化工学报 ›› 2024, Vol. 75 ›› Issue (9): 3320-3328.DOI: 10.11949/0438-1157.20240125
• 材料化学工程与纳米技术 • 上一篇
刘亚超( ), 谭晓杰, 李旭东, 王瑞, 王慧, 韩璇, 赵青山(
), 谭晓杰, 李旭东, 王瑞, 王慧, 韩璇, 赵青山( )
)
收稿日期:2024-01-29
修回日期:2024-05-20
出版日期:2024-09-25
发布日期:2024-10-10
通讯作者:
赵青山
作者简介:刘亚超(1997—),女,硕士研究生,13518629974@163.com
基金资助:
Yachao LIU( ), Xiaojie TAN, Xudong LI, Rui WANG, Hui WANG, Xuan HAN, Qingshan ZHAO(
), Xiaojie TAN, Xudong LI, Rui WANG, Hui WANG, Xuan HAN, Qingshan ZHAO( )
)
Received:2024-01-29
Revised:2024-05-20
Online:2024-09-25
Published:2024-10-10
Contact:
Qingshan ZHAO
摘要:
氢能因其热值高、清洁无污染等优势,被认为是实现碳中和最有效的能源载体之一。电解水制氢是实现可持续制氢的有效途径。其中,析氧反应(OER)缓慢动力学过程导致水分解效率低下,迫切需要开发高效、稳定的电催化剂。利用多元醇、尿素与CoCl2·6H2O之间的配位作用形成超分子三元低共熔溶剂(DES)体系,通过一锅溶剂热法构建表面粗糙的二维CoCO3纳米片,用于提升电催化OER效率,并针对醇羟基数量对CoCO3形貌及性能影响进行了探究。研究表明,丙三醇、尿素和CoCl2·6H2O三元DES体系制备的CoCO3-Gly催化剂,呈现更蓬松、更薄、更粗糙的片状结构,具有更加优异的OER性能,电流密度在10 mA·cm-2时过电位311 mV,经24 h稳定性测试电流保留率达99%。
中图分类号:
刘亚超, 谭晓杰, 李旭东, 王瑞, 王慧, 韩璇, 赵青山. DES合成高活性CoCO3纳米片及析氧反应性能研究[J]. 化工学报, 2024, 75(9): 3320-3328.
Yachao LIU, Xiaojie TAN, Xudong LI, Rui WANG, Hui WANG, Xuan HAN, Qingshan ZHAO. Synthesis of efficient cobalt carbonate nanosheets based on DES for oxygen evolution reaction[J]. CIESC Journal, 2024, 75(9): 3320-3328.
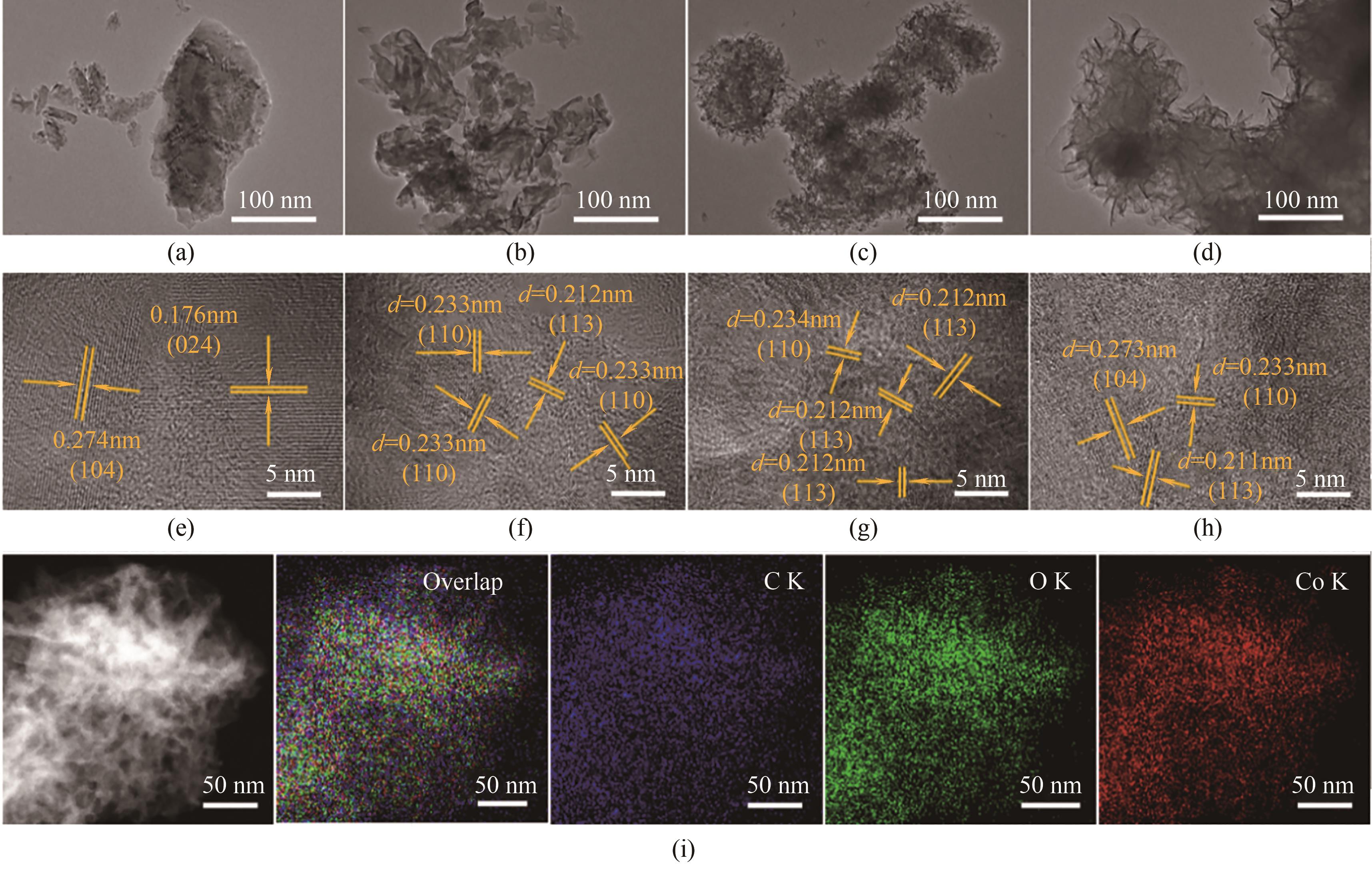
图2 CoCO3(a),CoCO3-PEG(b),CoCO3-EG(c)和CoCO3-Gly(d)的TEM图;CoCO3(e),CoCO3-PEG(f),CoCO3-EG(g)和CoCO3-Gly(h)的HRTEM图;CoCO3-Gly的EDX元素映射图(i)
