化工学报 ›› 2025, Vol. 76 ›› Issue (1): 18-39.DOI: 10.11949/0438-1157.20240659
收稿日期:2024-06-14
修回日期:2024-08-04
出版日期:2025-01-25
发布日期:2025-02-08
通讯作者:
张晓方
作者简介:纪之骄(1996—),女,博士,工程师,20089955@ceic.com
Zhijiao JI( ), Xiaofang ZHANG(
), Xiaofang ZHANG( ), Wen GAN, Yunpeng XUE
), Wen GAN, Yunpeng XUE
Received:2024-06-14
Revised:2024-08-04
Online:2025-01-25
Published:2025-02-08
Contact:
Xiaofang ZHANG
摘要:
电催化氮还原制氨(e-NRR)是一种低碳绿色的氨合成方法,高效电催化剂的开发是打破e-NRR热力学限制,推动该技术走向工业化的关键。单原子催化剂原子利用率高,有望用于e-NRR并实现高的法拉第效率和氨产率,但受限于单原子的高表面能,需要选择合适的载体以稳定单原子位点,并利用载体-金属强相互作用(SMSI)进一步提高催化活性。以e-NRR机理为基础,系统总结了单原子催化剂的合成与表征方法以及不同载体负载的单原子催化剂在e-NRR中的应用,归纳了单原子催化剂的优化与调控策略,分析了单原子催化剂在e-NRR领域的发展趋势。研究发现碳基材料负载的单原子催化剂应用最为广泛,而以氧化物、硫族化合物、MXenes为载体的单原子催化剂以及单原子合金催化剂在e-NRR领域的研究更多停留在理论,具有广阔的研发空间。在载体中构建缺陷以增强SMSI,或构建双单原子以实现协同催化是进一步提高e-NRR性能的有效策略。
中图分类号:
纪之骄, 张晓方, 甘汶, 薛云鹏. 载体对单原子电催化剂合成氨性能的影响与调控策略[J]. 化工学报, 2025, 76(1): 18-39.
Zhijiao JI, Xiaofang ZHANG, Wen GAN, Yunpeng XUE. Influence of support on the performance of single atom electrocatalyst for ammonia synthesis and the control strategy[J]. CIESC Journal, 2025, 76(1): 18-39.
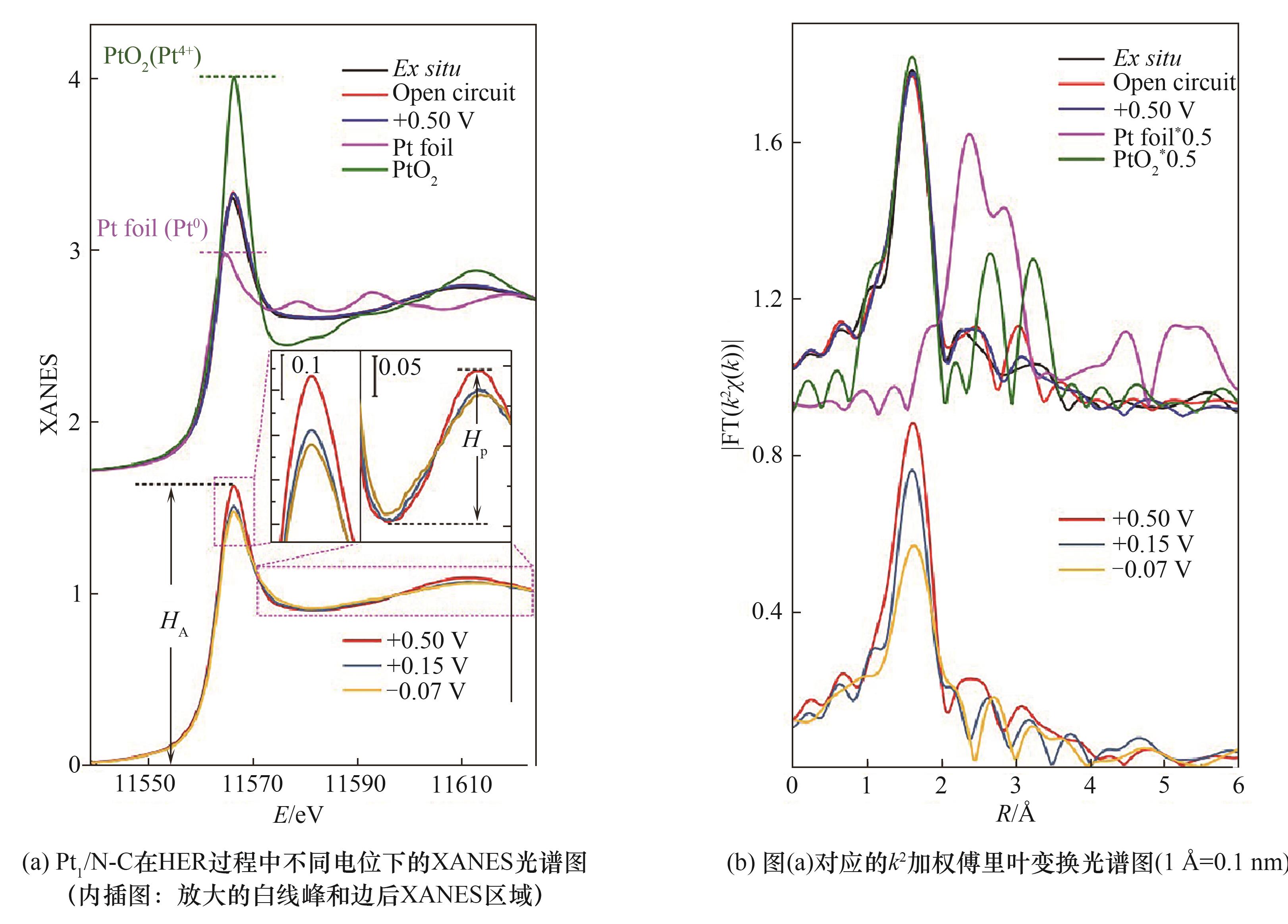
图8 Pt1/N-C的变电位XANES表征以及对应的k2加权傅里叶变换光谱[55]
Fig.8 XANES spectra of Pt1/N-C at different applied voltages and corresponding k2-weighted Fourier transform spectra[55]
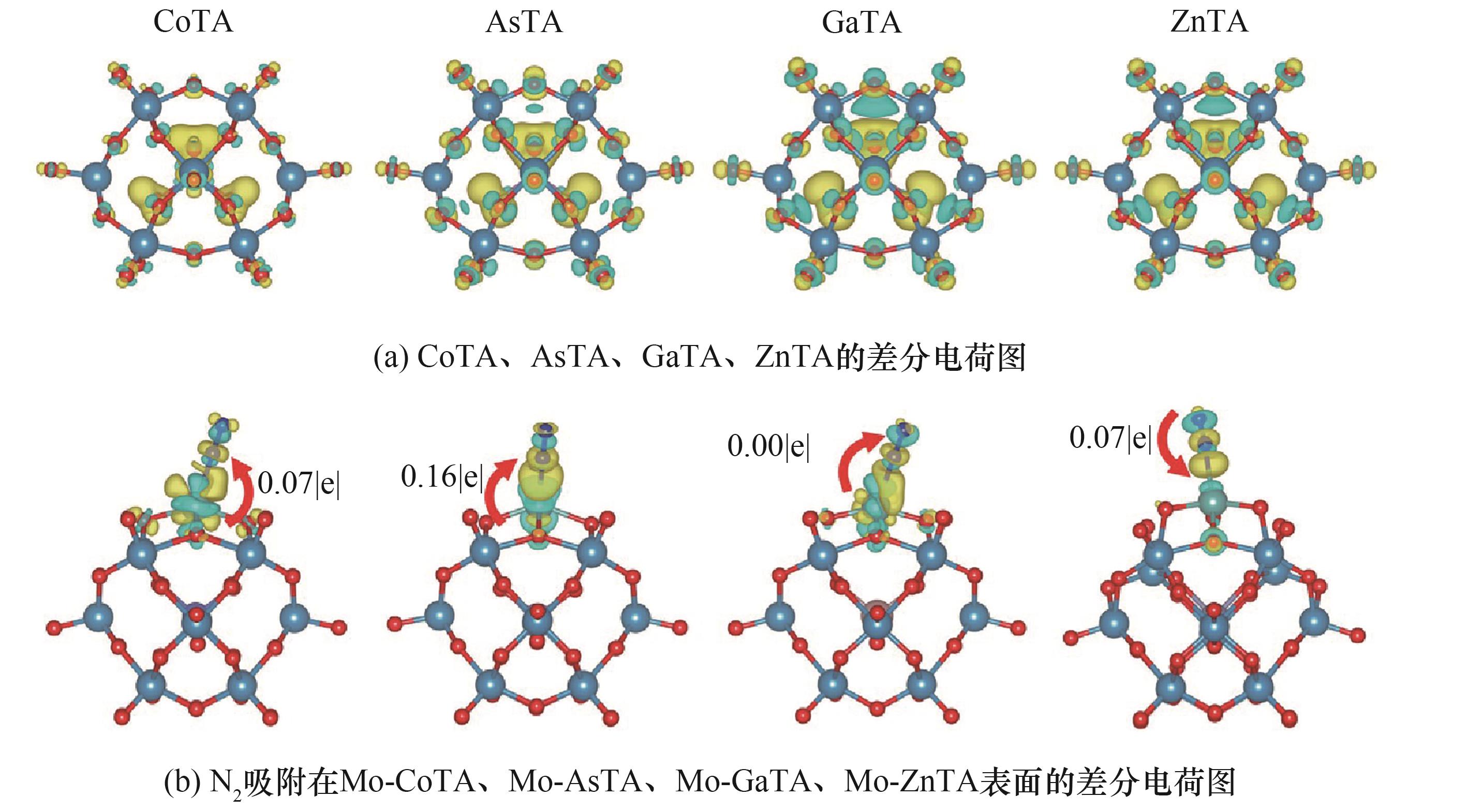
图10 CoTA、AsTA、GaTA、ZnTA和N2吸附在Mo-CoTA、Mo-AsTA、Mo-GaTA、Mo-ZnTA表面的差分电荷图[5]
Fig.10 Differential charge diagram of CoTA, AsTA, GaTA, ZnTA and N2 adsorbed on Mo-CoTA, Mo-AsTA, Mo-GaTA, Mo-ZnTA surfaces[5]
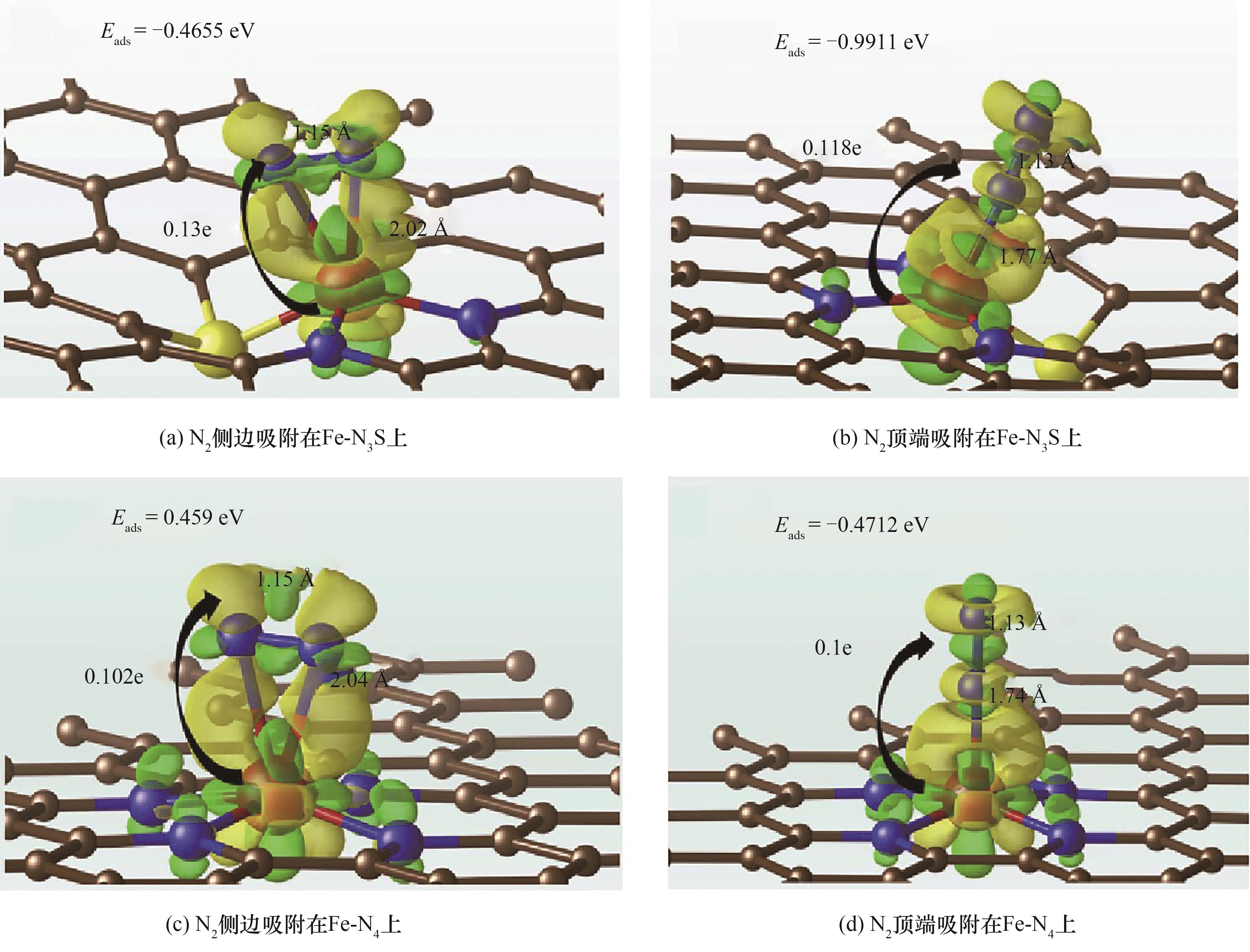
图11 Fe-N3S和Fe-N4活化N2的配位结构(负电荷和正电荷的变化分别显示为黄色和绿色)[72]
Fig.11 N2 activation on Fe-N3S and Fe-N4 coordination structure (negative and positive charge changes are displayed in yellow and green, respectively)[72]
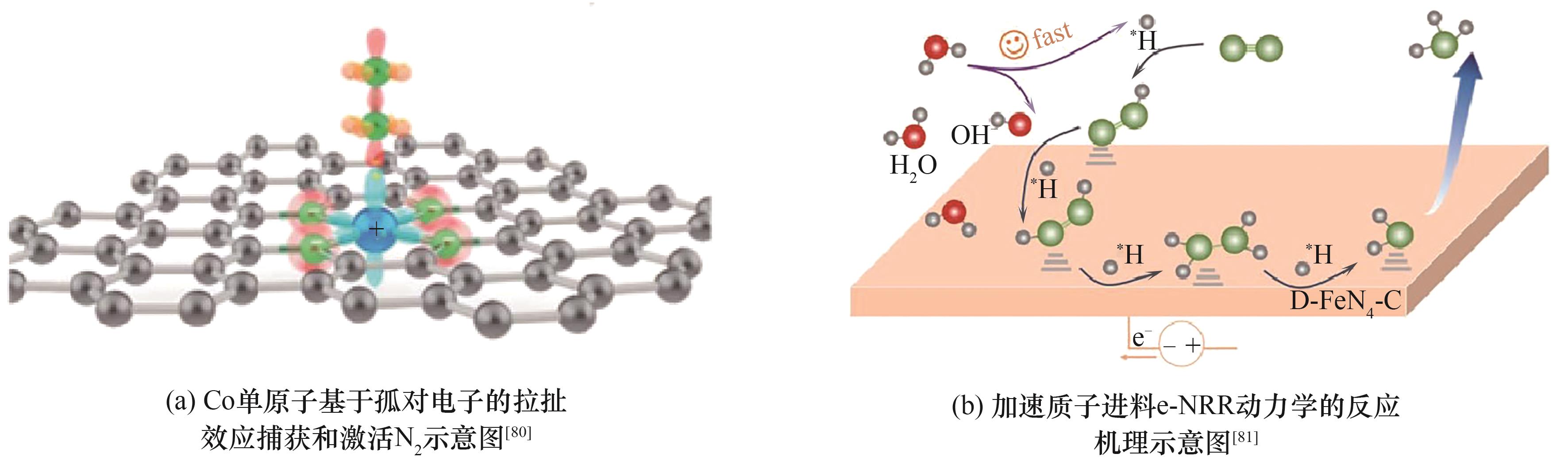
图12 非金属元素掺杂碳纤维载体负载的单原子催化剂催化机理示意图
Fig.12 Schematic diagram of catalytic mechanism of non-metallic element-doped carbon fiber carrier-loaded single-atom catalysts

图14 SA Ru-Mo2CT x 纳米片催化剂制备流程、电荷密度差分图以及N2在Ru单原子上的还原过程示意图[6](蓝色、紫色、棕色、红色、灰色、粉色球体分别代表Ru、Mo、C、O、N和H)
Fig.14 Preparation process of SA Ru-Mo2CT x nanosheet, charge density difference diagram, and the diagram of reduction process of N2 on Ru single atom[6]
| 序号 | 催化剂 | 电解液 | 最高氨产率/ | 最高FE/% | 负载量/ | 文献 | |
|---|---|---|---|---|---|---|---|
| 载体 | 组成 | (µg·h-1·mg -1) | %(质量分数) | ||||
| 1 | 金属氧化物 | Nb-TiO2(110) | 0.1 mol·L-1 Na2SO4 | 21.3 | 9.2 | 3.36 | [ |
| 2 | Cu SAs/TiO2 | 0.5 mol·L-1 K2SO4 | 6.3 | 12.9 | 1.44 | [ | |
| 3 | Cd/In2O3(VO) | 0.1 mol·L-1 KOH | 57.5 | 4.5 | 0.098 | [ | |
| 4 | FeSA-NO-C | 0.1 mol·L-1 HCl | 31.9 | 11.8 | 0.78 | [ | |
| 5 | D-FeN/C | 0.1 mol·L-1 KOH | 24.8 | 15.8 | 0.50 | [ | |
| 6 | Fe-N3S | 0.1 mol·L-1 KOH | 28.9 | 23.7 | 0.94 | [ | |
| 7 | FeMo/CN | 0.1 mol·L-1 Na2SO4 | 26.8 | 11.8 | — | [ | |
| 8 | FeRu-CNS | 0.1 mol·L-1 Na2SO4 | 43.9 | 29.3 | 1.00 | [ | |
| 9 | Co-SA/N-SCF | 0.01 mol·L-1 HCl | 67.6 | 56.9 | 4.66 | [ | |
| 10 | Zn1N-C | 0.1 mol·L-1 KOH | 16.1 | 11.8 | 1.64 | [ | |
| 11 | MoSA/ CMF-S | 0.1 mol·L-1 HCl | 46.6 | 28.9 | 0.83 | [ | |
| 12 | W-NO/NC | 0.5 mol·L-1 LiClO4 | 12.6 | 8.4 | 10.20 | [ | |
| 13 | Fe-B/N-C | 0.1 mol·L-1 HCl | 100.1 | 23.0 | — | [ | |
| 14 | MXenes | Ru SAs/Ti3C2O | 0.1 mol·L-1 HCl | 27.6 | 23.3 | 0.43 | [ |
| 15 | SA Ru-Mo2CT x | 0.5 mol·L-1 K2SO4 | 40.57 | 25.8 | 1.41 | [ | |
| 16 | Sb SA/N-Ti3C2T x | 0.5 mol·L-1 K2SO4 | 108.3 | 41.2 | 2.20 | [ | |
| 17 | 硒化物 | AuSA/npMoSe2 | 0.1 mol·L-1 Na2SO4 | 30.8 | 37.8 | 3.80 | [ |
| 18 | 单原子合金 | PdFe1 | 0.5 mol·L-1 LiClO4 | 111.9 | 37.8 | — | [ |
| 19 | PdFe3 | 0.1 mol·L-1 KOH | 29.07 | 22.8 | — | [ | |
| 20 | 钙钛矿 | LaFeO-Ru | 0.1 mol·L-1 K2SO4 | 约137.5 | 约56.9 | — | [ |
表1 不同载体负载单原子催化剂的e-NRR性能
Table 1 e-NRR performance of single atom catalysts supported by different support
| 序号 | 催化剂 | 电解液 | 最高氨产率/ | 最高FE/% | 负载量/ | 文献 | |
|---|---|---|---|---|---|---|---|
