化工学报 ›› 2024, Vol. 75 ›› Issue (5): 1903-1911.DOI: 10.11949/0438-1157.20231246
许茹枫1( ), 陈煜成1, 高丹2, 焦静雨2, 高栋2, 王海彬2, 姚善泾1, 林东强1(
), 陈煜成1, 高丹2, 焦静雨2, 高栋2, 王海彬2, 姚善泾1, 林东强1( )
)
收稿日期:2023-12-01
修回日期:2024-02-20
出版日期:2024-05-25
发布日期:2024-06-25
通讯作者:
林东强
作者简介:许茹枫(1999—),女,硕士研究生,528869431@qq.com
基金资助:
Rufeng XU1( ), Yucheng CHEN1, Dan GAO2, Jingyu JIAO2, Dong GAO2, Haibin WANG2, Shanjing YAO1, Dongqiang LIN1(
), Yucheng CHEN1, Dan GAO2, Jingyu JIAO2, Dong GAO2, Haibin WANG2, Shanjing YAO1, Dongqiang LIN1( )
)
Received:2023-12-01
Revised:2024-02-20
Online:2024-05-25
Published:2024-06-25
Contact:
Dongqiang LIN
摘要:
针对单抗电荷异质体分离,采用离子交换层析机理模型,预测洗脱分离行为,辅助工艺条件优化。设计了校准实验,拟合得到模型参数,模型计算与实验吻合良好,具有良好的预测能力。利用模型分析比较了不同洗脱方式,得到最优的两步阶跃洗脱方案,具有较高的收率,但发现该分离过程对盐浓度极为敏感。进一步针对第一步洗脱盐浓度进行过程稳健性约束的过程优化,发现盐浓度为108.5 mmol/L时过程稳健性增强。经实验验证,两步阶跃洗脱收率最高可达到85.3%,稳健约束优化后第一步等度洗脱盐浓度操作区间增大为98.9~117.5 mmol/L。结果表明,模型辅助的工艺优化可以进行复杂条件分析,促进难分离体系的分离过程优化,并能够针对过程稳健性给出合理解决方案。
中图分类号:
许茹枫, 陈煜成, 高丹, 焦静雨, 高栋, 王海彬, 姚善泾, 林东强. 离子交换层析分离单抗电荷异质体的模型辅助过程优化[J]. 化工学报, 2024, 75(5): 1903-1911.
Rufeng XU, Yucheng CHEN, Dan GAO, Jingyu JIAO, Dong GAO, Haibin WANG, Shanjing YAO, Dongqiang LIN. Model-assisted process optimization of ion-exchange chromatography for monoclonal antibody charge variant separation[J]. CIESC Journal, 2024, 75(5): 1903-1911.
| 参数类别 | 参数名称 | 参数符号 | 数值 | 单位 |
|---|---|---|---|---|
| 层析柱参数 | 柱径 | dcol | 10 | mm |
| 床高 | L | 196 | mm | |
| 总空隙率 | εt | 0.91 | — | |
| 离子交换容量 | Λ | 0.58 | mol/L | |
| 操作条件参数 | 单抗上样浓度 | cinj | 3.16 | mg/ml |
| 线性流速 | u | 0.467 | mm/s | |
| 表观轴向分散系数 | Dapp | 0.0989 | mm2/s |
表1 层析系统和层析柱参数
Table 1 Chromatographic system and column parameters
| 参数类别 | 参数名称 | 参数符号 | 数值 | 单位 |
|---|---|---|---|---|
| 层析柱参数 | 柱径 | dcol | 10 | mm |