Fig.2 TEM images of CoCO3 (a), CoCO3-PEG (b), CoCO3-EG (c) and CoCO3-Gly (d); HRTEM images of CoCO3 (e), CoCO3-PEG (f), CoCO3-EG (g) and CoCO3-Gly (h); EDX overlap and element mapping profiles of CoCO3-Gly (i)
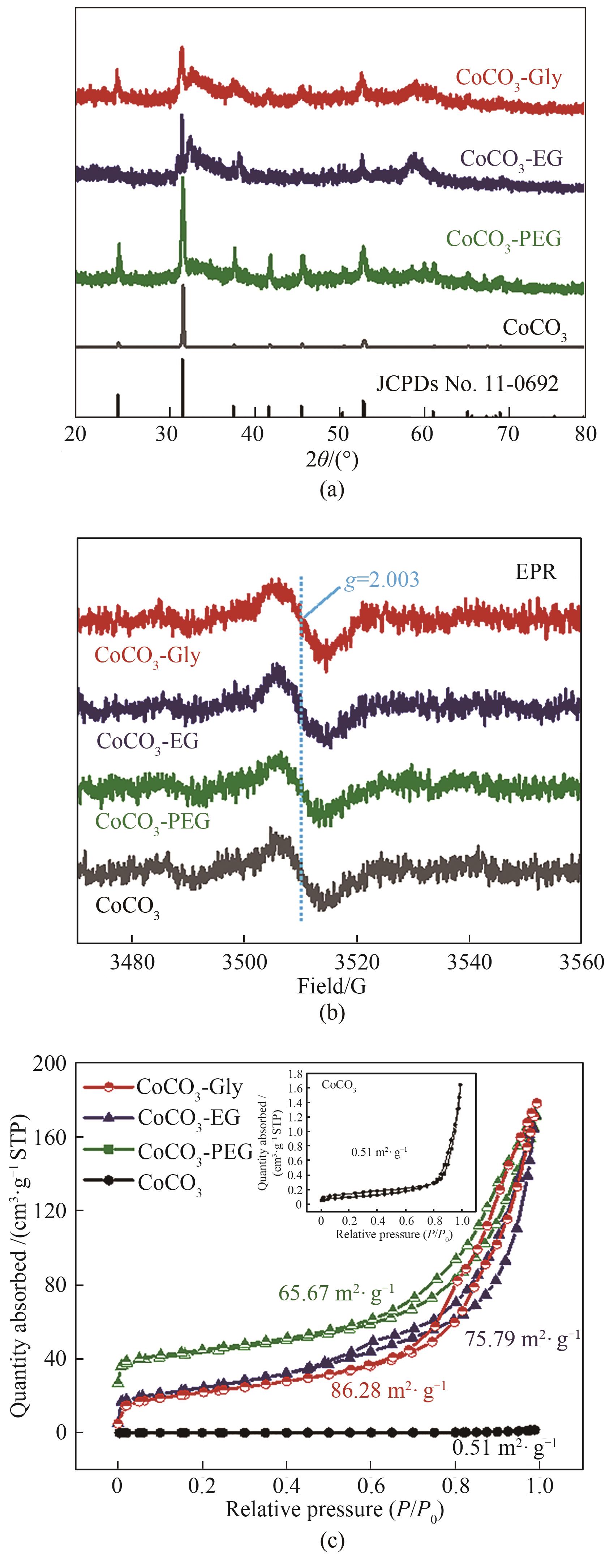
图3 CoCO3、CoCO3-PEG、CoCO3-EG和CoCO3-Gly的XRD表征图(a);EPR光谱图(b)和氮气吸脱附曲线(c)(1G=10-4T)
Fig.3 XRD patterns (a); EPR (b) and N2 adsorption-desorption isothermal (c) curves of CoCO3, CoCO3-PEG, CoCO3-EG and CoCO3-Gly
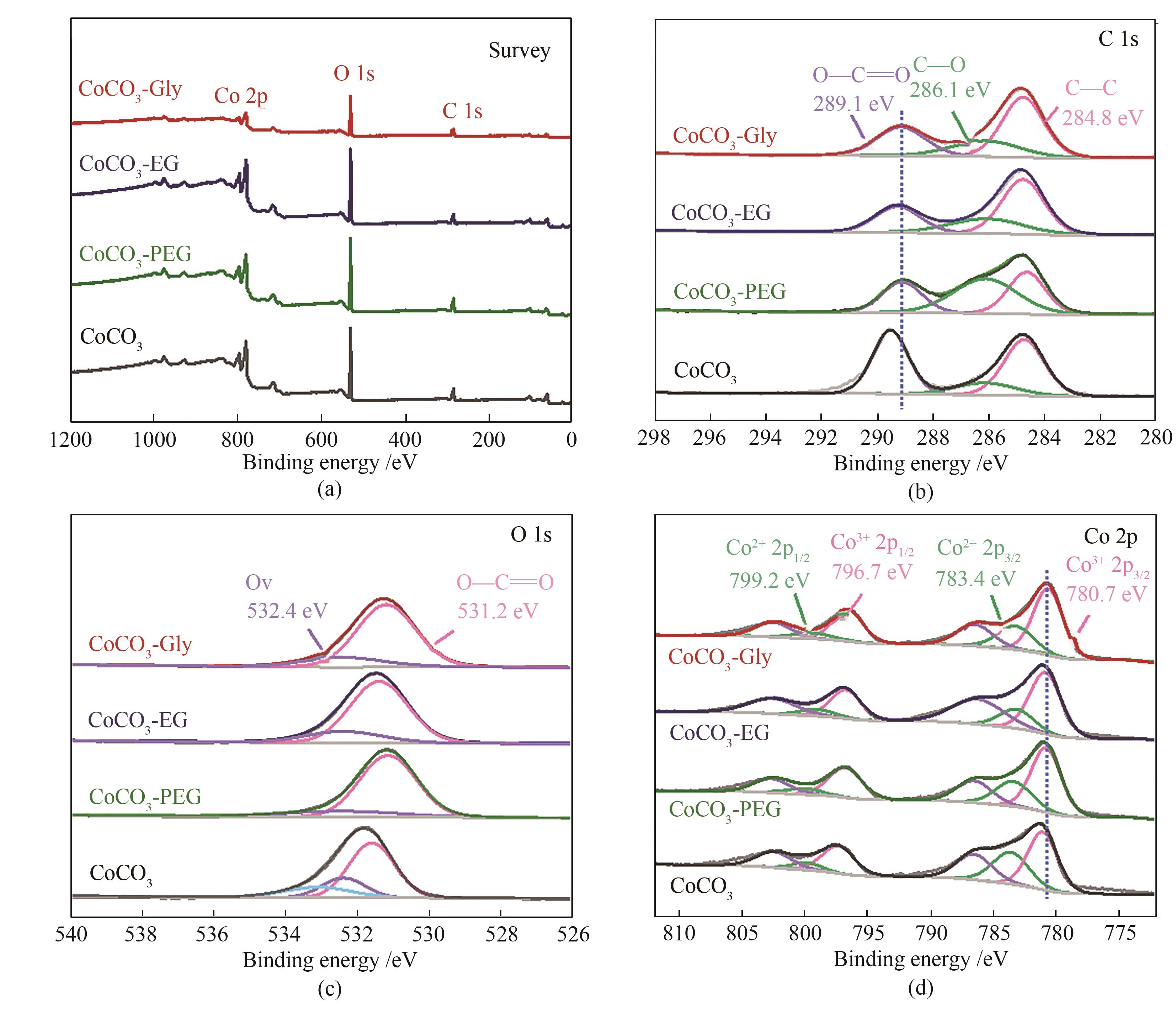
图4 CoCO3、CoCO3-PEG、CoCO3-EG和CoCO3-Gly的XPS全谱(a)及C 1s(b)、O 1s(c)、Co 2p(d)高分辨率XPS谱图
Fig.4 XPS survey spectra (a); high-resolution XPS C 1s spectra (b), O 1s spectra (c) and Co 2p spectra (d) of CoCO3, CoCO3-PEG, CoCO3-EG and CoCO3-Gly
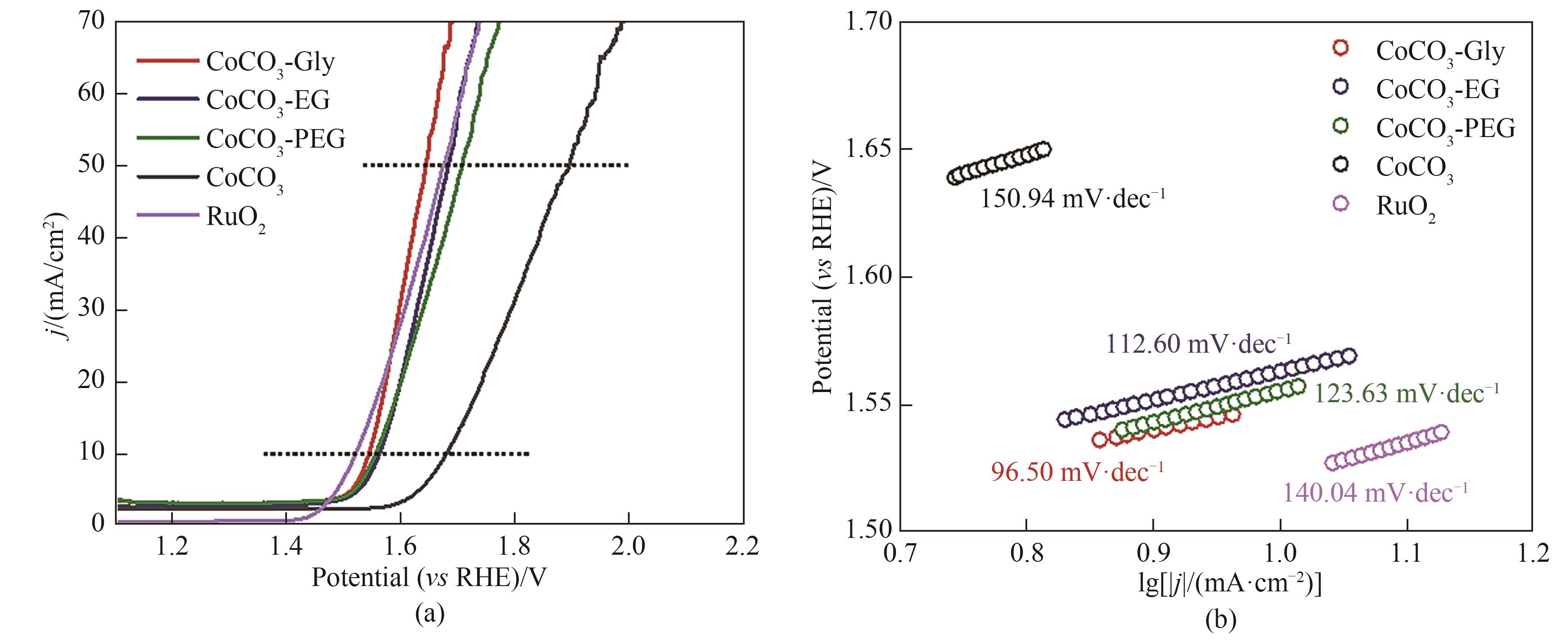
图5 CoCO3、CoCO3-PEG、CoCO3-EG、CoCO3-Gly和RuO2的OER极化曲线图(a); CoCO3、CoCO3-PEG、CoCO3-EG、CoCO3-Gly和RuO2的Tafel图(b)
Fig.5 OER polarization curves of CoCO3, CoCO3-PEG, CoCO3-EG, CoCO3-Gly and RuO2 (a); Tafel plots of CoCO3, CoCO3-PEG, CoCO3-EG, CoCO3-Gly and RuO2 (b)

图6 CoCO3(a),CoCO3-PEG(b),CoCO3-EG(c)和CoCO3-Gly(d)的CV扫描图(电位区间1.02~1.22 V,扫描速率分别为20、40、60、80、100、120 mV·s-1); CoCO3,CoCO3-PEG,CoCO3-EG,CoCO3-Gly在1.12 V电位处扫描速率与电流密度关系图及相应的双电层电容Cdl值(e); CoCO3,CoCO3-PEG,CoCO3-EG和CoCO3-Gly的电化学阻抗图(f)
Fig.6 Cyclic voltammograms of CoCO3 (a), CoCO3-PEG (b), CoCO3-EG (c) and CoCO3-Gly (d) in the region of 1.02—1.22 V (vs RHE) with different scan rates (20, 40, 60, 80, 100 and 120 mV·s-1); capacitive currents against scan rate and corresponding Cdl value of CoCO3, CoCO3-PEG, CoCO3-EG and CoCO3-Gly catalysts at 1.12 V (e); EIS Nyquist plots of CoCO3, CoCO3-PEG, CoCO3-EG and CoCO3-Gly (f)