| 载体 | 组成 | (µg·h-1·mg -1) | %(质量分数) | ||||
| 1 | 金属氧化物 | Nb-TiO2(110) | 0.1 mol·L-1 Na2SO4 | 21.3 | 9.2 | 3.36 | [ |
| 2 | Cu SAs/TiO2 | 0.5 mol·L-1 K2SO4 | 6.3 | 12.9 | 1.44 | [ | |
| 3 | Cd/In2O3(VO) | 0.1 mol·L-1 KOH | 57.5 | 4.5 | 0.098 | [ | |
| 4 | FeSA-NO-C | 0.1 mol·L-1 HCl | 31.9 | 11.8 | 0.78 | [ | |
| 5 | D-FeN/C | 0.1 mol·L-1 KOH | 24.8 | 15.8 | 0.50 | [ | |
| 6 | Fe-N3S | 0.1 mol·L-1 KOH | 28.9 | 23.7 | 0.94 | [ | |
| 7 | FeMo/CN | 0.1 mol·L-1 Na2SO4 | 26.8 | 11.8 | — | [ | |
| 8 | FeRu-CNS | 0.1 mol·L-1 Na2SO4 | 43.9 | 29.3 | 1.00 | [ | |
| 9 | Co-SA/N-SCF | 0.01 mol·L-1 HCl | 67.6 | 56.9 | 4.66 | [ | |
| 10 | Zn1N-C | 0.1 mol·L-1 KOH | 16.1 | 11.8 | 1.64 | [ | |
| 11 | MoSA/ CMF-S | 0.1 mol·L-1 HCl | 46.6 | 28.9 | 0.83 | [ | |
| 12 | W-NO/NC | 0.5 mol·L-1 LiClO4 | 12.6 | 8.4 | 10.20 | [ | |
| 13 | Fe-B/N-C | 0.1 mol·L-1 HCl | 100.1 | 23.0 | — | [ | |
| 14 | MXenes | Ru SAs/Ti3C2O | 0.1 mol·L-1 HCl | 27.6 | 23.3 | 0.43 | [ |
| 15 | SA Ru-Mo2CT x | 0.5 mol·L-1 K2SO4 | 40.57 | 25.8 | 1.41 | [ | |
| 16 | Sb SA/N-Ti3C2T x | 0.5 mol·L-1 K2SO4 | 108.3 | 41.2 | 2.20 | [ | |
| 17 | 硒化物 | AuSA/npMoSe2 | 0.1 mol·L-1 Na2SO4 | 30.8 | 37.8 | 3.80 | [ |
| 18 | 单原子合金 | PdFe1 | 0.5 mol·L-1 LiClO4 | 111.9 | 37.8 | — | [ |
| 19 | PdFe3 | 0.1 mol·L-1 KOH | 29.07 | 22.8 | — | [ | |
| 20 | 钙钛矿 | LaFeO-Ru | 0.1 mol·L-1 K2SO4 | 约137.5 | 约56.9 | — | [ |
| 1 | Liu H Z. Ammonia synthesis catalyst 100 years: practice, enlightenment and challenge[J]. Chinese Journal of Catalysis, 2014, 35(10): 1619-1640. |
| 2 | Liu M D, Zhang S, Chen M, et al. An isolated bimetallic Fe-Ru single-atom catalyst for efficient electrochemical nitrogen reduction[J]. Journal of Materials Chemistry A, 2023, 11(27): 14900-14910. |
| 3 | 刘恒源, 王海辉, 徐建鸿. 电催化氮还原合成氨电化学系统研究进展[J]. 化工学报, 2022, 73(1): 32-45. |
| Liu H Y, Wang H H, Xu J H. Advances in electrochemical systems for ammonia synthesis by electrocatalytic reduction of nitrogen[J]. CIESC Journal, 2022, 73(1): 32-45. | |
| 4 | Guo W H, Zhang K X, Liang Z B, et al. Electrochemical nitrogen fixation and utilization: theories, advanced catalyst materials and system design[J]. Chemical Society Reviews, 2019, 48(24): 5658-5716. |
| 5 | Lin L H, Wei F F, Jiang R, et al. The role of central heteroatom in electrochemical nitrogen reduction catalyzed by polyoxometalate-supported single-atom catalyst[J]. Nano Research, 2023, 16(1): 309-317. |
| 6 | Peng W, Luo M, Xu X D, et al. Spontaneous atomic ruthenium doping in Mo2CT X MXene defects enhances electrocatalytic activity for the nitrogen reduction reaction[J]. Advanced Energy Materials, 2020, 10(25): 2001364. |
| 7 | Zhai X W, Dong H X, Li Y F, et al. Termination effects of single-atom decorated v-Mo2CT x MXene for the electrochemical nitrogen reduction reaction[J]. Journal of Colloid and Interface Science, 2022, 605: 897-905. |
| 8 | Shi R, Zhang X R, Waterhouse G I N, et al. The journey toward low temperature, low pressure catalytic nitrogen fixation[J]. Advanced Energy Materials, 2020, 10(19): 2000659. |
| 9 | 王如意, 胡国艳, 王雪, 等. 合成氨电催化剂及其设计策略的研究进展[J]. 功能材料, 2023, 54(7): 7069-7079. |
| Wang R Y, Hu G Y, Wang X, et al. Recent developments and design strategies of electrocatalyts in ammonia synthesis[J]. Journal of Functional Materials, 2023, 54(7): 7069-7079. | |
| 10 | Dai Z C, Tian W J, Wang Z Q, et al. Ternary AuPS alloy mesoporous film for efficient electroreduction of nitrogen to ammonia[J]. ACS Applied Materials & Interfaces, 2021, 13(24): 28057-28063. |
| 11 | Wang Q Q, Zheng G K, Hao S Y, et al. Au1Co1 alloy supported on graphene oxide with enhanced performance for ambient electrolysis of nitrogen to ammonia[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(1): 44-49. |
| 12 | Niu Z Y, Jiao L, Zhang T, et al. Boosting electrocatalytic ammonia synthesis of bio-inspired porous Mo-doped hematite via nitrogen activation[J]. ACS Applied Materials & Interfaces, 2022, 14(50): 55559-55567. |