| 床高 | L | 196 | mm | |
| 总空隙率 | εt | 0.91 | — | |
| 离子交换容量 | Λ | 0.58 | mol/L | |
| 操作条件参数 | 单抗上样浓度 | cinj | 3.16 | mg/ml |
| 线性流速 | u | 0.467 | mm/s | |
| 表观轴向分散系数 | Dapp | 0.0989 | mm2/s |
| 参数名称 | 参数符号 | A1 | A2 | M |
|---|---|---|---|---|
| 特征电荷数 | υ | 11.58 | 11.75 | 11.89 |
| 平衡常数 | keq | 4.23×10-7 | 6.20×10-7 | 7.34×10-7 |
| 空间因子 | σ | 10.47 | 10.97 | 11.70 |
| 动力学常数 | kkin | 0.35×10-8 | 0.20×10-8 | 0.10×10-8 |
表2 SMA模型参数
Table 2 SMA model parameters
| 参数名称 | 参数符号 | A1 | A2 | M |
|---|---|---|---|---|
| 特征电荷数 | υ | 11.58 | 11.75 | 11.89 |
| 平衡常数 | keq | 4.23×10-7 | 6.20×10-7 | 7.34×10-7 |
| 空间因子 | σ | 10.47 | 10.97 | 11.70 |
| 动力学常数 | kkin | 0.35×10-8 | 0.20×10-8 | 0.10×10-8 |
| 洗脱方式 | 最大收率/%(纯度为89%) | |
|---|---|---|
| 单步洗脱 | 等度洗脱 | 59.2 |
| 梯度洗脱 | 61.9 | |
| 两步洗脱 | 两步阶跃 | 65.5 |
| 等度-梯度 | 64.2 | |
| 梯度-等度 | 65.6 | |
| 两步梯度 | 64.1 | |
| 三步洗脱 | 三步阶跃 | 65.5 |
| 等度-梯度-等度 | 65.1 | |
| 梯度-等度-梯度 | 65.0 | |
表3 不同洗脱方式的优化结果
Table 3 Process optimization of different elution methods
| 洗脱方式 | 最大收率/%(纯度为89%) | |
|---|---|---|
| 单步洗脱 | 等度洗脱 | 59.2 |
| 梯度洗脱 | 61.9 | |
| 两步洗脱 | 两步阶跃 | 65.5 |
| 等度-梯度 | 64.2 | |
| 梯度-等度 | 65.6 | |
| 两步梯度 | 64.1 | |
| 三步洗脱 | 三步阶跃 | 65.5 |
| 等度-梯度-等度 | 65.1 | |
| 梯度-等度-梯度 | 65.0 | |
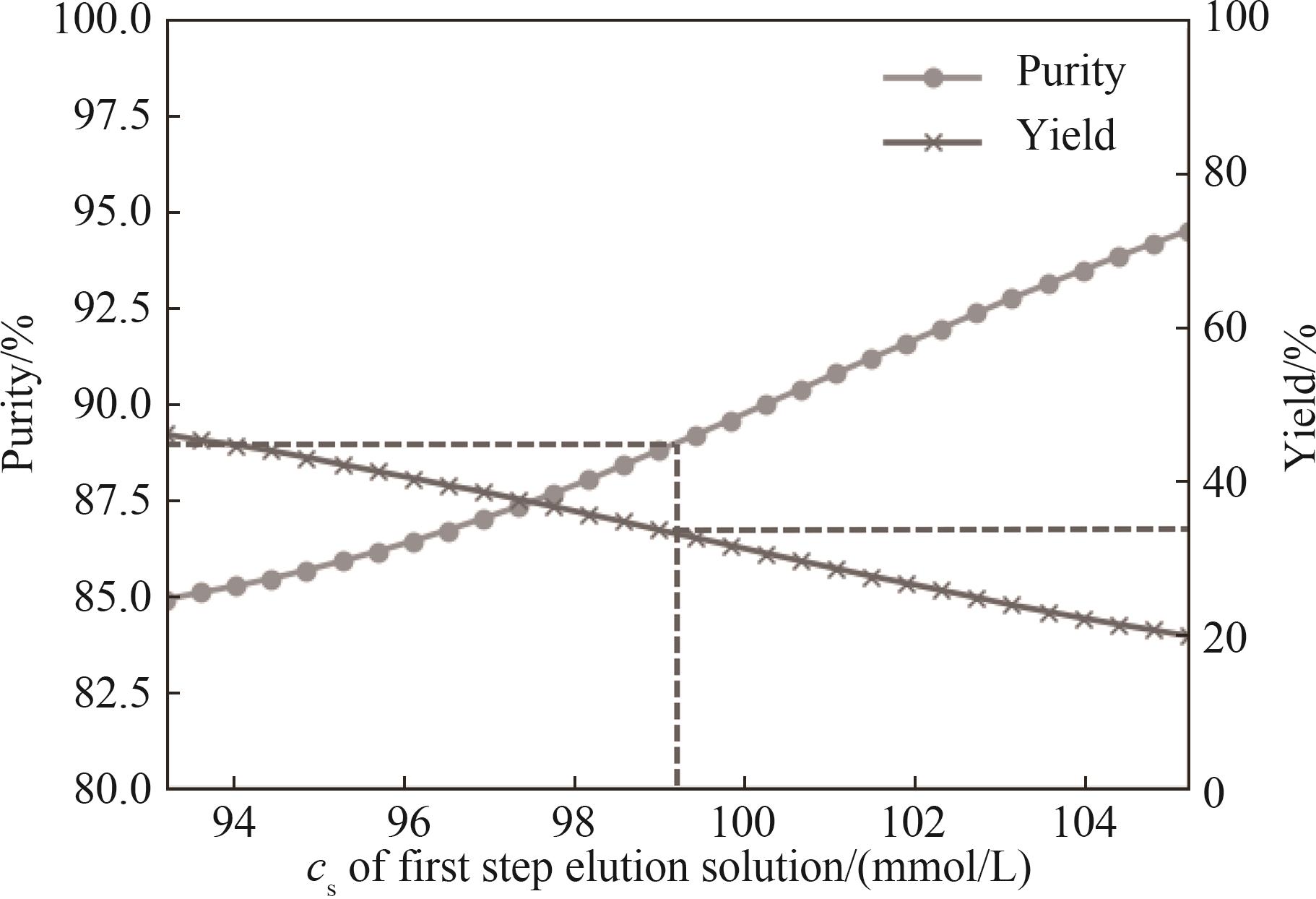
图6 第一步洗脱盐浓度对稳健优化及收集区间恒定的两步阶跃洗脱纯度和收率的影响
Fig.6 Effects of first-step elution salt concentration on purity and yield of optimized two-step stepwise elution under the robust constraints and fixed collection range
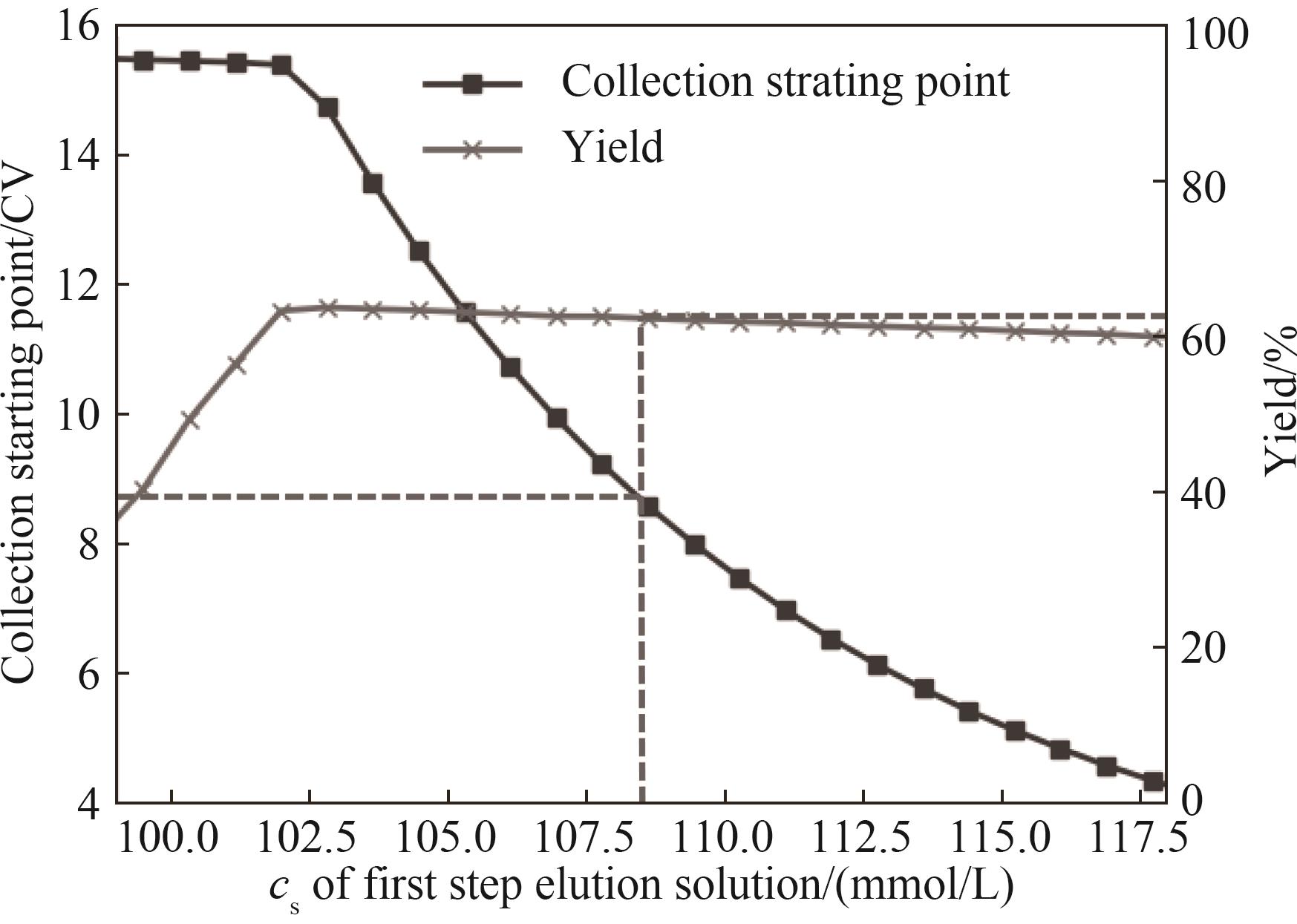
图8 第一步洗脱盐浓度对稳健优化及收集区间变化的两步阶跃洗脱收集起点和收率的影响
Fig.8 Effects of first-step elution salt concentration on purity and yield of optimized two-step stepwise elution under the robust constraints and changed collection range