图7 CoCO3(a)、CoCO3-PEG(b)、CoCO3-EG(c)和CoCO3-Gly(d)恒定电压测试24 h的计时电流曲线图;CoCO3(e)、CoCO3-PEG(f)、CoCO3-EG(g)和CoCO3-Gly(h)经过3000次CV扫描前后LSV曲线
Fig.7 Long-time stability test of CoCO3 (a), CoCO3-PEG (b), CoCO3-EG (c) and CoCO3-Gly (d) at a constant voltage for 24 h LSV curves of CoCO3 (e), CoCO3-PEG (f), CoCO3-EG (g) and CoCO3-Gly (h) before and after 3000 CV scans
| 1 | Le P A, Trung V D, Nguyen P L, et al. The current status of hydrogen energy: an overview[J]. RSC Advances, 2023, 13(40): 28262-28287. |
| 2 | Chen L, Wang H Y, Tian W W, et al. Enabling internal electric field in heterogeneous nanosheets to significantly accelerate alkaline hydrogen electrocatalysis[J]. Small, 2024, 20(18): e2307252. |
| 3 | Liao P S, Kang J W, Zhong Y C, et al. Recent advances of two-dimensional metal-organic frameworks in alkaline electrolysis water for hydrogen production[J]. Science China Chemistry, 2023, 66(7): 1924-1939. |
| 4 | Deng B H, Yu G Q, Zhao W, et al. A self-circulating pathway for the oxygen evolution reaction[J]. Energy & Environmental Science, 2023, 16(11): 5210-5219. |
| 5 | Hou Z Q, Cui C H, Li Y N, et al. Lattice-strain engineering for heterogenous electrocatalytic oxygen evolution reaction[J]. Advanced Materials, 2023, 35(39): e2209876. |