| 13 | Sun Y, Wang Q, Liu Z Y. Bifunctional OER/NRR catalysts based on a thin-layered Co3O4- x /GO sandwich structure[J]. ACS Applied Materials & Interfaces, 2022, 14(38): 43508-43516. |
| 14 | Xiao Y H, Tan X H, Guo Y Y, et al. Surface modification of CeO2- x nanorods with Sn doping for enhanced nitrogen electroreduction[J]. Journal of Energy Chemistry, 2023, 87: 400-407. |
| 15 | Wang J, Nan H F, Tian Y, et al. FeMo3S4 for efficient nitrogen reduction reaction[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(34): 12733-12740. |
| 16 | Kim H S, Choi J, Kong J M, et al. Regenerative electrocatalytic redox cycle of copper sulfide for sustainable NH3 production under ambient conditions[J]. ACS Catalysis, 2021, 11(1): 435-445. |
| 17 | Chen X Y, Wang Y R, Meng X C. Fabrication of NiS x /MoS2 interface for accelerated charge transfer with greatly improved electrocatalytic activity in nitrogen reduction to produce ammonia[J]. Chemical Engineering Journal, 2024, 479: 147701. |
| 18 | Johnson D, Hunter B, Christie J, et al. Ti2N nitride MXene evokes the Mars-van Krevelen mechanism to achieve high selectivity for nitrogen reduction reaction[J]. Scientific Reports, 2022, 12(1): 657. |
| 19 | Ding Z, Li X S, Kang C X, et al. Single Ru atoms confined into MOF/C3N4 for dual improved photocatalytic carbon dioxide reduction and nitrogen fixation[J]. Chemical Engineering Journal, 2023, 473: 145256. |
| 20 | Qi J M, Gao L Y, Wei F F, et al. Design of a high-performance electrocatalyst for N2 conversion to NH3 by trapping single metal atoms on stepped CeO2 [J]. ACS Applied Materials & Interfaces, 2019, 11(50): 47525-47534. |
| 21 | Peng G M, Wu J W, Wang M Z, et al. Nitrogen-defective polymeric carbon nitride nanolayer enabled efficient electrocatalytic nitrogen reduction with high faradaic efficiency[J]. Nano Letters, 2020, 20(4): 2879-2885. |
| 22 | Jiang M, Zhu Y X, Zhong X, et al. Frustrated Lewis pairs-engineered boron-doped 3D carbon nitride for improved electrocatalytic nitrogen reduction[J]. Chemical Engineering Journal, 2023, 466: 143256. |
| 23 | Su J N, Zhuang L Z, Zhang S S, et al. Single atom catalyst for electrocatalysis[J]. Chinese Chemical Letters, 2021, 32(10): 2947-2962. |
| 24 | Zhao P W, Wang H Z, Huang Z, et al. High-throughput screening of nitrogen reduction reaction on single atom@1T'-MoS2 [J]. Applied Surface Science, 2023, 631: 157480. |
| 25 | Zhou Y X, Wei B, Cao H J, et al. Electroreduction of nitrogen to ammonia by single-atom catalysis with synergistic boron-carbon nitrogen nanotubes[J]. Journal of Environmental Chemical Engineering, 2022, 10(3): 107752. |
| 26 | Ying Y R, Fan K, Luo X, et al. Transition metal-tetracyanoquinodimethane monolayers as single-atom catalysts for the electrocatalytic nitrogen reduction reaction[J]. Materials Advances, 2020, 1(5): 1285-1292. |
| 27 | Abghoui Y, Garden A L, Hlynsson V F, et al. Enabling electrochemical reduction of nitrogen to ammonia at ambient conditions through rational catalyst design[J]. Physical Chemistry Chemical Physics, 2015, 17(7): 4909-4918. |
| 28 | Abghoui Y, Garden A L, Howalt J G, et al. Electroreduction of N2 to ammonia at ambient conditions on mononitrides of Zr, Nb, Cr, and V: a DFT guide for experiments[J]. ACS Catalysis, 2016, 6(2): 635-646. |
| 29 | Abghoui Y, Skúlason E. Electrochemical synthesis of ammonia via Mars-van Krevelen mechanism on the (111) facets of group Ⅲ—Ⅶ transition metal mononitrides[J]. Catalysis Today, 2017, 286: 78-84. |
| 30 | Zhao X, Hu G Z, Chen G F, et al. Comprehensive understanding of the thriving ambient electrochemical nitrogen reduction reaction[J]. Advanced Materials, 2021, 33(33): e2007650. |
| 31 | Ling C Y, Zhang Y H, Li Q, et al. New mechanism for N2 reduction: the essential role of surface hydrogenation[J]. Journal of the American Chemical Society, 2019, 141(45): 18264-18270. |
