化工学报 ›› 2023, Vol. 74 ›› Issue (5): 1862-1874.DOI: 10.11949/0438-1157.20230135
郭旭1,2( ), 张永政3, 夏厚兵1,2, 杨娜4, 朱真珍1,2, 齐晶瑶1,2(
), 张永政3, 夏厚兵1,2, 杨娜4, 朱真珍1,2, 齐晶瑶1,2( )
)
收稿日期:2023-02-21
修回日期:2023-04-20
出版日期:2023-05-05
发布日期:2023-06-29
通讯作者:
齐晶瑶
作者简介:郭旭(1996—),男,博士研究生,guox96@163.com
基金资助:
Xu GUO1,2( ), Yongzheng ZHANG3, Houbing XIA1,2, Na YANG4, Zhenzhen ZHU1,2, Jingyao QI1,2(
), Yongzheng ZHANG3, Houbing XIA1,2, Na YANG4, Zhenzhen ZHU1,2, Jingyao QI1,2( )
)
Received:2023-02-21
Revised:2023-04-20
Online:2023-05-05
Published:2023-06-29
Contact:
Jingyao QI
摘要:
电极材料是电氧化技术的核心关键。碳基材料具有稳定性好、结构可调、导电性佳、来源广泛等优势。从碳基材料电氧化过程出发,简要描述了碳材料在阳极电流下的降解行为及其可复合活性材料增强自由基产生能力。重点综述了碳纳米管、石墨烯、生物质碳为代表的碳基材料通过缺陷调控、表面修饰、界面工程等方式调控电氧化性能的研究进展,最后对发展碳基阳极材料提出展望。
中图分类号:
郭旭, 张永政, 夏厚兵, 杨娜, 朱真珍, 齐晶瑶. 碳基材料电氧化去除水体污染物的研究进展[J]. 化工学报, 2023, 74(5): 1862-1874.
Xu GUO, Yongzheng ZHANG, Houbing XIA, Na YANG, Zhenzhen ZHU, Jingyao QI. Research progress in the removal of water pollutants by carbon-based materials via electrooxidation[J]. CIESC Journal, 2023, 74(5): 1862-1874.
| 项目 | 反应式 | 序号 |
|---|---|---|
| 间接氧化 | (2) | |
| (3) | ||
| 析氧反应 | (4) | |
| (5) | ||
| (6) | ||
| (7) |
表1 间接氧化(·OH介导)及析氧反应对比
Table 1 Comparison of indirect oxidation (·OH mediated) and oxygen evolution reactions
| 项目 | 反应式 | 序号 |
|---|---|---|
| 间接氧化 | (2) | |
| (3) | ||
| 析氧反应 | (4) | |
| (5) | ||
| (6) | ||
| (7) |

图1 (a)酸预处理的碳纳米管海绵电氧化去除全氟辛酸[26];(b)碳纳米管/聚氨酯海绵电化学过滤器运行示意图[27]
Fig.1 (a) Acid pretreated carbon nanotube sponge electro-oxidation for removal of PFOA[26]; (b) Schematic diagram of carbon nanotube/urethane sponge electrochemical filter operation[27]

图2 具有纳米异质结结构的Ni@Ni3S2/CNT电极用于低能耗的析氢(阴极)与电催化去除盐水中的乙醇胺污染物(阳极)的示意图[34]
Fig.2 Schematic diagram of Ni@Ni3S2/CNT electrode with nanoheterojunction structure for low energy consumption of hydrogen precipitation (cathode) and electrocatalytic removal of ethanolamine contaminants from brine (anode)[34]
| 材料名称 | 析氧过电位 | 电解质 | 污染物 | 去除率/% | 矿化度 | 活性位点 | 活性物种 | 能耗 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
酸处理后 SWCNT海绵 | — | Na2SO4 | 100 μg·L-1 PFOA | 90(60 min) | — | 含氧官能团、 疏水碳骨架 | — | — | [ |
| 聚氨酯海绵负载MWCNT | — | Na2SO4 | 0.2 mmol·L-1 TCH | 92 | — | — | — | 5~100 kW·h·kg-1 | [ |
| 0.06 mmol·L-1 MO | 94 | — | |||||||
| CNT-EO | — | Na2SO4 | 1.0 mmol·L-1 PhOH | — | 去除0.22 mg·h-1·cm-2 | — | ·OH | — | [ |
| CNT-HNO3 | — | — | 去除0.06 mg·h-1·cm-2 | — | |||||
| Pt/CNT | — | NaOH | 1 mmol·L-1 DCF | 74(6 h) | 48%(8 h) | Pt | 直接氧化 | — | [ |
| Ru/CNT | — | NaHCO3/ Na2CO3 | 88(8 h) | 27%(8 h) | Ru | 直接氧化 | — | ||
| Ni@Ni3S2/MWCNT | — | NaCl | 0.5 mol·L-1 ETA | 约38(20 h) | — | Ni | 直接氧化 | — | [ |
| Ti/SnO2-Sb2O3/ CNT-PbO2 | 1.79 V (vs SCE) | Na2SO4 | 100 mg·L-1 MO | 78.6(2 h) | 58.2%(2 h) | — | ·OH | 225.40 kW·h·kg-1 | [ |
| Ti/SnO2-Sb2O3/ Bi-CNT-PbO2 | 1.89 V (vs SCE) | 84.8(2 h) | 81.8%(2 h) | — | ·OH | 165.57 kW·h·kg-1 | |||
| Bi-Sb-SnO2-CNT | — | Na2SO4 | 0.5 mmol·L-1 PhOH | 约40 (100 min) | 22%(100 min) | — | ·OH | — | [ |
| Sb-SnO2-CNT | 约30 (100 min) | 13%(100 min) | |||||||
| Ti/MWCNTs/ SnO2-Sb-Er | 2.15 V (vs SCE) | Na2SO4 | 50 mg·L-1 Cefotaxime | 83.7 (60 min) | 约80% (60 min) | — | ·OH | — | [ |
| Ti/MWCNTs/ SnO2-Sb | 约1.9 V (vs SCE) | 100 (60 min) | 约65% (60 min) |
表2 典型碳纳米管基阳极材料及其降解污染物相关参数对比
Table 2 Comparison of typical carbon nanotube-based anode materials and their parameters related to degradation of pollutants
| 材料名称 | 析氧过电位 | 电解质 | 污染物 | 去除率/% | 矿化度 | 活性位点 | 活性物种 | 能耗 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
酸处理后 SWCNT海绵 | — | Na2SO4 | 100 μg·L-1 PFOA | 90(60 min) | — | 含氧官能团、 疏水碳骨架 | — | — | [ |
| 聚氨酯海绵负载MWCNT | — | Na2SO4 | 0.2 mmol·L-1 TCH | 92 | — | — | — | 5~100 kW·h·kg-1 | [ |
| 0.06 mmol·L-1 MO | 94 | — | |||||||
| CNT-EO | — | Na2SO4 | 1.0 mmol·L-1 PhOH | — | 去除0.22 mg·h-1·cm-2 | — | ·OH | — | [ |