| 序号 | 洗脱总长度/CV | 收集 起点/CV | 第一步等度洗脱 | 纯度 | 收率 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 洗脱长度/CV | 盐浓度/(mmol/L) | 预测值/% | 实验值/% | 偏差/% | 预测值/% | 实验值/% | 偏差/% | |||
| Ⅰ | 15.0 | 13.80 | 14.42 | 103.4 | 89.0 | 92.2 | 3.47 | 65.5 | 64.2 | 2.02 |
| Ⅱ | 15.0 | 11.00 | 14.38 | 108.5 | 89.0 | 91.1 | 2.31 | 63.4 | 59.8 | 6.02 |
| Ⅲ | 15.0 | 16.06 | 14.38 | 98.9 | 89.0 | 91.7 | 2.94 | 43.8 | 43.5 | 0.69 |
| Ⅳ | 15.0 | 4.40 | 14.38 | 117.5 | 89.0 | 89.0 | 0 | 60.0 | 57.5 | 4.35 |
表4 层析分离验证实验与模型预测结果比较
Table 4 Comparison of chromatographic separation verification experiments and model predicted results
| 序号 | 洗脱总长度/CV | 收集 起点/CV | 第一步等度洗脱 | 纯度 | 收率 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 洗脱长度/CV | 盐浓度/(mmol/L) | 预测值/% | 实验值/% | 偏差/% | 预测值/% | 实验值/% | 偏差/% | |||
| Ⅰ | 15.0 | 13.80 | 14.42 | 103.4 | 89.0 | 92.2 | 3.47 | 65.5 | 64.2 | 2.02 |
| Ⅱ | 15.0 | 11.00 | 14.38 | 108.5 | 89.0 | 91.1 | 2.31 | 63.4 | 59.8 | 6.02 |
| Ⅲ | 15.0 | 16.06 | 14.38 | 98.9 | 89.0 | 91.7 | 2.94 | 43.8 | 43.5 | 0.69 |
| Ⅳ | 15.0 | 4.40 | 14.38 | 117.5 | 89.0 | 89.0 | 0 | 60.0 | 57.5 | 4.35 |
| 1 | Mullard A. FDA approves 100th monoclonal antibody product[J]. Nature Reviews Drug Discovery, 2021, 20: 491-495. |
| 2 | Fisher A C, Kamga M H, Agarabi C, et al. The current scientific and regulatory landscape in advancing integrated continuous biopharmaceutical manufacturing[J]. Trends in Biotechnology, 2019, 37(3): 253-267. |
| 3 | Kaplon H, Chenoweth A, Crescioli S, et al. Antibodies to watch in 2022[J]. MAbs, 2022, 14(1): 2014296. |
| 4 | Rathore A S, Winkle H. Quality by design for biopharmaceuticals[J]. Nature Biotechnology, 2009, 27: 26-34. |