| 32 | Yao Z B, Liu S Q, Liu H H, et al. Pre-adsorbed H-assisted N2 activation on single-atom cadmium-O5 decorated In2O3 for efficient NH3 electrosynthesis[J]. Advanced Functional Materials, 2023, 33(5): 2209843. |
| 33 | Hao Y C, Guo Y, Chen L W, et al. Promoting nitrogen electroreduction to ammonia with bismuth nanocrystals and potassium cations in water[J]. Nature Catalysis, 2019, 2: 448-456. |
| 34 | Gu W L, Pei A, Zhang S Y, et al. Atomic-interface effect of single-atom Ru/CoO x for selective electrooxidation of 5-hydroxymethylfurfural[J]. ACS Applied Materials & Interfaces, 2023, 15(23): 28036-28043. |
| 35 | Shi L, Bi S N, Qi Y, et al. Anchoring Mo single-atom sites on B/N codoped porous carbon nanotubes for electrochemical reduction of N2 to NH3 [J]. ACS Catalysis, 2022, 12(13): 7655-7663. |
| 36 | Huang K, Zhang L, Xu T, et al. -60℃ solution synthesis of atomically dispersed cobalt electrocatalyst with superior performance[J]. Nature Communications, 2019, 10: 606. |
| 37 | Wang H T, Wang Q X, Cheng Y C, et al. Doping monolayer graphene with single atom substitutions[J]. Nano Letters, 2012, 12(1): 141-144. |
| 38 | Zhou L H, Chang X, Zheng W, et al. Single atom Rh-sensitized SnO2 via atomic layer deposition for efficient formaldehyde detection[J]. Chemical Engineering Journal, 2023, 475: 146300. |
| 39 | Gu F B, Di M Y, Han D M, et al. Atomically dispersed Au on In2O3 nanosheets for highly sensitive and selective detection of formaldehyde[J]. ACS Sensors, 2020, 5(8): 2611-2619. |
| 40 | Zhang Z R, Feng C, Liu C X, et al. Electrochemical deposition as a universal route for fabricating single-atom catalysts[J]. Nature Communications, 2020, 11(1): 1215. |
| 41 | Jin H Y, Sultan S, Ha M R, et al. Simple and scalable mechanochemical synthesis of noble metal catalysts with single atoms toward highly efficient hydrogen evolution[J]. Advanced Functional Materials, 2020, 30(25): 2000531. |
| 42 | Dong Y H, Zhang D, Li D G, et al. Control of Ostwald ripening[J]. Science China Materials, 2023, 66(3): 1249-1255. |
| 43 | Jones J, Xiong H F, DeLaRiva A T, et al. Thermally stable single-atom platinum-on-ceria catalysts via atom trapping[J]. Science, 2016, 353(6295): 151-154. |
| 44 | Zhao C M, Wang Y, Li Z J, et al. Solid-diffusion synthesis of single-atom catalysts directly from bulk metal for efficient CO2 reduction[J]. Joule, 2019, 3(2): 584-594. |
| 45 | Li J Z, Chen M J, Cullen D A, et al. Atomically dispersed manganese catalysts for oxygen reduction in proton-exchange membrane fuel cells[J]. Nature Catalysis, 2018, 1: 935-945. |
| 46 | Wang Y C, Chu F L, Zeng J, et al. Single atom catalysts for fuel cells and rechargeable batteries: principles, advances, and opportunities[J]. ACS Nano, 2021, 15(1): 210-239. |
| 47 | Zhang S, Wu J H, Zheng M T, et al. Fe/Cu diatomic catalysts for electrochemical nitrate reduction to ammonia[J]. Nature Communications, 2023, 14(1): 3634. |
| 48 | Wan J, Liu D, Feng C Z, et al. Efficient N2 electroreduction enabled by linear charge transfer over atomically dispersed W sites[J]. Chemical Science, 2024, 15(32): 12796-12805. |
| 49 | Palazov A, Chang C C, Kokes R J. The infrared spectrum of carbon monoxide on reduced and oxidized palladium[J]. Journal of Catalysis,1975, 36(3): 338-350. |
| 50 | Yates J T, Duncan T M, Worley S D, et al. Infrared spectra of chemisorbed CO on Rh[J]. The Journal of Chemical Physics, 1979, 70(3): 1219-1224. |
| 51 | Matsubu J C, Yang V N, Christopher P. Isolated metal active site concentration and stability control catalytic CO2 reduction selectivity[J]. Journal of the American Chemical Society, 2015, 137(8): 3076-3084. |
| 52 | Wang F Z, Shang S Y, Sun Z Y, et al. P-block antimony-copper single-atom alloys for selective nitrite electroreduction to ammonia[J]. ACS Nano, 2024, 18(20): 13141-13149. |
