化工学报 ›› 2025, Vol. 76 ›› Issue (1): 231-240.DOI: 10.11949/0438-1157.20240629
收稿日期:2024-06-06
修回日期:2024-09-20
出版日期:2025-01-25
发布日期:2025-02-08
通讯作者:
王朋,刘治明
作者简介:郭珊(2000—),女,硕士研究生,19861834561@163.com
基金资助:
Shan GUO( ), Yu TIAN, Yongbin XU, Peng WANG(
), Yu TIAN, Yongbin XU, Peng WANG( ), Zhiming LIU(
), Zhiming LIU( )
)
Received:2024-06-06
Revised:2024-09-20
Online:2025-01-25
Published:2025-02-08
Contact:
Peng WANG, Zhiming LIU
摘要:
以从废旧三元锂离子电池中回收的镍钴锰(NCM)有价金属与钯(Pd)盐为原料,采用快速高温轰击方法制备含氮碳基体负载钯镍钴锰中熵合金纳米颗粒(Pd MEA@N-C)作为锂氧电池双功能催化剂,以实现废弃三元锂离子电池的循环使用,优化在氧还原/析出过程(ORR/OER)中放电产物过氧化锂(Li2O2)的可逆生成与分解。XRD、TEM表明Pd MEA成功制备,XPS表明Pd的引入有利于实现合金颗粒中电子排布的精准调控。以Pd MEA@N-C为正极材料组装锂氧电池测试性能,结果显示,在限制1000 mAh·g-1的容量,200 mA·g-1的电流密度条件下,过电位低至0.49 V;200 mA·g-1的电流密度条件下进行深度充放电测试,充放电容量高达15491 mAh·g-1;在循环82圈之后仍保持稳定状态。
中图分类号:
郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240.