| CNT-HNO3 | — | — | 去除0.06 mg·h-1·cm-2 | — | |||||
| Pt/CNT | — | NaOH | 1 mmol·L-1 DCF | 74(6 h) | 48%(8 h) | Pt | 直接氧化 | — | [ |
| Ru/CNT | — | NaHCO3/ Na2CO3 | 88(8 h) | 27%(8 h) | Ru | 直接氧化 | — | ||
| Ni@Ni3S2/MWCNT | — | NaCl | 0.5 mol·L-1 ETA | 约38(20 h) | — | Ni | 直接氧化 | — | [ |
| Ti/SnO2-Sb2O3/ CNT-PbO2 | 1.79 V (vs SCE) | Na2SO4 | 100 mg·L-1 MO | 78.6(2 h) | 58.2%(2 h) | — | ·OH | 225.40 kW·h·kg-1 | [ |
| Ti/SnO2-Sb2O3/ Bi-CNT-PbO2 | 1.89 V (vs SCE) | 84.8(2 h) | 81.8%(2 h) | — | ·OH | 165.57 kW·h·kg-1 | |||
| Bi-Sb-SnO2-CNT | — | Na2SO4 | 0.5 mmol·L-1 PhOH | 约40 (100 min) | 22%(100 min) | — | ·OH | — | [ |
| Sb-SnO2-CNT | 约30 (100 min) | 13%(100 min) | |||||||
| Ti/MWCNTs/ SnO2-Sb-Er | 2.15 V (vs SCE) | Na2SO4 | 50 mg·L-1 Cefotaxime | 83.7 (60 min) | 约80% (60 min) | — | ·OH | — | [ |
| Ti/MWCNTs/ SnO2-Sb | 约1.9 V (vs SCE) | 100 (60 min) | 约65% (60 min) |

图3 (a)通过调控前体和制备方式获得含有边缘硫和骨架硫掺杂石墨烯[20];(b)掺硫还原氧化石墨烯作为活性层提升Ti/Ce-Mn/SnO2-Sb-La电极电氧化性能和稳定性[48]
Fig.3 (a) The edge sulfur containing and skeletal sulfur doped graphene by modulating the precursors and preparation[20]; (b) Sulfur doped reduced graphene oxide as an active layer to enhance the electrooxidation performance and stability of Ti/Ce-Mn/SnO2-Sb-La electrodes[48]
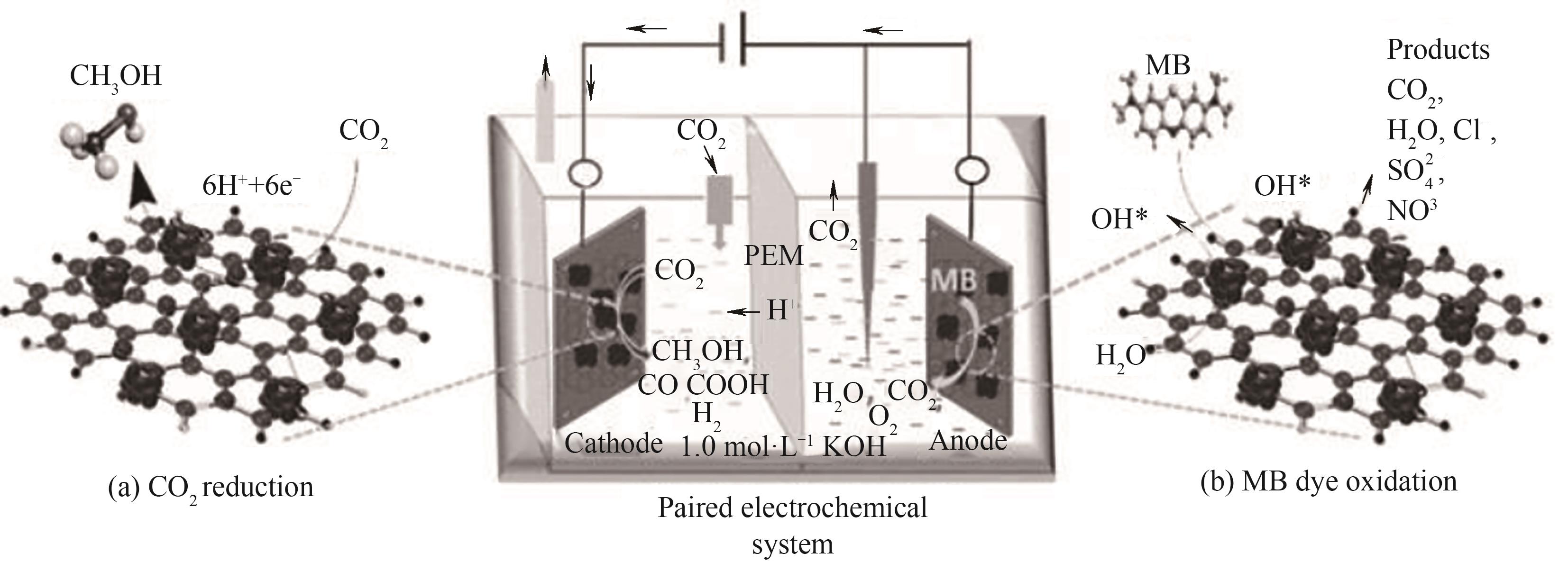
图4 双功能负载Co3O4纳米球的氮掺杂还原氧化石墨烯电极电氧化去除亚甲基蓝同时还原CO2选择性生产甲醇的示意图[53]
Fig.4 Schematic diagram of a bifunctional Co3O4 nanosphere-loaded nitrogen-doped reduced graphene oxide electrode for simultaneous electro-oxidation of methylene blue removal and CO2 reduction for selective methanol production[53]
| 材料名称 | 析氧过电位 | 电解质 | 污染物 | 去除率/% | 矿化度/% | 活性位点 | 活性物种 | 能耗 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
| P-N-GN | — | NaCl | 10 mg·L-1 APAP | 98.2±1.8 (60 min) | 78.5(180 min) | 含磷、氮元素 的官能团 | 活性氯和 | 0.017 kW·h·g-1 | [ |
| A-SGO | — | NaCl | 10×10-6 BPA | 97(120 min) | 78.5(60 min) | —COOH,—OH和C—SO3 | 活性氯和 | — | [ |
| S-GO/Pt/TiO2 | — | NaCl | 10 mg·L-1 APAP | 98(90 min) | 44.1(4 h) | —SO x 旁边的 碳原子 | 直接氧化 | 0.069 kW·h·g-1 | [ |
| —SO x | 活性氯 和·OH | ||||||||
| Ti/Ce-Mn/ SnO2-Sb-La-S-rGO | 2.12 V | Na2SO4 | 100 mg·L-1 PhOH | 89.5(120 min) | 79.8(120 min) | — | ·OH | — | [ |
| GNP-PbO2 | 2.05 V (vs SCE) | Na2SO4 | 100 mg·L-1 ENO | 92.69(120 min) | 62.5(120 min) | — | ·OH | — | [ |
| Borophene-rGO | — | 磷酸盐缓冲液和NaCl | 1 µmol·L-1 DTR | 89±1 | — | —O—C— B—N— | ·OH和1O2 | 4.13 kW·h·m-3 | [ |
| hBN-rGO | 76±1 | — | 5.73 kW·h·m-3 | ||||||
| Cu-rGO-PC | — | Na2SO4 | 20 mg·L-1 DCF | 100(60 min) | — | rGO | 直接氧化 | — | [ |
| Cu | ·OH和 活性氯 | ||||||||
| Co3O4/N-rGO | — | KOH | 100 mg·L-1 MB | 100(30 min) | — | Co | ·OH | — | [ |
表3 典型石墨烯基阳极材料及其降解污染物相关参数对比
Table 3 Comparison of typical graphene-based anode materials and their parameters related to degradation of pollutants
| 材料名称 | 析氧过电位 | 电解质 | 污染物 | 去除率/% | 矿化度/% | 活性位点 | 活性物种 | 能耗 | 文献 |
|---|---|---|---|---|---|---|---|---|---|
| P-N-GN | — | NaCl | 10 mg·L-1 APAP | 98.2±1.8 (60 min) | 78.5(180 min) | 含磷、氮元素 的官能团 | 活性氯和 | 0.017 kW·h·g-1 | [ |
| A-SGO | — | NaCl | 10×10-6 BPA | 97(120 min) | 78.5(60 min) | —COOH,—OH和C—SO3 | 活性氯和 | — | [ |
| S-GO/Pt/TiO2 | — | NaCl | 10 mg·L-1 APAP | 98(90 min) | 44.1(4 h) | —SO x 旁边的 碳原子 | 直接氧化 | 0.069 kW·h·g-1 | [ |
| —SO x | 活性氯 和·OH | ||||||||
| Ti/Ce-Mn/ SnO2-Sb-La-S-rGO | 2.12 V | Na2SO4 | 100 mg·L-1 PhOH | 89.5(120 min) | 79.8(120 min) | — | ·OH | — | [ |
| GNP-PbO2 | 2.05 V (vs SCE) | Na2SO4 | 100 mg·L-1 ENO | 92.69(120 min) | 62.5(120 min) | — | ·OH | — | [ |
| Borophene-rGO | — | 磷酸盐缓冲液和NaCl | 1 µmol·L-1 DTR | 89±1 | — | —O—C— B—N— | ·OH和1O2 | 4.13 kW·h·m-3 | [ |
| hBN-rGO | 76±1 | — | 5.73 kW·h·m-3 | ||||||
| Cu-rGO-PC | — | Na2SO4 | 20 mg·L-1 DCF | 100(60 min) | — | rGO | 直接氧化 | — | [ |
| Cu | ·OH和 活性氯 | ||||||||
| Co3O4/N-rGO | — | KOH | 100 mg·L-1 MB | 100(30 min) | — | Co | ·OH | — | [ |