| 53 | Nie Y F, Yan H P, Lu S W, et al. Theory-guided construction of Cu—O—Ti—Ov active sites on Cu/TiO2 catalysts for efficient electrocatalytic nitrate reduction[J]. Chinese Journal of Catalysis, 2024, 59: 293-302. |
| 54 | Liu X, Jing S J, Wang K W, et al. Double bonuses achieved in single-atom catalysts for efficient oxygen evolution: enhanced reaction kinetics and reinforced electrochemical reconstruction[J]. Advanced Functional Materials, 2024, 34(13): 2309824. |
| 55 | Fang S, Zhu X R, Liu X K, et al. Uncovering near-free platinum single-atom dynamics during electrochemical hydrogen evolution reaction[J]. Nature Communications, 2020, 11(1): 1029. |
| 56 | Su H, Zhou W L, Zhou W, et al. In-situ spectroscopic observation of dynamic-coupling oxygen on atomically dispersed iridium electrocatalyst for acidic water oxidation[J]. Nature Communications, 2021, 12(1): 6118. |
| 57 | Qiao B T, Wang A Q, Yang X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeO x [J]. Nature Chemistry, 2011, 3(8): 634-641. |
| 58 | Li R Z, Luo L, Ma X L, et al. Single atoms supported on metal oxides for energy catalysis[J]. Journal of Materials Chemistry, 2022, 10(11): 5717-5742. |
| 59 | Hoang S, Guo Y B, Binder A J, et al. Activating low-temperature diesel oxidation by single-atom Pt on TiO2 nanowire array[J]. Nature Communications, 2020, 11(1): 1062. |
| 60 | van Deelen T W, Hernández Mejía C, de Jong K P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity[J]. Nature Catalysis, 2019, 2: 955-970. |
| 61 | 朱锐杰, 康磊磊, 李林, 等. WO3-TiO2负载的Pt单原子催化剂光热协同催化丙烷和丙烯氧化[J]. 物理化学学报, 2024, 40(1): 2303003. |
| Zhu R J, Kang L L, Li L, et al. Photo-thermo catalytic oxidation of C3H8 and C3H6 over the WO3-TiO2 supported Pt single-atom catalyst[J]. Acta Physico-Chimica Sinica, 2024, 40(1): 2303003. | |
| 62 | Liu X F, Luo Y N, Ling C C, et al. Rare earth La single atoms supported MoO3- x for efficient photocatalytic nitrogen fixation[J]. Applied Catalysis B: Environmental, 2022, 301: 120766. |
| 63 | Yang X F, Wang A Q, Qiao B T, et al. Single-atom catalysts: a new frontier in heterogeneous catalysis[J]. Accounts of Chemical Research, 2013, 46(8): 1740-1748. |
| 64 | Gao Y N, Yang Y, Hao L D, et al. Single Nb atom modified anatase TiO2(110) for efficient electrocatalytic nitrogen reduction reaction[J]. Chem Catalysis, 2022, 2(9): 2275-2288. |
| 65 | Zhao Z Q, Li K, Liu J, et al. Light field-enhanced single-site Cu electrocatalyst for nitrogen fixation[J]. Small, 2023, 19(10): e2206626. |
| 66 | Hu B, Wang B H, Chen L, et al. Electronic modulation of the interaction between Fe single atoms and WO2.72- x for photocatalytic N2 reduction[J]. ACS Catalysis, 2022, 12(19): 11860-11869. |
| 67 | 陈余涛, 陈治江, 宋煜, 等. 碳基材料电催化合成氨的研究进展[J]. 山东化工, 2024, 53(3): 115-117. |
| Chen Y T, Chen Z J, Song Y, et al. Research progress on carbon-based materials in electrocatalytic ammonia synthesis[J]. Shandong Chemical Industry, 2024, 53(3): 115-117. | |
| 68 | Yang S K, Zhang C N, Rao D W, et al. Synergistic interaction of Nb atoms anchored on g-C3N4 and H+ promoting high-efficiency nitrogen reduction reaction[J]. Chinese Journal of Catalysis, 2022, 43(4): 1139-1147. |
| 69 | Senthamaraikannan T G, Lim D H, Lim D. Nitrogen reduction reaction enhanced by single-atom transition metal catalysts on functionalized graphene: a first-principles study[J]. International Journal of Hydrogen Energy, 2024, 72: 449-461. |
| 70 | Wei Z X, Feng Y Z, Ma J M. Co-doped graphene edge for enhanced N2-to-NH3 conversion[J]. Journal of Energy Chemistry, 2020, 48: 322-327. |
| 71 | Zhao W H, Chen L L, Zhang W H, et al. Single Mo1(W1, Re1) atoms anchored in pyrrolic-N3 doped graphene as efficient electrocatalysts for the nitrogen reduction reaction[J]. Journal of Materials Chemistry A, 2021, 9(10): 6547-6554. |
| 72 | Chen S N, Bu M K, Zhou Z Y, et al. Boosting nitrogen reduction to ammonia on Fe-N3S sites by introduction S into defect graphene[J]. Materials Today Energy, 2022, 25: 100954. |