| 6 | Li H X, Han X, Zhao W, et al. Electrochemical preparation of nano/micron structure transition metal-based catalysts for the oxygen evolution reaction[J]. Materials Horizons, 2022, 9(7): 1788-1824. |
| 7 | Zhang X, Yi H, An Q, et al. Recent advances in engineering cobalt carbonate hydroxide for enhanced alkaline water splitting[J]. Journal of Alloys and Compounds, 2021, 887: 161405. |
| 8 | Chen Y Q, Mao J N, Zhou H, et al. Coordination shell dependent activity of cuco diatomic catalysts for oxygen reduction, oxygen evolution, and hydrogen evolution reaction[J]. Advanced Functional Materials, 2024, 34(10): 2311664. |
| 9 | Pan J B, Liu X, Wang B H, et al. Conductive MOFs coating on hematite photoanode for activity boost via surface state regulation[J]. Applied Catalysis B: Environmental, 2022, 315: 121526. |
| 10 | Pan J B, Wang B H, Shen S, et al. Introducing bidirectional axial coordination into BiVO4@metal phthalocyanine core-shell photoanodes for efficient water oxidation[J]. Angewandte Chemie International Edition, 2023, 62(38): e202307246. |
| 11 | Abbott A P, Capper G, Davies D L, et al. Novel solvent properties of choline chloride/urea mixtures[J]. Chemical Communications, 2003(1): 70-71. |
| 12 | Prabhune A, Dey R. Green and sustainable solvents of the future: deep eutectic solvents[J]. Journal of Molecular Liquids, 2023, 379: 121676. |