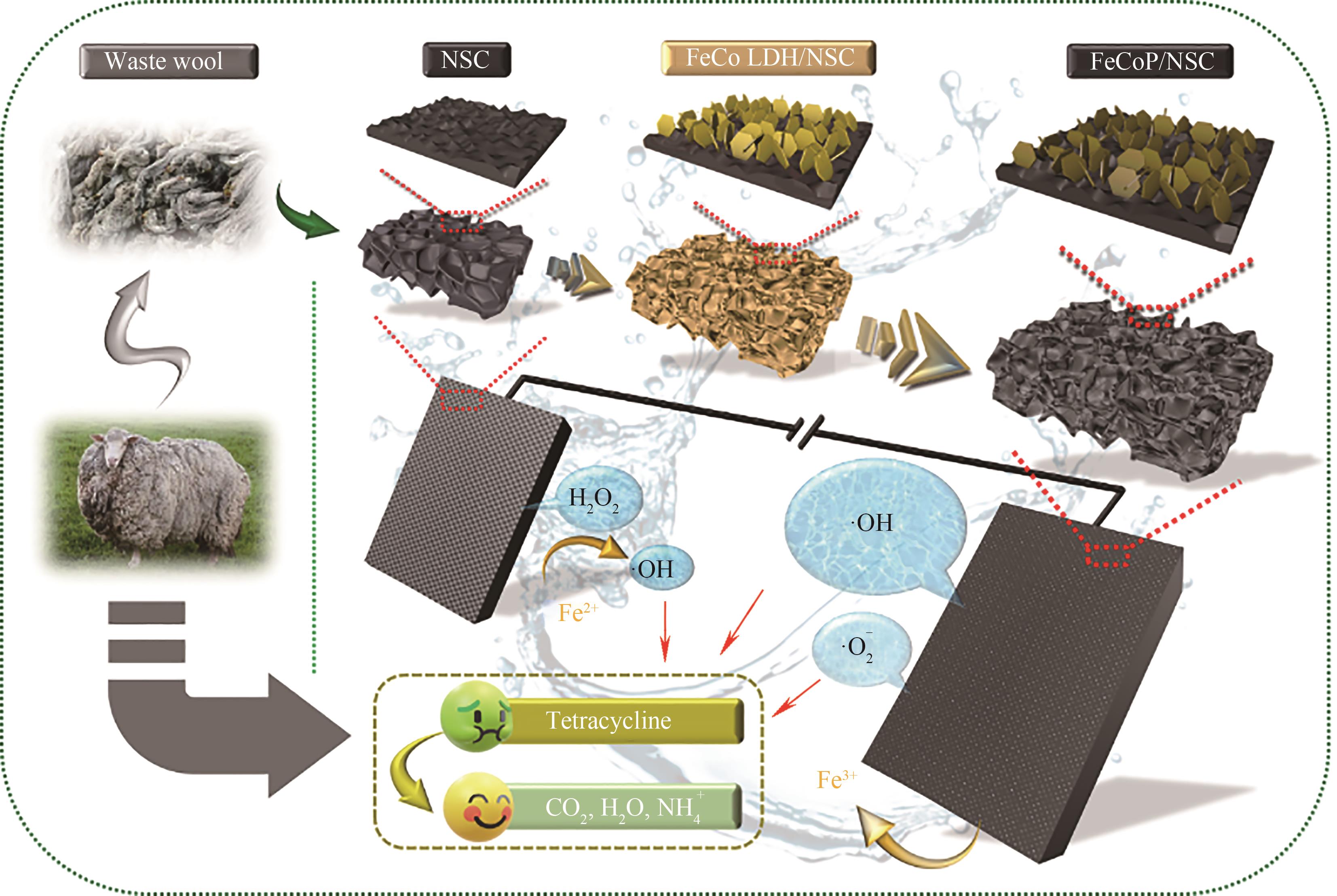
图5 废弃羊毛衍生碳负载磷化铁钴去除盐酸四环素示意图[70]
Fig.5 Schematic diagram of the removal of tetracycline hydrochloride by waste wool-derived carbon-loaded iron-cobalt[70]
| 1 | Zhuo Q F, Lu J C, Niu J F, et al. Electrocatalytic oxidation processes for treatment of halogenated organic pollutants in aqueous solution: a critical review[J]. ACS ES&T Engineering, 2022, 2(10): 1756-1775. |
| 2 | Wu J C, Chuang Y H, Liou S Y H, et al. In situ engineering of highly conductive TiO2/carbon heterostructure fibers for enhanced electrocatalytic degradation of water pollutants[J]. Journal of Hazardous Materials, 2022, 429: 128328. |
| 3 | Urtiaga A. Electrochemical technologies combined with membrane filtration[J]. Current Opinion in Electrochemistry, 2021, 27: 100691. |
| 4 | Ren G B, Li R X, Zhao M C, et al. Membrane electrodes for electrochemical advanced oxidation processes: preparation, self-cleaning mechanisms and prospects[J]. Chemical Engineering Journal, 2023, 451: 138907. |
| 5 | 周雨珺, 吉庆华, 胡承志, 等. 电化学氧化水处理技术研究进展[J]. 土木与环境工程学报, 2022, 44(3): 104-118. |
| Zhou Y J, Ji Q H, Hu C Z, et al. Recent advances in electro-oxidation technology for water treatment[J]. Journal of Civil and Environmental Engineering, 2022, 44(3): 104-118. | |
| 6 | Marinho B A, Suhadolnik L, Likozar B, et al. Photocatalytic, electrocatalytic and photoelectrocatalytic degradation of pharmaceuticals in aqueous media: analytical methods, mechanisms, simulations, catalysts and reactors[J]. Journal of Cleaner Production, 2022, 343: 131061. |
| 7 | Liu Y, Pang D, Wang L Y, et al. Electrochemically reduced phytic acid-doped TiO2 nanotubes for the efficient electrochemical degradation of toxic pollutants[J]. Journal of Hazardous Materials, 2021, 414: 125600. |
| 8 | Singh N, Goldsmith B R. Role of electrocatalysis in the remediation of water pollutants[J]. ACS Catalysis, 2020, 10(5): 3365-3371. |
| 9 | Wei L, Chen Y. Degradation of carbon materials in electrocatalysis[J]. Current Opinion in Electrochemistry, 2022, 36: 101159. |
| 10 | Zhang G Y, Liu X, Wang L, et al. Recent advances of biomass derived carbon-based materials for efficient electrochemical energy devices[J]. Journal of Materials Chemistry A, 2022, 10(17): 9277-9307. |
| 11 | 唐长斌, 卢宇轩, 王飞, 等. 用于水体中有机污染物电催化降解的非贵金属氧化物阳极的研究进展[J]. 材料工程, 2020, 48(6): 62-72. |
| Tang C B, Lu Y X, Wang F, et al. Research progress of non-precious metal oxide coated anodes for electrocatalytic degradation of organic pollutants in water[J]. Journal of Materials Engineering, 2020, 48(6): 62-72. | |
| 12 | Zeng Y X, Almatrafi E, Xia W, et al. Nitrogen-doped carbon-based single-atom Fe catalysts: synthesis, properties, and applications in advanced oxidation processes[J]. Coordination Chemistry Reviews, 2023, 475: 214874. |
| 13 | Yi Y, Tornow J, Willinger E, et al. Electrochemical degradation of multiwall carbon nanotubes at high anodic potential for oxygen evolution in acidic media[J]. ChemElectroChem, 2015, 2(12): 1929-1937. |
| 14 | 冯玉杰, 崔玉虹, 孙丽欣, 等. 电化学废水处理技术及高效电催化电极的研究与进展[J]. 哈尔滨工业大学学报, 2004, 36(4): 450-455. |
| Feng Y J, Cui Y H, Sun L X, et al. Development of electro-chemical technology and high efficiency catalytic electrode for wastewater treatment[J]. Journal of Harbin Institute of Technology, 2004, 36(4): 450-455. | |
| 15 | Li L, Yin Z, Cheng M, et al. Insights into reactive species generation and organics selective degradation in Fe-based heterogeneous Fenton-like systems: a critical review[J]. Chemical Engineering Journal, 2023, 454: 140126. |
| 16 | Guo Z J, Zhang Y, Jia H, et al. Electrochemical methods for landfill leachate treatment: a review on electrocoagulation and electrooxidation[J]. Science of the Total Environment, 2022, 806: 150529. |