| 73 | Wang Y, Cui X Q, Zhao J X, et al. Rational design of Fe-N/C hybrid for enhanced nitrogen reduction electrocatalysis under ambient conditions in aqueous solution[J]. ACS Catalysis, 2019, 9(1): 336-344. |
| 74 | Liao W R, Qi L, Wang Y L, et al. Interfacial engineering promoting electrosynthesis of ammonia over Mo/phosphotungstic acid with high performance[J]. Advanced Functional Materials, 2021, 31(22): 2009151. |
| 75 | Zhao J, Liu L J, Yang Y, et al. Insights into electrocatalytic nitrate reduction to ammonia via Cu-based bimetallic catalysts[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(6): 2468-2475. |
| 76 | Fan J W, Chen Y Y, Chen X Q, et al. Atomically dispersed iron enables high-efficiency electrocatalytic conversion of nitrate to dinitrogen on a N-coordinated mesoporous carbon architecture[J]. Applied Catalysis B: Environmental, 2023, 320: 121983. |
| 77 | Frank E, Hermanutz F, Buchmeiser M R. Carbon fibers: precursors, manufacturing, and properties[J]. Macromolecular Materials and Engineering, 2012, 297(6): 493-501. |
| 78 | Li S, Yue G C, Li H K, et al. Pd single atom stabilized on multiscale porous hollow carbon fibers for phenylacetylene semi-hydrogenation reaction[J]. Chemical Engineering Journal, 2023, 454: 140031. |
| 79 | Zhao Y F, Guo Y, Lu X F, et al. Exposing single Ni atoms in hollow S/N-doped carbon macroporous fibers for highly efficient electrochemical oxygen evolution[J]. Advanced Materials, 2022, 34(35): 2203442. |
| 80 | Tran N Q, Liu X H, Cho Y, et al. Efficient ambient ammonia synthesis by Lewis acid pair over cobalt single atom catalyst with suppressed proton reduction[J]. Journal of Materials Chemistry A, 2022, 10(15): 8432-8439. |
| 81 | Kong Y, Wu L, Yang X X, et al. Accelerating protonation kinetics for ammonia electrosynthesis on single iron sites embedded in carbon with intrinsic defects[J]. Advanced Functional Materials, 2022, 32(44): 2205409. |
| 82 | Li L, Yu W K, Gong W B, et al. Sulfur-induced electron redistribution of single molybdenum atoms promotes nitrogen electroreduction to ammonia[J]. Applied Catalysis B: Environmental, 2023, 321: 122038. |
| 83 | Chen L, He C Z, Wang R, et al. Potential active sites of Mo single atoms for electrocatalytic reduction of N2 [J]. Chinese Chemical Letters, 2021, 32(1): 53-56. |
| 84 | Sun Z Z, Lin J W, Lu S W, et al. Interfacial engineering boosting the activity and stability of MIL-53(Fe) toward electrocatalytic nitrogen reduction[J]. Langmuir, 2024, 40(10): 5469-5478. |
| 85 | Johnson L R, Sridhar S, Zhang L, et al. MXene materials for the electrochemical nitrogen reduction functionalized or not?[J]. ACS Catalysis, 2020, 10(1): 253-264. |
| 86 | Ren Y F, Tian F G, Jin L M, et al. Fluidic MXene electrode functionalized with iron single atoms for selective electrocatalytic nitrate transformation to ammonia[J]. Environmental Science & Technology, 2023, 57(28): 10458-10466. |
| 87 | Chen Z G, Fan X L, Shen Z H, et al. Cu anchored Ti2NO2 as high performance electrocatalyst for oxygen evolution reaction: a density functional theory study[J]. ChemCatChem, 2020, 12(16): 4059-4066. |
| 88 | Shen Z H, Fan X L, Ma S G, et al. 3D transitional-metal single atom catalysis toward hydrogen evolution reaction on MXenes supports[J]. International Journal of Hydrogen Energy, 2020, 45(28): 14396-14406. |
| 89 | Cheng Y W, Dai J H, Song Y, et al. Single molybdenum atom anchored on 2D Ti2NO2 MXene as a promising electrocatalyst for N2 fixation[J]. Nanoscale, 2019, 11(39): 18132-18141. |
| 90 | Qu J L, Xiao J W, Chen H T, et al. Orbital symmetry matching: achieving superior nitrogen reduction reaction over single-atom catalysts anchored on Mxene substrates[J]. Chinese Journal of Catalysis, 2021, 42(2): 288-296. |