| 13 | Zhang C Y, Fu Y Q, Gao W, et al. Deep eutectic solvent-mediated electrocatalysts for water splitting[J]. Molecules, 2022, 27(22): 8098. |
| 14 | Makoś-Chełstowska P, Kaykhaii M, Płotka-Wasylka J, et al. Magnetic deep eutectic solvents—fundamentals and applications[J]. Journal of Molecular Liquids, 2022, 365: 120158. |
| 15 | Abbott A, Barron J, Ryder K, et al. Eutectic-based ionic liquids with metal-containing anions and cations[J]. Chemistry-A European Journal, 2007, 13(22): 6495-6501. |
| 16 | Rachiero G P, Berton P, Shamshina J. Deep eutectic solvents: alternative solvents for biomass-based waste valorization[J]. Molecules, 2022, 27(19): 6606. |
| 17 | Płotka-Wasylka J, de la Guardia M, Andruch V, et al. Deep eutectic solvents vs ionic liquids: similarities and differences[J]. Microchemical Journal, 2020, 159: 105539. |
| 18 | 荣凯. 基于低共熔溶剂体系的电化学应用探索[D]. 合肥: 中国科学技术大学, 2020. |
| Rong K. Exploration of electrochemical applications based on deep eutectic solvents[D]. Hefei: University of Science and Technology of China, 2020. | |
| 19 | Długosz O, Banach M. Green methods for obtaining deep eutectic solvents (DES)[J]. Journal of Cleaner Production, 2024, 434: 139914. |
| 20 | Ren S H, Mu T C, Wu W Z. Advances in deep eutectic solvents: new green solvents[J]. Processes, 2023, 11(7): 1920. |
| 21 | Długosz O. Natural deep eutectic solvents in the synthesis of inorganic nanoparticles[J]. Materials, 2023, 16(2): 627. |
| 22 | 陈钰, 牟天成. 低共熔溶剂在电池和电催化中的应用[J]. 化工学报, 2020, 71(1): 106-121. |