Shan GUO, Yu TIAN, Yongbin XU, Peng WANG, Zhiming LIU. Synthesis of a high-efficacy medium-entropy alloy catalyst via the recycling of spent batteries and its subsequent performance evaluation[J]. CIESC Journal, 2025, 76(1): 231-240.
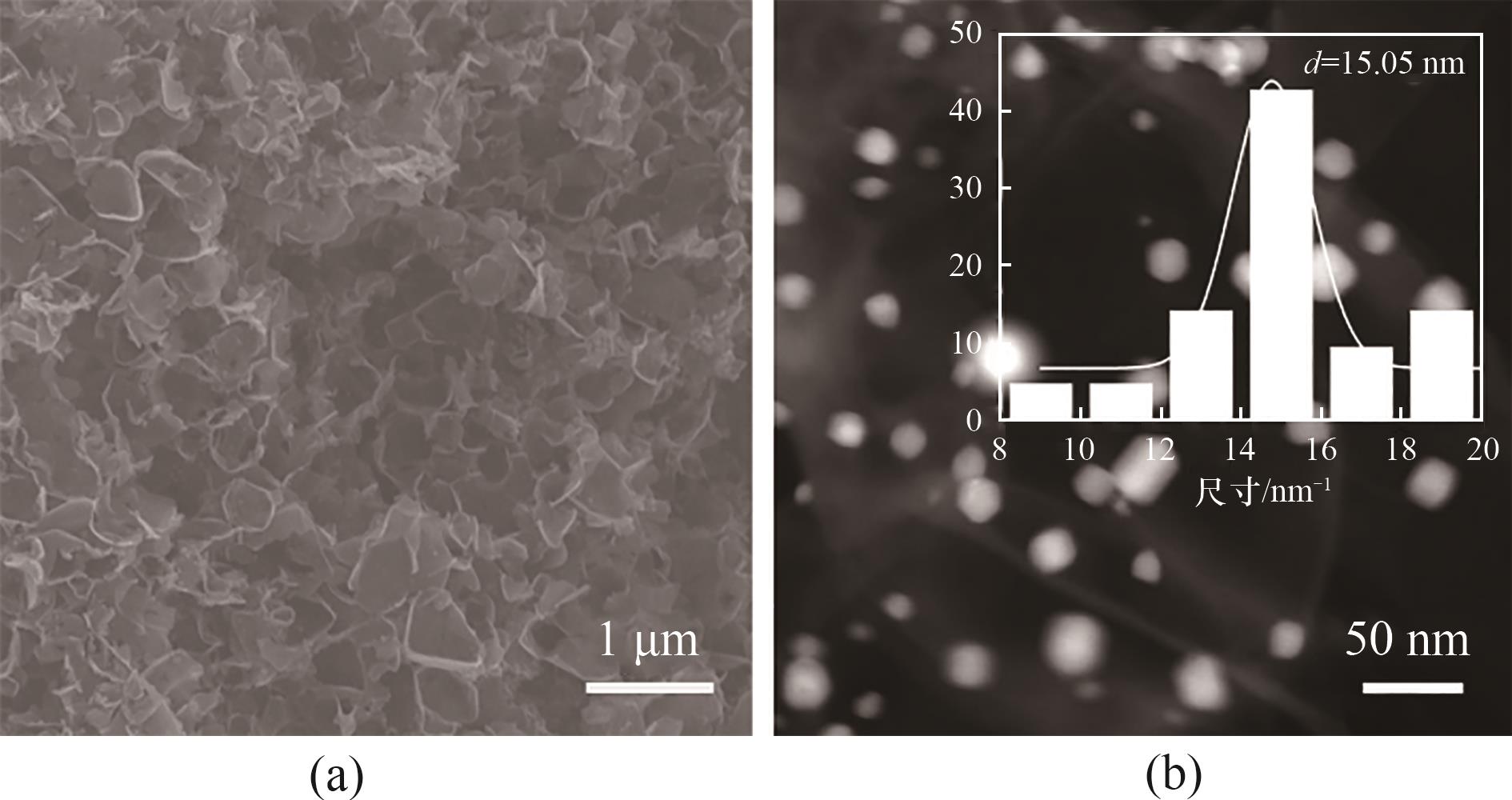
图1 (a) Pd MEA@N-C的SEM图像;(b) Pd MEA@N-C的HAADF-STEM图像和粒径分布
Fig.1 (a) SEM image of Pd MEA@N-C; (b) TEM image (inset shows particle size distribution).of Pd MEA@N-C
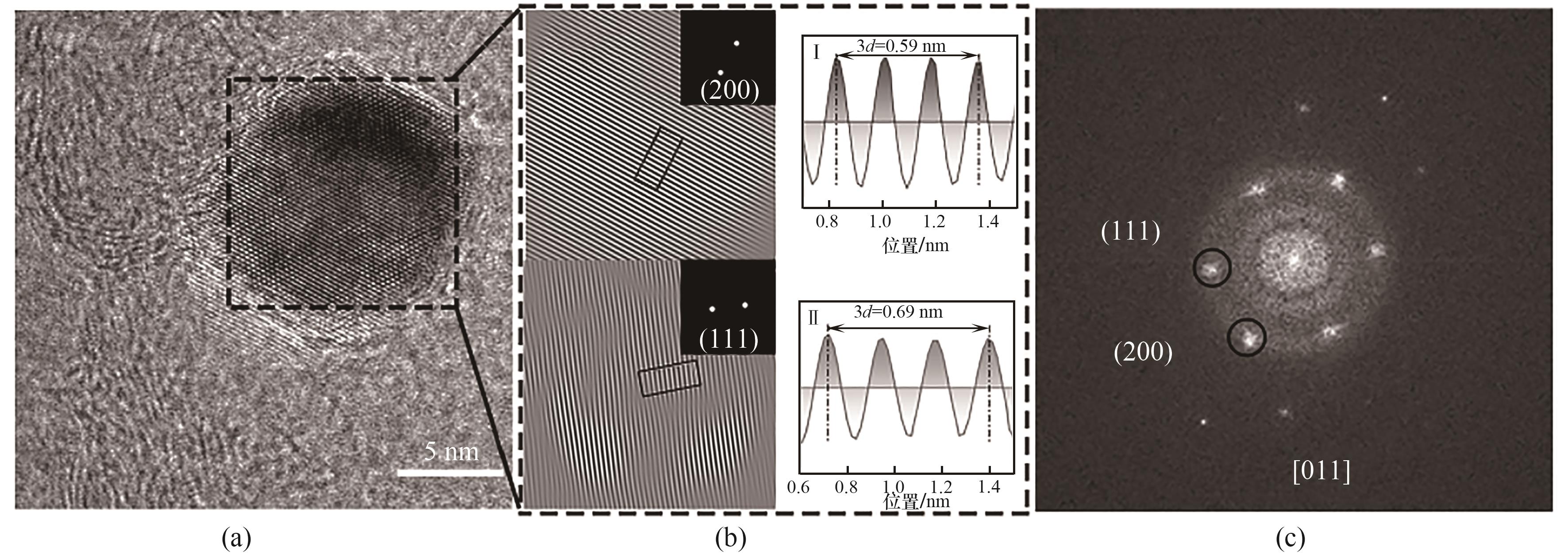
图2 (a) Pd MEA@N-C纳米结构的HR-TEM图像;(b) Pd MEA@N-C(200)、(111)晶面的晶面间距;(c) Pd MEA@N-C纳米结构的FFT图像