| 91 | Chen G, Ding M M, Zhang K, et al. Single-atomic ruthenium active sites on Ti3C2 MXene with oxygen-terminated surface synchronize enhanced activity and selectivity for electrocatalytic nitrogen reduction to ammonia[J]. ChemSusChem, 2022, 15(3): e202102352. |
| 92 | Tang S B, Liu T Y, Dang Q, et al. Synergistic effect of surface-terminated oxygen vacancy and single-atom catalysts on defective MXenes for efficient nitrogen fixation[J]. The Journal of Physical Chemistry Letters, 2020, 11(13): 5051-5058. |
| 93 | Li L, Wang X Y, Guo H R, et al. Theoretical screening of single transition metal atoms embedded in MXene defects as superior electrocatalyst of nitrogen reduction reaction[J]. Small Methods, 2019, 3(11): 1900337. |
| 94 | Wang S, Li L, San Hui K, et al. Computational screening of single atoms anchored on defective Mo2CO2 MXene nanosheet as efficient electrocatalysts for the synthesis of ammonia[J]. Advanced Engineering Materials, 2021, 23(10): 2100405. |
| 95 | Gu H F, Li J N, Niu X F, et al. Symmetry-breaking p-block antimony single atoms trigger N-bridged titanium sites for electrocatalytic nitrogen reduction with high efficiency[J]. ACS Nano, 2023, 17(21): 21838-21849. |
| 96 | Zhang M M, Lai C, Li B S, et al. MXenes as superexcellent support for confining single atom: properties, synthesis, and electrocatalytic applications[J]. Small, 2021, 17(29): 2007113. |
| 97 | Huang B, Yang J, Ren G Y, et al. Design of single-atom catalysts on S-functionalized Mxenes for enhanced activity and selectivity in N2 electroreduction[J]. Applied Catalysis A: General, 2022, 646: 118886. |
| 98 | Liao W R, Xie K, Liu L J, et al. Triggering in-plane defect cluster on MoS2 for accelerated dinitrogen electroreduction to ammonia[J]. Journal of Energy Chemistry, 2021, 62: 359-366. |
| 99 | 宋悦, 张启成, 彭文朝, 等. MoS2基单原子催化剂的合成及其在电催化中的应用[J]. 化工学报, 2023, 74(2): 535-545. |
| Song Y, Zhang Q C, Peng W C, et al. Synthesis of MoS2-based single atom catalyst and its application in electrocatalysis[J]. CIESC Journal, 2023, 74(2): 535-545. | |
| 100 | Dang Q, Tang S B, Liu T T, et al. Regulating electronic spin moments of single-atom catalyst sites via single-atom promoter tuning on S-vacancy MoS2 for efficient nitrogen fixation[J]. The Journal of Physical Chemistry Letters, 2021, 12(34): 8355-8362. |
| 101 | Chen D C, Luo M, Ning S C, et al. Single-atom gold isolated onto nanoporous MoSe2 for boosting electrochemical nitrogen reduction[J]. Small, 2022, 18(4): 2104043. |
| 102 | Ma X G, Hu J S, Zheng M K, et al. N2 reduction using single transition-metal atom supported on defective WS2 monolayer as promising catalysts: a DFT study[J]. Applied Surface Science, 2019, 489: 684-692. |
| 103 | Bao A Y, Xu Y, Cao Y, et al. Theoretical screening and investigation on electrocatalytic nitrogen fixation of single transition metal atom supported by monolayer SnS2 [J]. Applied Surface Science, 2023, 615: 156362. |
| 104 | Liu Y X, Fan X, Bian W Y, et al. High-loading Fe1 sites on vanadium disulfides: a scalable and non-defect-stabilized single atom catalyst for electrochemical nitrogen reduction[J]. Journal of Materials Chemistry A, 2022, 10(39): 21142-21148. |
| 105 | Kyriakou G, Boucher M B, Jewell A D, et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations[J]. Science, 2012, 335(6073): 1209-1212. |
| 106 | Dai T Y, Wang Z L, Lang X Y, et al. "Sabatier principle" of d electron number for describing the nitrogen reduction reaction performance of single-atom alloy catalysts[J]. Journal of Materials Chemistry A, 2022, 10(32): 16900-16907. |
| 107 | Zheng G K, Li Y L, Qian X, et al. High-throughput screening of a single-atom alloy for electroreduction of dinitrogen to ammonia[J]. ACS Applied Materials & Interfaces, 2021, 13(14): 16336-16344. |
| 108 | Mu J J, Zhao Z W, Gao X W, et al. Bimetallic PdFe3 nano-alloy with tunable electron configuration for boosting electrochemical nitrogen fixation[J]. Advanced Energy Materials, 2024, 14(8): 2303558. |