| Chen Y, Mu T C. Application of deep eutectic solvents in battery and electrocatalysis[J]. CIESC Journal, 2020, 71(1): 106-121. | |
| 23 | El Achkar T, Greige-Gerges H, Fourmentin S. Basics and properties of deep eutectic solvents: a review[J]. Environmental Chemistry Letters, 2021, 19(4): 3397-3408. |
| 24 | 张晨韵, 张国新, 沈璟虹, 等. 基于(类)离子液体的镍基催化剂研究进展[J]. 日用化学工业(中英文), 2023, 53(3): 316-324. |
| Zhang C Y, Zhang G X, Shen J H, et al. Research progress of nickel-based catalysts mediated by (quasi) ionic liquids[J]. China Surfactant Detergent & Cosmetics, 2023, 53(3): 316-324. | |
| 25 | Pariiska O, Mazur D, Cherchenko K, et al. Efficient Co-N-C electrocatalysts for oxygen reduction derived from deep eutectic solvents[J]. Electrochimica Acta, 2022, 413: 140132. |
| 26 | Guan S Q, Xu B E, Wu J C, et al. High-entropy materials based on deep eutectic solvent for boosting oxygen evolution reaction[J]. Fuel, 2024, 358: 130315. |
| 27 | Zhang C Y, Xin B W, Chen T T, et al. Deep eutectic solvent strategy enables an octahedral Ni-Co precursor for creating high-performance NiCo2O4 catalyst toward oxygen evolution reaction[J]. Green Energy & Environment, 2022, 7(6): 1217-1227. |
| 28 | Samage A, Pramoda K, Halakarni M, et al. One-step rapid conversion of electroactive comno nanostructures using a deep eutectic solvent as the template, solvent, and source[J]. ACS Applied Energy Materials, 2023, 6(4): 2412-2422. |