| 17 | Feng H P, Yu J F, Tang L, et al. Tuning electron density endows Fe1- x Co x P with exceptional capability of electrooxidation of organic pollutants[J]. Environmental Science & Technology, 2019, 53(23): 13878-13887. |
| 18 | Gao S Y, Chen Y, Su J Z, et al. Triboelectric nanogenerator powered electrochemical degradation of organic pollutant using Pt-free carbon materials[J]. ACS Nano, 2017, 11(4): 3965-3972. |
| 19 | Duan P Z, Chen D D, Hu X. Tin dioxide decorated on Ni-encapsulated nitrogen-doped carbon nanotubes for anodic electrolysis and persulfate activation to degrade cephalexin: mineralization and degradation pathway[J]. Chemosphere, 2021, 269: 128740. |
| 20 | Zhang Q, Wang B X, Chen S, et al. S edge/center-selectively doped graphene oxide for bisphenol A electro-degradation: preparation, efficiency and mechanism[J]. Chemical Engineering Journal, 2021, 420: 127669. |
| 21 | Biswas B, Goel S. Electrocoagulation and electrooxidation technologies for pesticide removal from water or wastewater: a review[J]. Chemosphere, 2022, 302: 134709. |
| 22 | Iijima S. Helical microtubules of graphitic carbon[J]. Nature, 1991, 354: 56-58. |
| 23 | Wilson M E, Rukh M G, Ashraf M A. The role of nanotechnology, based on carbon nanotubes in water and wastewater treatment[J]. Desalination and Water Treatment, 2021, 242: 12-21. |
| 24 | Li S Z, Liu J Y, Liang J S, et al. Tuning oxygen vacancy in SnO2 inhibits Pt migration and agglomeration towards high-performing fuel cells[J]. Applied Catalysis B-Environmental, 2023, 320: 122017. |
| 25 | Tan T Y, Zeng Z T, Zeng G M, et al. Electrochemically enhanced simultaneous degradation of sulfamethoxazole, ciprofloxacin and amoxicillin from aqueous solution by multi-walled carbon nanotube filter[J]. Separation and Purification Technology, 2020, 235: 116167. |
| 26 | Xue A, Yuan Z W, Sun Y, et al. Electro-oxidation of perfluorooctanoic acid by carbon nanotube sponge anode and the mechanism[J]. Chemosphere, 2015, 141: 120-126. |
| 27 | Liu Y B, Li F, Xia Q, et al. Conductive 3D sponges for affordable and highly-efficient water purification[J]. Nanoscale, 2018, 10(10): 4771-4778. |
| 28 | Jame S A, Zhou Z. Electrochemical carbon nanotube filters for water and wastewater treatment[J]. Nanotechnology Reviews, 2016, 5(1): 41-50. |
| 29 | Chu Y B, Li Y X, Ni X Y, et al. Effect of the presence of various natural organic matters on anodic oxidation of electrified carbon nanotube membrane[J]. Environmental Science and Pollution Research, 2022, 29(47): 71179-71189. |
| 30 | Lin S H, Zhou Z, Wu H X, et al. Electrochemical oxidation of aniline using a high-flux CNT filter[J]. Journal of Water Process Engineering, 2022, 46: 102536. |
| 31 | Gao G D, Pan M L, Vecitis C D. Effect of the oxidation approach on carbon nanotube surface functional groups and electrooxidative filtration performance[J]. Journal of Materials Chemistry A, 2015, 3(14): 7575-7582. |
| 32 | Ferreira M, Kuzniarska-Biernacka I, Fonseca A M, et al. Electrochemical oxidation of amoxicillin on carbon nanotubes and carbon nanotube supported metal modified electrodes[J]. Catalysis Today, 2020, 357: 322-331. |
| 33 | Ferreira M, Guney S, Kuzniarska-Biernacka I, et al. Electrochemical oxidation of diclofenac on CNT and M/CNT modified electrodes[J]. New Journal of Chemistry, 2021, 45(28): 12622-12633. |
| 34 | Zhao B, Liu J, Feng R, et al. Less-energy consumed hydrogen evolution coupled with electrocatalytic removal of ethanolamine pollutant in saline water over Ni@Ni3S2/CNT nano-heterostructured electrocatalysts[J]. Small Methods, 2022, 6(3): e2101195. |
| 35 | Zhao W, Xing J T, Chen D H, et al. Comparative studies on the performance of porous Ti/SnO2-Sb2O3/PbO2 enhanced by CNT and Bi co-doped electrodes for methyl orange oxidation[J]. Journal of Advanced Oxidation Technologies, 2017, 20(1): 20160181. |