| 109 | Li X C, Shen P, Luo Y J, et al. PdFe single-atom alloy metallene for N2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 61(28): e202205923. |
| 110 | Zheng Y N, Zhang S S, Yao H, et al. A-site substitution motivating the phase transformation of LaNiO3 perovskite for high-performance artificial N2 fixation[J]. ChemCatChem, 2022, 14(20): e202200920. |
| 111 | Han Z Y, Tranca D, Rodríguez-Hernández F, et al. Embedding Ru clusters and single atoms into perovskite oxide boosts nitrogen fixation and affords ultrahigh ammonia yield rate[J]. Small, 2023, 19(17): e2208102. |
| 112 | Liu D, Yan H P, Lin J W, et al. Regulation of cerium species in Keggin structure of phosphotungstic acid for efficient nitrogen electroreduction to ammonia[J]. Chemical Engineering Science, 2024, 283: 119448. |
| 113 | Liao W R, Liu H X, Qi L, et al. Lithium/bismuth co-functionalized phosphotungstic acid catalyst for promoting dinitrogen electroreduction with high Faradaic efficiency[J]. Cell Reports Physical Science, 2021, 2(9): 100557. |
| 114 | Li Y, Li J W, Huang J H, et al. Boosting electroreduction kinetics of nitrogen to ammonia via tuning electron distribution of single-atomic iron sites[J]. Angewandte Chemie International Edition, 2021, 60(16): 9078-9085. |
| 115 | Cui W J, Geng B K, Chu X, et al. Coupling Fe and Mo single atom on hierarchical N-doped carbon nanotubes enhances electrochemical nitrogen reduction reaction performance[J]. Nano Research, 2023, 16(4): 5743-5749. |
| 116 | Kong Y, Li Y, Sang X H, et al. Atomically dispersed zinc(Ⅰ) active sites to accelerate nitrogen reduction kinetics for ammonia electrosynthesis[J]. Advanced Materials, 2022, 34(2): e2103548. |
| 117 | Gu Y, Xi B J, Tian W Z, et al. Boosting selective nitrogen reduction via geometric coordination engineering on single-tungsten-atom catalysts[J]. Advanced Materials, 2021, 33(25): e2100429. |
| 118 | Zhao Y C, Zhang S, Han C, et al. Effect of boron and nitrogen modulation in metal atoms anchoring on flower-like carbon superstructure for efficient ammonia electrosynthesis[J]. Chemical Engineering Journal, 2023, 468: 143517. |
| 119 | Ji Z J, Song Y J, Zhao S H, et al. Pathway manipulation via Ni, Co, and V ternary synergism to realize high efficiency for urea electrocatalytic oxidation[J]. ACS Catalysis, 2022, 12(1): 569-579. |
| 120 | Mu J J, Gao X W, Yu T, et al. Ambient electrochemical ammonia synthesis: from theoretical guidance to catalyst design[J]. Advanced Science, 2024, 11(15): e2308979. |
| [1] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [2] | 徐子易, 席阳, 宋泽文, 周海骏. 碳纳米材料在锌离子电池中的应用研究进展[J]. 化工学报, 2025, 76(1): 40-52. |
| [3] | 邹吉军, 刘宝宏, 史成香, 潘伦, 张香文. 综纤维素衍生物转化合成生物航空燃料的非均相催化剂研究进展[J]. 化工学报, 2025, 76(1): 1-17. |
| [4] | 杨晨, 毛伟, 董兴宗, 田松, 赵锋伟, 吕剑. 选择性加氢脱氯合成烯烃研究进展[J]. 化工学报, 2025, 76(1): 53-70. |
| [5] | 陈彦霖, 周爱国, 郑家乐, 杨川箬, 葛天舒. 载体对于胺浸渍类DAC吸附剂性能的影响[J]. 化工学报, 2024, 75(S1): 217-222. |
| [6] | 石美琳, 赵连达, 邓行健, 王静松, 左海滨, 薛庆国. 催化甲烷重整工艺的研究进展[J]. 化工学报, 2024, 75(S1): 25-39. |
| [7] | 吴德威, 汪郑鹏, 周玥, 李晓宁, 陈招, 李卓, 刘成伟, 李学刚, 肖文德. 固定床法制备锂离子电池硅碳负极材料及其储锂性能研究[J]. 化工学报, 2024, 75(S1): 300-308. |
| [8] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| [9] | 王树振, 王玉婷, 马梦茜, 张巍, 向江南, 鲁海莹, 王琰, 范彬彬, 郑家军, 代卫炯, 李瑞丰. 两步晶化合成ZSM-22分子筛及其临氢异构反应性能[J]. 化工学报, 2024, 75(9): 3176-3187. |
| [10] | 王舒英, 左涛, 石志伟, 范小明, 张卫新. 阳离子交换树脂基介孔石墨化碳合成与储钠性能[J]. 化工学报, 2024, 75(9): 3338-3347. |
| [11] | 王冉, 王焕, 熊晓云, 关慧敏, 郑云锋, 陈彩琳, 秦玉才, 宋丽娟. FCC催化剂传质强化活性位利用效率的可视化分析[J]. 化工学报, 2024, 75(9): 3198-3209. |
| [12] | 刘亚超, 谭晓杰, 李旭东, 王瑞, 王慧, 韩璇, 赵青山. DES合成高活性CoCO3纳米片及析氧反应性能研究[J]. 化工学报, 2024, 75(9): 3320-3328. |
| [13] | 张梦婷, 王书林, 桑熙, 元兴昊, 徐刚. 人工Cu-TM1459金属酶催化不对称迈克尔加成反应[J]. 化工学报, 2024, 75(9): 3255-3265. |
| [14] | 彭丹, 卢俊杰, 倪文静, 杨媛, 汪靖伦. 高电压钴酸锂电池电解液研究进展[J]. 化工学报, 2024, 75(9): 3028-3040. |
| [15] | 刘旭升, 李泽洋, 杨宇森, 卫敏. 电催化二氧化碳还原制备气态产物的研究进展[J]. 化工学报, 2024, 75(7): 2385-2408. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号