| 5 | Saleh D, Wang G, Rischawy F, et al. In silico process characterization for biopharmaceutical development following the quality by design concept[J]. Biotechnology Progress, 2021, 37(6): e3196. |
| 6 | Hong G, Bazin-Redureau M I, Scherrmann J M. Pharmacokinetics and organ distribution of cationized colchicine-specific IgG and Fab fragments in rat[J]. Journal of Pharmaceutical Sciences, 1999, 88(1): 147-153. |
| 7 | Chung S, Tian J, Tan Z J, et al. Industrial bioprocessing perspectives on managing therapeutic protein charge variant profiles[J]. Biotechnology and Bioengineering, 2018, 115(7): 1646-1665. |
| 8 | Ahmed S, Atia N N, Rageh A H. Selectivity enhanced cation exchange chromatography for simultaneous determination of peptide variants[J]. Talanta, 2019, 199: 347-354. |
| 9 | Fekete S, Beck A, Veuthey J L, et al. Ion-exchange chromatography for the characterization of biopharmaceuticals[J]. Journal of Pharmaceutical and Biomedical Analysis, 2015, 113: 43-55. |
| 10 | Benner S W, Welsh J P, Rauscher M A, et al. Prediction of lab and manufacturing scale chromatography performance using mini-columns and mechanistic modeling[J]. Journal of Chromatography. A, 2019, 1593: 54-62. |
| 11 | Jakobsson N, Degerman M, Stenborg E, et al. Model based robustness analysis of an ion-exchange chromatography step[J]. Journal of Chromatography. A, 2007, 1138(1/2): 109-119. |
| 12 | Close E J, Salm J R, Iskra T, et al. Fouling of an anion exchange chromatography operation in a monoclonal antibody process: visualization and kinetic studies[J]. Biotechnology and Bioengineering, 2013, 110(9): 2425-2435. |
| 13 | Andris S, Hubbuch J. Modeling of hydrophobic interaction chromatography for the separation of antibody-drug conjugates and its application towards quality by design[J]. Journal of Biotechnology, 2020, 317: 48-58. |