| 36 | Moyo M, Florence L R, Okonkwo J O. Improved electro-oxidation of triclosan at nano-zinc oxide-multiwalled carbon nanotube modified glassy carbon electrode[J]. Sensors and Actuators B: Chemical, 2015, 209: 898-905. |
| 37 | Yang S Y, Vecitis C D, Park H. Electrocatalytic water treatment using carbon nanotube filters modified with metal oxides[J]. Environmental Science and Pollution Research, 2019, 26(2): 1036-1043. |
| 38 | Lei J W, Duan P Z, Liu W J, et al. Degradation of aqueous cefotaxime in electro-oxidation-electro-Fenton-persulfate system with Ti/CNT/SnO2-Sb-Er anode and Ni@NCNT cathode[J]. Chemosphere, 2020, 250: 126163. |
| 39 | Zheng W T, Liu Y B, Liu W, et al. A novel electrocatalytic filtration system with carbon nanotube supported nanoscale zerovalent copper toward ultrafast oxidation of organic pollutants[J]. Water Research, 2021, 194: 116961. |
| 40 | Qin W L, Chen Z F, Liu X Y, et al. BiPO4-coated carbon microtube electrodes: preparation and characterization of their properties and electrocatalytic degradation of methylene blue[J]. Environmental Science and Pollution Research, 2023, 30(11): 29190-29205. |
| 41 | Wu W X, Zhao Z Y, Li M H, et al. Electrified nanohybrid filter for enhanced phosphorus removal from water[J]. Chemosphere, 2022, 303: 135226. |
| 42 | Hadrup N, Knudsen K B, Carriere M, et al. Safe-by-design strategies for lowering the genotoxicity and pulmonary inflammation of multiwalled carbon nanotubes: reduction of length and the introduction of COOH groups[J]. Environmental Toxicology and Pharmacology, 2021, 87: 103702. |
| 43 | Yao H Y, Zhu M Z, Wang P, et al. Combination of mussel inspired method and “thiol-Michael” click reaction for biocompatible alginate-modified carbon nanotubes[J]. Nanomaterials, 2021, 11(9): 2191. |
| 44 | Zhang J, Cui Z X, Liu J, et al. Bifunctional oxygen electrocatalysts for rechargeable zinc-air battery based on MXene and beyond[J]. Frontiers of Physics, 2023, 18(1): 13603. |
| 45 | 孟亮, 孙阳, 公晗, 等. 石墨烯基材料应用于水污染物治理领域的研究进展[J]. 新型炭材料, 2019, 34(3): 220-237. |
| Meng L, Sun Y, Gong H, et al. Research progress of the application of graphene-based materials in the treatment of water pollutants[J]. New Carbon Materials, 2019, 34(3): 220-237. | |
| 46 | Zhang Q, Yu Y B, Hong J M. Mechanism and efficiency research of P- and N-codoped graphene for enhanced paracetamol electrocatalytic degradation[J]. Environmental Science and Pollution Research, 2022, 29(53): 80281-80296. |
| 47 | Zhang Q, Huang W, Hong J M, et al. Deciphering acetaminophen electrical catalytic degradation using single-form S doped graphene/Pt/TiO2 [J]. Chemical Engineering Journal, 2018, 343: 662-675. |
| 48 | Bi Q, Wang Z Q, Dang C X, et al. A study on the effect of introducing S-doped GO into the active layer on the performance of wire mesh Ti/Ce-Mn/SnO2-Sb-La electrodes[J]. Journal of Alloys and Compounds, 2021, 862: 158033. |
| 49 | Dai J S, Feng H J, Shi K F, et al. Electrochemical degradation of antibiotic enoxacin using a novel PbO2 electrode with a graphene nanoplatelets inter-layer: characteristics, efficiency and mechanism[J]. Chemosphere, 2022, 307: 135833. |
| 50 | Lumbaque E C, Baptista-Pires L, Radjenovic J. Functionalization of graphene sponge electrodes with two-dimensional materials for tailored electrocatalytic activity towards specific contaminants of emerging concern[J]. Chemical Engineering Journal, 2022, 446: 137057. |
| 51 | Kumar A, Omar R A, Verma N. Efficient electro-oxidation of diclofenac persistent organic pollutant in wastewater using carbon film-supported Cu-rGO electrode[J]. Chemosphere, 2020, 248: 126030. |
