Chemistry Research, 2011, 50(19): 11406-11421. |
| 23 | Wang H N, Chen H F, Chen W H, et al. Vapor-liquid equilibrium study of LiBr + H2O and LiBr + CaCl2 + H2O systems[J]. Frontiers in Chemistry, 2020, 7: 890. |
| 24 | Chen W, Liang S Q, Guo Y X, et al. Thermodynamic performances of [mmim]DMP/methanol absorption refrigeration[J]. Journal of Thermal Science, 2012, 21(6): 557-563. |
| 25 | 陈伟, 李兰兰, 梁世强, 等. [mmim]DMP/CH3OH吸收式制冷热力性能研究[J]. 工程热物理学报, 2013, 34(4): 689-693. |
| Chen W, Li L L, Liang S Q, et al. Investigation of [mmim]DMP/CH3OH absorption refrigeration thermodynamic performances[J]. Journal of Engineering Thermophysics, 2013, 34(4): 689-693. | |
| 26 | Zheng W X, Liu X J, Zhu L Y, et al. Pretreatment with γ-valerolactone/[mmim]DMP and enzymatic hydrolysis on corncob and its application in immobilized butyric acid fermentation[J]. Journal of Agricultural and Food Chemistry, 2018, 66(44): 11709-11717. |
| 27 | 王建召, 郑丹星, 董丽,等. 氯化1,3-二甲基咪唑的合成及其水溶液饱和蒸气压[C]//第五届全国化学工程与生物化工年会. 西安, 2008. |
| Wang J Z, Zheng D X, Dong L, et al. Synthesis of 1,3-dimethylimidazole chloride and its saturated vapor pressure in aqueous solution[C]//The 5th Annual National Chemical Engineering and Biochemical Conference. Xi'an, 2008. | |
| 28 | Dommert F, Holm C. Refining classical force fields for ionic liquids: theory and application to [MMIM][Cl[J]. Physical Chemistry Chemical Physics, 2013, 15(6): 2037-2049. |
| 29 | He Y, Zhang X B, Chen W, et al. Experimental study and thermal analysis of the combustion characteristics of powder-activated cokes[J]. Powder Technology, 2019, 356: 640-648. |
| 30 | Zhu L H, Gu J J. Second law-based thermodynamic analysis of ammonia/sodium thiocyanate absorption system[J]. Renewable Energy, 2010, 35(9): 1940-1946. |
| 31 | 王凡. 第二类LiBr-H2O吸收式热泵系统的模拟与实验研究[D]. 济南: 山东建筑大学, 2014. |
| Wang F. Simulative and experimental study on the second class LiBr-H2O absorption heat pump[D]. Jinan: Shandong Jianzhu University, 2014. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [4] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [5] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [6] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [7] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [8] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [9] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [10] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [11] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [12] | 张缘良, 栾昕奇, 苏伟格, 李畅浩, 赵钟兴, 周利琴, 陈健民, 黄艳, 赵祯霞. 离子液体复合萃取剂选择性萃取尼古丁的研究及DFT计算[J]. 化工学报, 2023, 74(7): 2947-2956. |
| [13] | 龙臻, 王谨航, 任俊杰, 何勇, 周雪冰, 梁德青. 离子液体协同PVCap抑制天然气水合物生成实验研究[J]. 化工学报, 2023, 74(6): 2639-2646. |
| [14] | 毛磊, 刘冠章, 袁航, 张光亚. 可捕集CO2的纳米碳酸酐酶粒子的高效制备及性能研究[J]. 化工学报, 2023, 74(6): 2589-2598. |
| [15] | 杨灿, 孙雪琦, 尚明华, 张建, 张香平, 曾少娟. 相变离子液体体系吸收分离CO2的研究现状及展望[J]. 化工学报, 2023, 74(4): 1419-1432. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