| 14 | Lin D Q, Zhang Q L, Yao S J. Model-assisted approaches for continuous chromatography: current situation and challenges[J]. Journal of Chromatography. A, 2021, 1637: 461855. |
| 15 | Forrer N, Butté A, Morbidelli M. Chromatographic behavior of a polyclonal antibody mixture on a strong cation exchanger column (part Ⅰ): Adsorption characterization[J]. Journal of Chromatography. A, 2008, 1214(1/2): 59-70. |
| 16 | Brooks C A, Cramer S M, Rosano T G. Preparative chromatographic purification of cyclosporine metabolites[J]. Clinical Chemistry, 1993, 39(3): 457-466. |
| 17 | Yamamoto S. Plate height determination for gradient elution chromatography of proteins[J]. Biotechnology and Bioengineering, 1995, 48(5): 444-451. |
| 18 | Keller W R, Evans S T, Ferreira G, et al. Use of MiniColumns for linear isotherm parameter estimation and prediction of benchtop column performance[J]. Journal of Chromatography. A, 2015, 1418: 94-102. |
| 19 | Saleh D, Wang G, Mueller B, et al. Cross-scale quality assessment of a mechanistic cation exchange chromatography model[J]. Biotechnology Progress, 2021, 37(1): e3081. |
| 20 | Creasy A, Reck J, Pabst T, et al. Systematic interpolation method predicts antibody monomer-dimer separation by gradient elution chromatography at high protein loads[J]. Biotechnology Journal, 2019, 14(3): e1800132. |
| 21 | Saleh D, Wang G, Müller B, et al. Straightforward method for calibration of mechanistic cation exchange chromatography models for industrial applications[J]. Biotechnology Progress, 2020, 36(4): e2984. |
| 22 | Chen Y C, Yao S J, Lin D Q. Parameter-by-parameter method for steric mass action model of ion exchange chromatography: theoretical considerations and experimental verification[J]. Journal of Chromatography. A, 2022, 1680: 463418. |