Fig.2 (a) HR-TEM image of Pd MEA@N-C nanoparticle; (b) IFFT image of (200), (111) plane of Pd MEA nanoparticle; (c) FFT image of Pd MEA@N-C nanoparticle
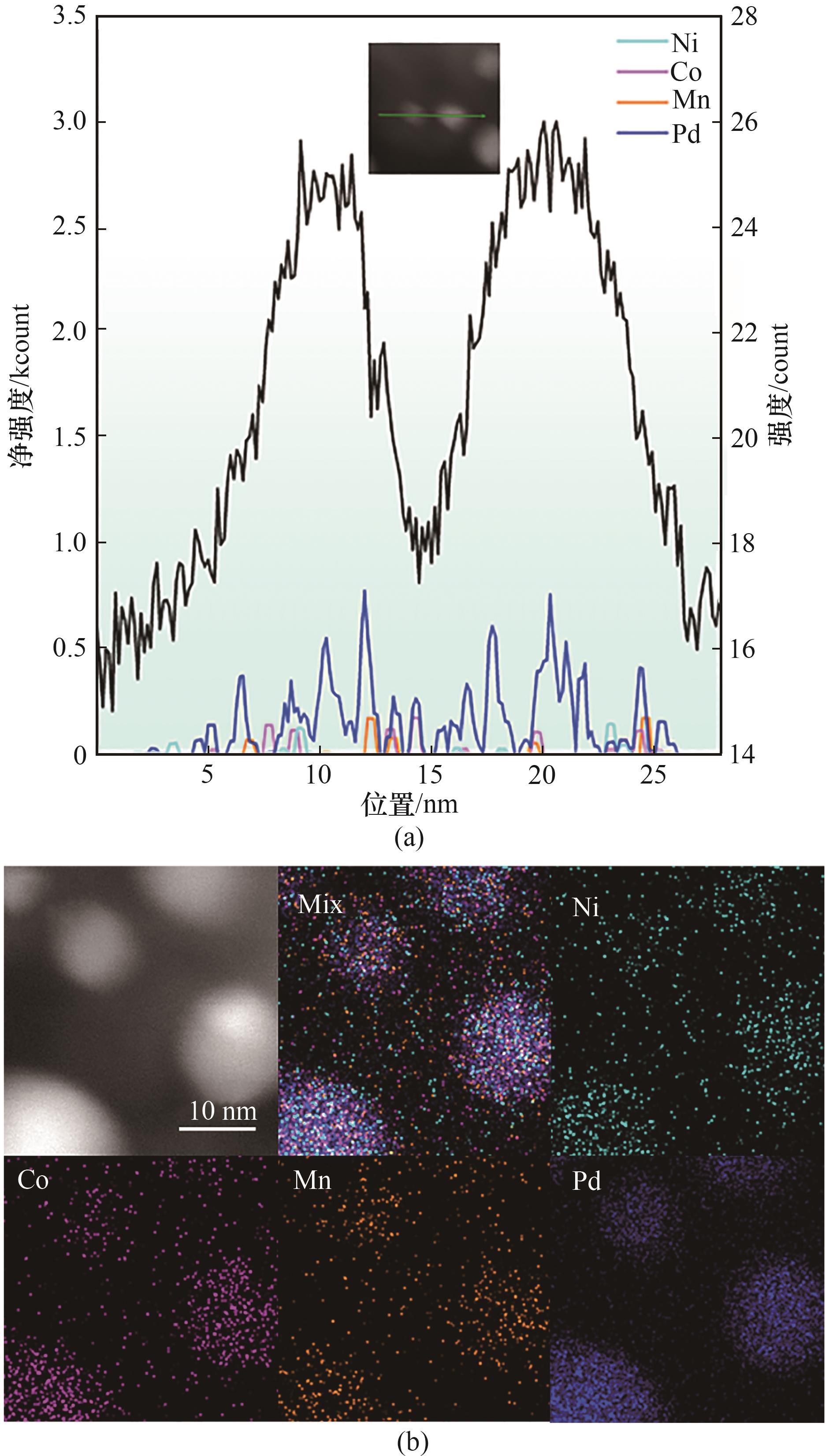
图3 (a) Pd MEA@N-C纳米颗粒上的Ni、Co、Mn和Pd的线扫描信号;(b) Pd MEA@N-C的元素映射图
Fig.3 (a) The line scan signals of Ni, Co, Mn, and Pd across the Pd MEA@N-C nanoparticle; (b) Elemental mapping images of Pd MEA@N-C
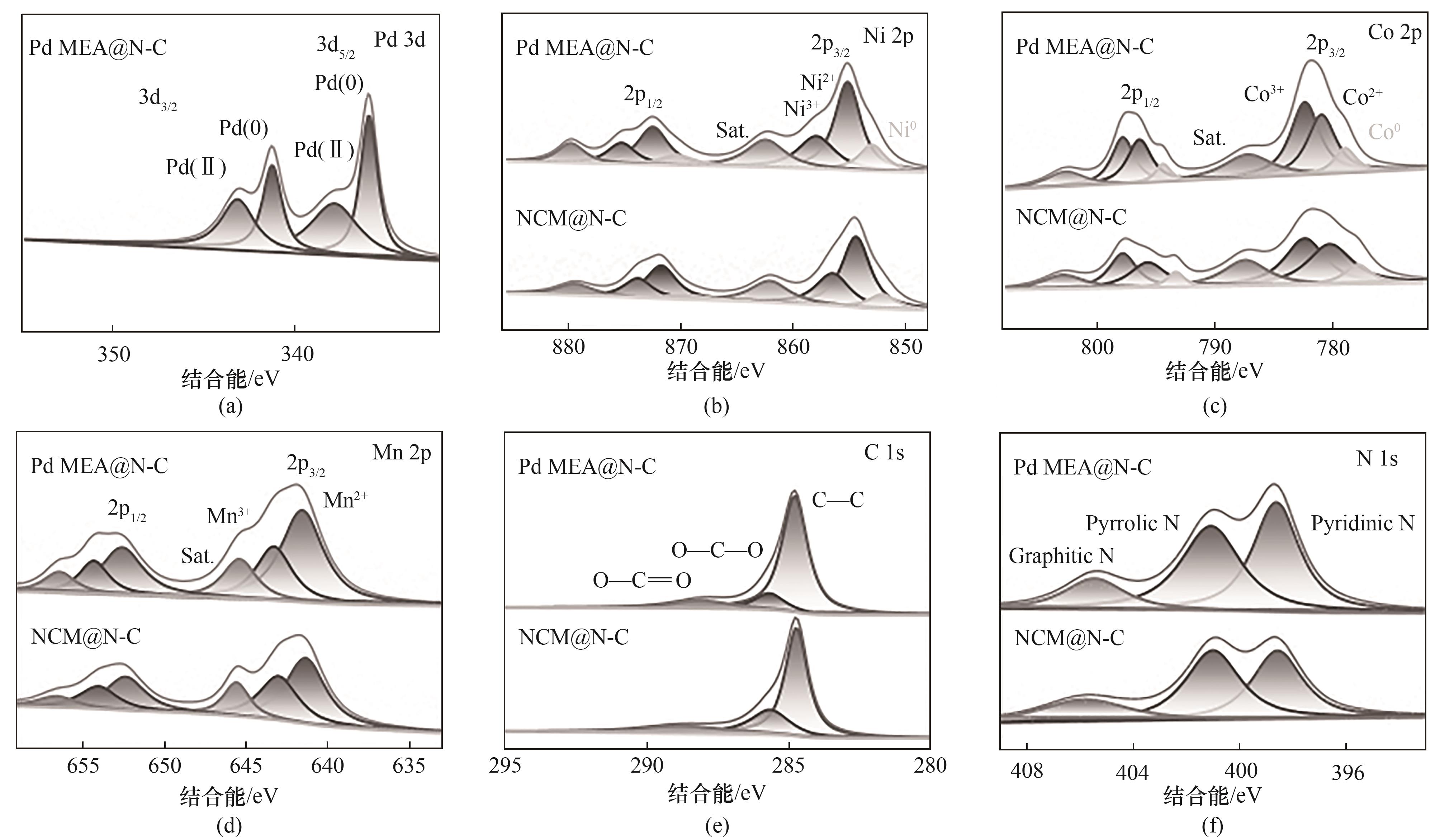