| 29 | Zhang Y C, Han C D, Gao J, et al. NiCo-based electrocatalysts for the alkaline oxygen evolution reaction: a review[J]. ACS Catalysis, 2021, 11(20): 12485-12509. |
| 30 | Wei Y H, Jiang J Y, Dong J L, et al. Designable synthesis of reactive deep eutectic solvents (RDES) in regulating Ni-based materials for an efficient oxygen evolution reaction[J]. Green Chemistry, 2022, 24(20): 8014-8020. |
| 31 | Zhang D L, Mou H Y, Lu F, et al. A novel strategy for 2D/2D NiS/graphene heterostructures as efficient bifunctional electrocatalysts for overall water splitting[J]. Applied Catalysis B: Environmental, 2019, 254: 471-478. |
| 32 | Zhang D, Mou H, Chen L, et al. Surface/interface engineering N-doped carbon/NiS2 nanosheets for efficient electrocatalytic H2O splitting[J]. Nanoscale, 2020, 12(5): 3370-3376. |
| 33 | Liu S, Zhang C, Zhang B, et al. All-in-one deep eutectic solvent toward cobalt-based electrocatalyst for oxygen evolution reaction[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(9): 8964-8971. |
| 34 | Pan J B, Wang B H, Wang J B, et al. Activity and stability boosting of an oxygen-vacancy-rich BiVO4 photoanode by NiFe-MOFs thin layer for water oxidation[J]. Angewandte Chemie International Edition, 2021, 60(3): 1433-1440. |
| [1] | 张梦婷, 王书林, 桑熙, 元兴昊, 徐刚. 人工Cu-TM1459金属酶催化不对称迈克尔加成反应[J]. 化工学报, 2024, 75(9): 3255-3265. |
| [2] | 王树振, 王玉婷, 马梦茜, 张巍, 向江南, 鲁海莹, 王琰, 范彬彬, 郑家军, 代卫炯, 李瑞丰. 两步晶化合成ZSM-22分子筛及其临氢异构反应性能[J]. 化工学报, 2024, 75(9): 3176-3187. |