| 23 | Chen Y C, Yao S J, Lin D Q. Parameter-by-parameter method for steric mass action model of ion exchange chromatography: simplified estimation for steric shielding factor[J]. Journal of Chromatography. A, 2023, 1687: 463655. |
| 24 | Huuk T C, Briskot T, Hahn T, et al. A versatile noninvasive method for adsorber quantification in batch and column chromatography based on the ionic capacity[J]. Biotechnology Progress, 2016, 32(3): 666-677. |
| 25 | Hahn T, Huuk T, Osberghaus A, et al. Calibration-free inverse modeling of ion-exchange chromatography in industrial antibody purification[J]. Engineering in Life Sciences, 2016, 16(2): 107-113. |
| 26 | Rüdt M, Gillet F, Heege S, et al. Combined Yamamoto approach for simultaneous estimation of adsorption isotherm and kinetic parameters in ion-exchange chromatography[J]. Journal of Chromatography. A, 2015, 1413: 68-76. |
| 27 | Heymann W, Glaser J, Schlegel F, et al. Advanced score system and automated search strategies for parameter estimation in mechanistic chromatography modeling[J]. Journal of Chromatography A, 2022, 1661: 462693. |
| 28 | Saleh D, Hess R, Ahlers-Hesse M, et al. Modeling the impact of amino acid substitution in a monoclonal antibody on cation exchange chromatography[J]. Biotechnology and Bioengineering, 2021, 118(8): 2923-2933. |
| 29 | Natarajan V, Ghose S, Cramer S M. Comparison of linear gradient and displacement separations in ion-exchange systems[J]. Biotechnology and Bioengineering, 2002, 78(4): 365-375. |
| 30 | Hahn T, Baumann P, Huuk T, et al. UV absorption-based inverse modeling of protein chromatography[J]. Engineering in Life Sciences, 2016, 16(2): 99-106. |