| 52 | Li X L, Xia T. Characterization and preparation of metallic nanoparticles decorated graphene oxide/carbon nanotube on polytetrafluoroethylene membrane as an electrochemical filter for wastewater treatment[J]. International Journal of Electrochemical Science, 2020, 15(8): 7119-7135. |
| 53 | Bharath G, Rambabu K, Aubry C, et al. Self-assembled Co3O4 nanospheres on N-doped reduced graphene oxide (Co3O4/N-RGo) bifunctional electrocatalysts for cathodic reduction of CO2 and anodic oxidation of organic pollutants[J]. ACS Applied Energy Materials, 2021, 4(10): 11408-11418. |
| 54 | Mustafa B, Mehmood T, Wang Z Y, et al. Next-generation graphene oxide additives composite membranes for emerging organic micropollutants removal: separation, adsorption and degradation[J]. Chemosphere, 2022, 308: 136333. |
| 55 | Ren L, Ma J, Chen M, et al. Recent advances in electrocatalytic membrane for the removal of micropollutants from water and wastewater[J]. Iscience, 2022, 25(5): 104342. |
| 56 | Priyadharshini S D, Manikandan S, Kiruthiga R, et al. Graphene oxide-based nanomaterials for the treatment of pollutants in the aquatic environment: recent trends and perspectives—a review[J]. Environmental Pollution, 2022, 306: 119377. |
| 57 | De Marchi L, Pretti C, Gabriel B, et al. An overview of graphene materials: properties, applications and toxicity on aquatic environments[J]. Science of the Total Environment, 2018, 631/632: 1440-1456. |
| 58 | Gwóźdź M, Brzeczek-Szafran A. Carbon-based electrocatalyst design with phytic acid—a versatile biomass-derived modifier of functional materials[J]. International Journal of Molecular Sciences, 2022, 23(19): 11282. |
| 59 | Zhang B, Jiang Y Q, Balasubramanian R. Synthesis, formation mechanisms and applications of biomass-derived carbonaceous materials: a critical review[J]. Journal of Materials Chemistry A, 2021, 9(44): 24759-24802. |
| 60 | Yan M D, Qin Y C, Wang L X, et al. Recent advances in biomass-derived carbon materials for sodium-ion energy storage devices[J]. Nanomaterials, 2022, 12(6): 930. |
| 61 | Gopalakrishnan A, Badhulika S. Effect of self-doped heteroatoms on the performance of biomass-derived carbon for supercapacitor applications[J]. Journal of Power Sources, 2020, 480: 228830. |
| 62 | Zhou J Q, Zhang S L, Zhou Y N, et al. Biomass-derived carbon materials for high-performance supercapacitors: current status and perspective[J]. Electrochemical Energy Reviews, 2021, 4(2): 219-248. |
| 63 | Sekhon S S, Lee J, Park J S. Biomass-derived bifunctional electrocatalysts for oxygen reduction and evolution reaction: a review[J]. Journal of Energy Chemistry, 2022, 65: 149-172. |
| 64 | Yang H B, Miao J W, Hung S F, et al. Identification of catalytic sites for oxygen reduction and oxygen evolution in N-doped graphene materials: development of highly efficient metal-free bifunctional electrocatalyst[J]. Science Advances, 2016, 2(4): e1501122. |
| 65 | Guo F, Lou Y Y, Yan Q, et al. Insight into the Fe-Ni/biochar composite supported three-dimensional electro-Fenton removal of electronic industry wastewater[J]. Journal of Environmental Management, 2023, 325: 116466. |
| 66 | Ge Y H, Ke J, Li X, et al. Electro-activating persulfate via biochar catalytic cathode for sulfamethazine degradation: performance and mechanism insight[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 109020. |
| 67 | Xie S M, Li M, Liao Y X, et al. In-situ preparation of biochar-loaded particle electrode and its application in the electrochemical degradation of 4-chlorophenol in wastewater[J]. Chemosphere, 2021, 273: 128506. |
| 68 | Ren X, Tang P X, Xu L H, et al. Review on treating refractory, organics-laden wastewater using three-dimensional electrochemical reactor[J]. Desalination and Water Treatment, 2022, 267: 1-12. |