| [3] | 王冉, 王焕, 熊晓云, 关慧敏, 郑云锋, 陈彩琳, 秦玉才, 宋丽娟. FCC催化剂传质强化活性位利用效率的可视化分析[J]. 化工学报, 2024, 75(9): 3198-3209. |
| [4] | 王寅, 初鹏飞, 刘虎, 吕静, 黄守莹, 王胜平, 马新宾. 不同pH铝溶胶对二甲醚羰基化成型丝光沸石催化剂性能的影响[J]. 化工学报, 2024, 75(7): 2533-2543. |
| [5] | 杨露, 刘聪聪, 孟彤彤, 张博远, 杨腾飞, 邓文安, 王晓斌. 分散型催化剂在煤/重油共炼体系中的加氢抑焦作用[J]. 化工学报, 2024, 75(7): 2556-2564. |
| [6] | 罗莉, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 氧化铝结构与表面性质调控及其催化甲醇脱水制二甲醚性能研究[J]. 化工学报, 2024, 75(7): 2522-2532. |
| [7] | 刘旭升, 李泽洋, 杨宇森, 卫敏. 电催化二氧化碳还原制备气态产物的研究进展[J]. 化工学报, 2024, 75(7): 2385-2408. |
| [8] | 王天闻, 闫肃, 赵梦园, 杨天让, 刘建国. 固体氧化物电池空气电极铬中毒机理及抗铬性能研究进展[J]. 化工学报, 2024, 75(6): 2091-2108. |
| [9] | 丁禹, 杨昌泽, 李军, 孙会东, 商辉. 原子尺度钼系加氢脱硫催化剂的研究进展与展望[J]. 化工学报, 2024, 75(5): 1735-1749. |
| [10] | 裴欣哲, 孙朱行, 林钰翔, 张朝阳, 钱勇, 吕兴才. 电催化分解液氨阳极材料的研究[J]. 化工学报, 2024, 75(5): 1843-1854. |
| [11] | 赵亭亭, 鄢立祥, 唐福利, 肖敏之, 谭烨, 宋刘斌, 肖忠良, 李灵均. 光辅助锂-二氧化碳电池催化剂的设计策略与反应机理研究进展[J]. 化工学报, 2024, 75(5): 1750-1764. |
| [12] | 莫锦洪, 韩雪, 朱毅翔, 李菁, 王旭裕, 纪红兵. Pt-Ga/CeO2-ZrO2-Al2O3脱氢裂解双功能催化剂用于正丁烷催化制烯烃研究[J]. 化工学报, 2024, 75(5): 1855-1869. |
| [13] | 范以薇, 刘威, 李盈盈, 王培霞, 张吉松. 有机液体储氢中全氢化乙基咔唑催化脱氢研究进展[J]. 化工学报, 2024, 75(4): 1198-1208. |
| [14] | 贾旭东, 杨博龙, 程前, 李雪丽, 向中华. 分步负载金属法制备铁钴双金属位点高效氧还原电催化剂[J]. 化工学报, 2024, 75(4): 1578-1593. |
| [15] | 严孝清, 赵瑛, 张宇哲, 欧鸿辉, 黄起中, 胡华贵, 杨贵东. 五重孪晶铜纳米线@聚吡咯制备及其电催化硝酸盐还原制氨[J]. 化工学报, 2024, 75(4): 1519-1532. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号