| 31 | Cherra D E, Khattabi S, Guiochon G. Adsorption behavior and prediction of the band profiles of the enantiomers of 3-chloro-1-phenyl-1-propanol. Influence of the mass transfer kinetics[J]. Journal of Chromatography. A, 2000, 877(1/2): 109-122. |
| [1] | 汪威, 白旭, 赵翔, 马学良, 林纬, 喻九阳. 基于响应面法的气浮旋流分离条件优化[J]. 化工学报, 2024, 75(5): 1929-1938. |
| [2] | 李添翼, 武玉泰, 王永胜, 顾佳锐, 宋沂恒, 杨丰铖, 郝广平. 轻同位素分离纯化与催化标记研究进展[J]. 化工学报, 2024, 75(4): 1284-1301. |
| [3] | 李俊, 赵亮, 高金森, 徐春明. 不同馏分油分级分质加工中萃取技术研究进展[J]. 化工学报, 2024, 75(4): 1065-1080. |
| [4] | 吕田田, 原敏, 王江, 高美珍, 杨佳辉, 徐红, 董晋湘, 石琪. ZTIF基疏水微介孔碳的制备及5-羟甲基糠醛吸附分离性能[J]. 化工学报, 2024, 75(4): 1642-1654. |
| [5] | 张子佳, 仇昕月, 孙翔, 罗志斌, 罗海中, 贺高红, 阮雪华. 聚酰亚胺膜材料分子结构设计强化CO2渗透性研究进展[J]. 化工学报, 2024, 75(4): 1137-1152. |
| [6] | 莫滨宇, 张雅馨, 刘国振, 刘公平, 金万勤. 面向一/二价离子分离的金属有机骨架膜研究进展[J]. 化工学报, 2024, 75(4): 1183-1197. |
| [7] | 文一如, 付佳, 刘大欢. 基于机器学习的MOFs材料研究进展:能源气体吸附分离[J]. 化工学报, 2024, 75(4): 1370-1381. |
| [8] | 董霄, 白志山, 杨晓勇, 殷伟, 刘宁普, 于启凡. CHPPO工艺氧化液耦合除杂技术的研究与工业应用[J]. 化工学报, 2024, 75(4): 1630-1641. |
| [9] | 刘莹, 郑芳, 杨启炜, 张治国, 任其龙, 鲍宗必. 二甲苯异构体吸附分离研究进展[J]. 化工学报, 2024, 75(4): 1081-1095. |
| [10] | 张凯博, 沈佳新, 李玉霞, 谈朋, 刘晓勤, 孙林兵. Y沸石中Cu(Ⅰ)的可控构筑及其乙烯/乙烷吸附分离性能研究[J]. 化工学报, 2024, 75(4): 1607-1615. |
| [11] | 李宁, 朱朋飞, 张立峰, 卢栋臣. 基于非凸与不可分离正则化算法的电容层析成像图像重建[J]. 化工学报, 2024, 75(3): 836-846. |
| [12] | 邢雷, 关帅, 蒋明虎, 赵立新, 蔡萌, 刘海龙, 陈德海. 高气液比井下气液旋流分离器结构设计与性能分析[J]. 化工学报, 2024, 75(3): 900-913. |
| [13] | 陶明清, 慕明昊, 程滕, 王博. 喷雾耦合降温强化旋风分离器脱除细颗粒物的研究[J]. 化工学报, 2024, 75(2): 584-592. |
| [14] | 王尤佳, 赵亮, 高金森, 徐春明. 柴油烃类族组成分离技术研究进展[J]. 化工学报, 2024, 75(1): 20-32. |
| [15] | 郑雨婷, 方冠东, 张梦波, 张浩淼, 王靖岱, 阳永荣. 微化工精馏分离技术研究进展[J]. 化工学报, 2024, 75(1): 47-59. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号