| 69 | Zhang C, Li H Q, Yang X, et al. Characterization of electrodes modified with sludge-derived biochar and its performance of electrocatalytic oxidation of azo dyes[J]. Journal of Environmental Management, 2022, 324: 116445. |
| 70 | Guo X, Yang N, Zhu Z Z, et al. Iron-cobalt phosphide nanoarrays grown on waste wool-derived carbon: an efficient electrocatalyst for degradation of tetracycline[J]. Journal of Environmental Chemical Engineering, 2022, 10(6): 108788. |
| 71 | Safian M T U, Haron U S, Ibrahim M N M. A review on bio-based graphene derived from biomass wastes[J]. Bioresources, 2020, 15(4): 9756-9785. |
| 72 | Omoriyekomwan J E, Tahmasebi A, Dou J X, et al. A review on the recent advances in the production of carbon nanotubes and carbon nanofibers via microwave-assisted pyrolysis of biomass[J]. Fuel Processing Technology, 2021, 214: 106686. |
| 73 | Kang C, Huang Y, Yang H, et al. A review of carbon dots produced from biomass wastes[J]. Nanomaterials, 2020, 10(11): 2316. |
| 74 | Tabac S, Eisenberg D. Pyrolyze this paper: can biomass become a source for precise carbon electrodes?[J]. Current Opinion in Electrochemistry, 2021, 25: 100638. |
| 75 | 李莲莲, 陈冠钦. 高性能掺硼金刚石电极的研究进展[J]. 金刚石与磨料磨具工程, 2022, 42(5): 543-551. |
| Li L L, Chen G Q. Preparation methods of boron-doped diamond electrode and its research progresses[J]. Diamond & Abrasives Engineering, 2022, 42(5): 543-551. | |
| 76 | Tonanon P, Webster R D. Recent electrode and electrolyte choices for use in small scale water treatment applications—a short review[J]. Current Opinion in Electrochemistry, 2023, 38: 101211. |
| 77 | Li Z S, Zhou B, Yang W L, et al. The effect of boron doping concentration on the electrochemical oxidation of chlorine using BDD electrode[J]. Journal of the Electrochemical Society, 2023, 170(3): 33502. |
| 78 | Chen Y H, Gao X L, Liu G S, et al. Correlation of the role of boron concentration on the microstructure and electrochemical properties of diamond electrodes[J]. Functional Diamond, 2021, 1(1): 197-204. |
| 79 | Pierpaoli M, Szopińska M, Wilk B K, et al. Electrochemical oxidation of PFOA and PFOS in landfill leachates at low and highly boron-doped diamond electrodes[J]. Journal of Hazardous Materials, 2021, 403: 123606. |
| 80 | Espinoza L C, Candia-Onfray C, Vidal J, et al. Influence of the chemical nature of boron-doped diamond anodes on wastewater treatments[J]. Current Opinion in Solid State and Materials Science, 2021, 25(6): 100963. |
| 81 | Wei Q P, Liu G S, Zhu C W, et al. Ordered structures with functional units (OSFU) enabled highly robust diamond anode for electrochemical decomposing of organic pollutants[J]. Chemical Engineering Journal, 2020, 397: 125465. |
| 82 | Yang W L, Tan J L, Chen Y H, et al. Relationship between substrate type and BDD electrode structure, performance and antibiotic tetracycline mineralization[J]. Journal of Alloys and Compounds, 2022, 890: 161760. |
| 83 | Lu X R, Ding M H, Zhang C, et al. Comparative study on stability of boron doped diamond coated titanium and niobium electrodes[J]. Diamond and Related Materials, 2019, 93: 26-33. |
| 84 | Li H C, Yang W L, Ma L, et al. 3D-printed highly ordered Ti networks-based boron-doped diamond: an unprecedented robust electrochemical oxidation anode for decomposition of refractory organics[J]. Chemical Engineering Journal, 2021, 426: 131479. |
| 85 | Chen W P, Li W, Liu F M, et al. Microstructure of boron doped diamond electrodes and studies on its basic electrochemical characteristics and applicability of dye degradation[J]. Journal of Environmental Chemical Engineering, 2020, 8(5): 104348. |
| 86 | Yang S N, Liu Y B, Shen C S, et al. Rapid decontamination of tetracycline hydrolysis product using electrochemical CNT filter: mechanism, impacting factors and pathways[J]. Chemosphere, 2020, 244: 125525. |
| 87 | Pan Z L, Yu F P, Li L, et al. Low-cost electrochemical filtration carbon membrane prepared from coal via self-bonding[J]. Chemical Engineering Journal, 2020, 385: 123928. |
| 88 | Shi C D, Yu S Y, Wang L, et al. Degradation of tetracycline/oxytetracycline by electrospun aligned polyacrylonitrile-based carbon nanofibers as anodic electrocatalysis microfiltration membrane[J]. Journal of Environmental Chemical Engineering, 2021, 9(6): 106540. |
| 89 | Li C, Feng G Q, Sun M H, et al. Preparation and application of high-performance and acid-tolerant TiO2/carbon electrocatalytic membrane for organic wastewater treatment[J]. Chemosphere, 2022, 296: 134017. |
| 90 | Huang Q, Jiang X, Xiong J, et al. Aspartic acid derivative-based MOFs: a promising green material for simultaneous removal of phosphorus and arsenic(Ⅴ) in contaminated spring water[J]. Journal of Water Process Engineering, 2023, 52: 103547. |
| 91 | Huang P F, Lei J W, Sun Z R, et al. Fabrication of MOF-derivated CuO x -C electrode for electrochemical degradation of ceftazidime from aqueous solution[J]. Chemosphere, 2021, 268: 129157. |
| 92 | Shih Y J, Huang C P, Chan Y H, et al. Electrochemical degradation of oxalic acid over highly reactive nano-textured γ- and α-MnO2/carbon electrode fabricated by KMnO4 reduction on loofah sponge-derived active carbon[J]. Journal of Hazardous Materials, 2019, 379: 120759. |
| 93 | Zhang W W, Ye W J, Hu X X, et al. Electrocatalytic degradation of humic acid using particle electrodes of activated carbon loaded with metallic cobalt[J]. Chemosphere, 2021, 263: 128200. |
| 94 | Xie W H, Shi Y L, Wang Y X, et al. Electrospun iron/cobalt alloy nanoparticles on carbon nanofibers towards exhaustive electrocatalytic degradation of tetracycline in wastewater[J]. Chemical Engineering Journal, 2021, 405: 126585. |
| 95 | Jakóbczyk P, Skowierzak G, Kaczmarzyk I, et al. Electrocatalytic performance of oxygen-activated carbon fibre felt anodes mediating degradation mechanism of acetaminophen in aqueous environments[J]. Chemosphere, 2022, 304: 135381. |
| 96 | Chen D, Shen J Y, Jiang X B, et al. Fabrication of polypyrrole/β-MnO2 modified graphite felt anode for enhancing recalcitrant phenol degradation in a bioelectrochemical system[J]. Electrochimica Acta, 2017, 244: 119-128. |
| 97 | Hernández R, Olvera-Rodríguez I, Guzmán C, et al. Microwave-assisted sol-gel synthesis of an Au-TiO2 photoanode for the advanced oxidation of paracetamol as model pharmaceutical pollutant[J]. Electrochemistry Communications, 2018, 96: 42-46. |
| 98 | Thangamani R, Periyaraman P M, Thanarasu A, et al. Electrooxidation of coragen-contaminated wastewater using graphite electrodes and sorbent nano-hydroxyapatite[J]. Environmental Technology, 2022, 43(11): 1603-1612. |
| [1] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [2] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [3] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [4] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [5] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [6] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [7] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [8] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [9] | 涂玉明, 邵高燕, 陈健杰, 刘凤, 田世超, 周智勇, 任钟旗. 钙基催化剂的设计合成及应用研究进展[J]. 化工学报, 2023, 74(7): 2717-2734. |
| [10] | 张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772. |
| [11] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [12] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [13] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [14] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [15] | 李盼, 马俊洋, 陈志豪, 王丽, 郭耘. Ru/α-MnO2催化剂形貌对NH3-SCO反应性能的影响[J]. 化工学报, 2023, 74(7): 2908-2918. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号