图5 Pd MEA@N-C、NCM@N-C和N-C Pd 3d、Ni 2p、Co 2p、Mn 2p、C 1s和N 1s的XPS光谱
Fig.5 High-resolution XPS spectra of Pd 3d, Ni 2p, Co 2p, Mn 2p, C 1s, and N 1s of Pd MEA@N-C, NCM@N-C and N-C
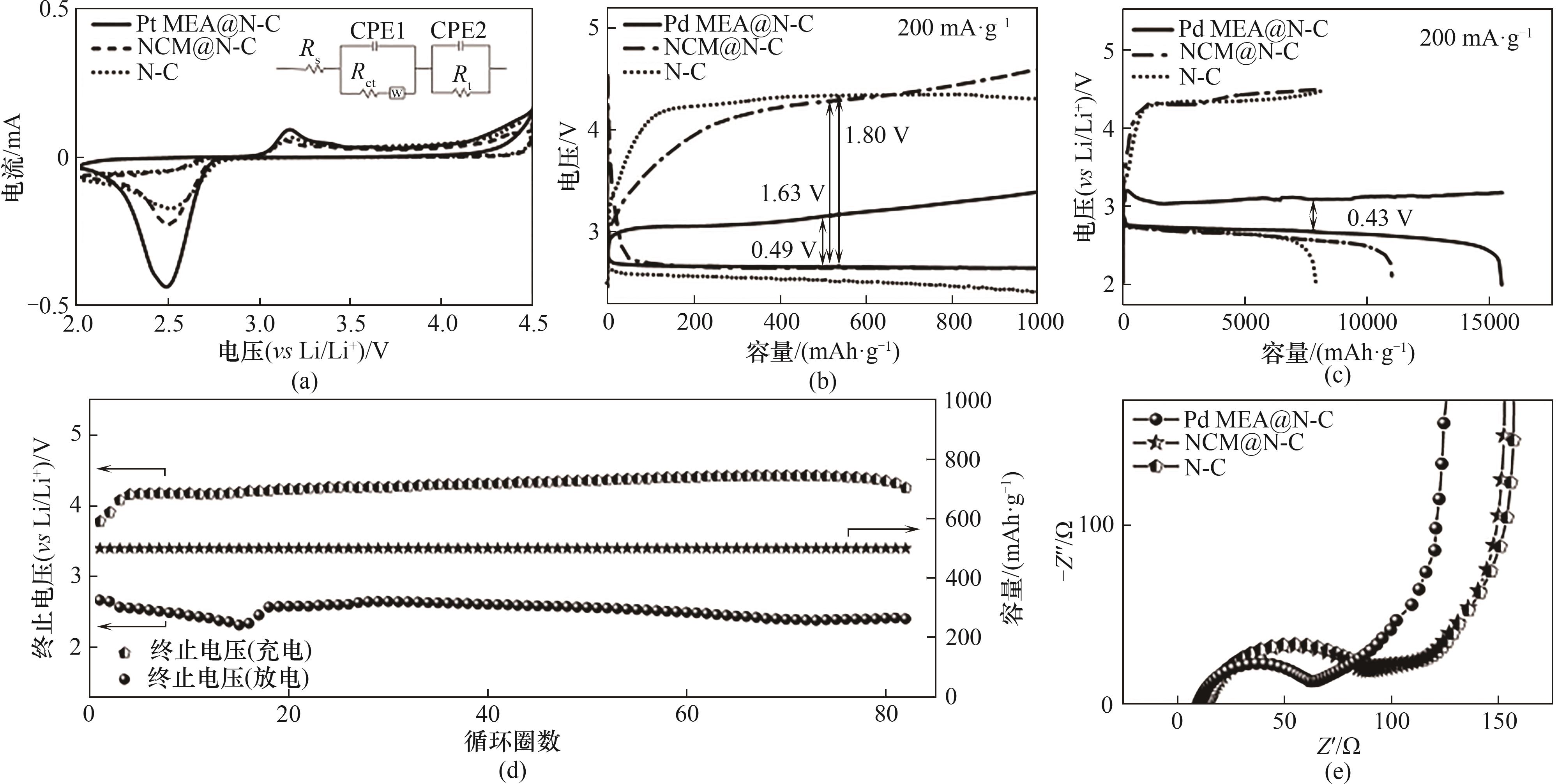
图8 电化学性能:(a) 0.1mV·s-1下Pd MEA@N-C、NCM@N-C和N-C电极的CV曲线;(b)三个电极在限制1000 mAh·g-1容量下200 mA·g-1电流密度的充放电曲线;(c)三个电极在200 mA·g-1下的初始深度放电-充电曲线;(d) Pd MEA@N-C电极在200 mA·g-1电流密度限制容量为500 mAh·g-1时的循环稳定性;(e) Pd MEA@N-C、NCM@N-C和N-C原始样品的EIS
Fig.8 Electrochemical performance: (a) CV curves of Pd MEA@N-C, NCM@N-C and N-C electrodes at 0.1 mV·s-1; (b) Discharge-charge curves of the three electrodes at a curtailed capacity of 1000 mAh·g-1 at 200 mA·g-1; (c) The initial deep discharge-charge curves of the three electrodes at 200 mA·g-1; (d) Cycling stability of Pd MEA@N-C electrode at 200 mA·g-1 with a limited capacity of 500 mAh·g-1; (e) EIS spectra of Pd MEA@N-C, NCM@N-C and N-C electrodes in the pristine
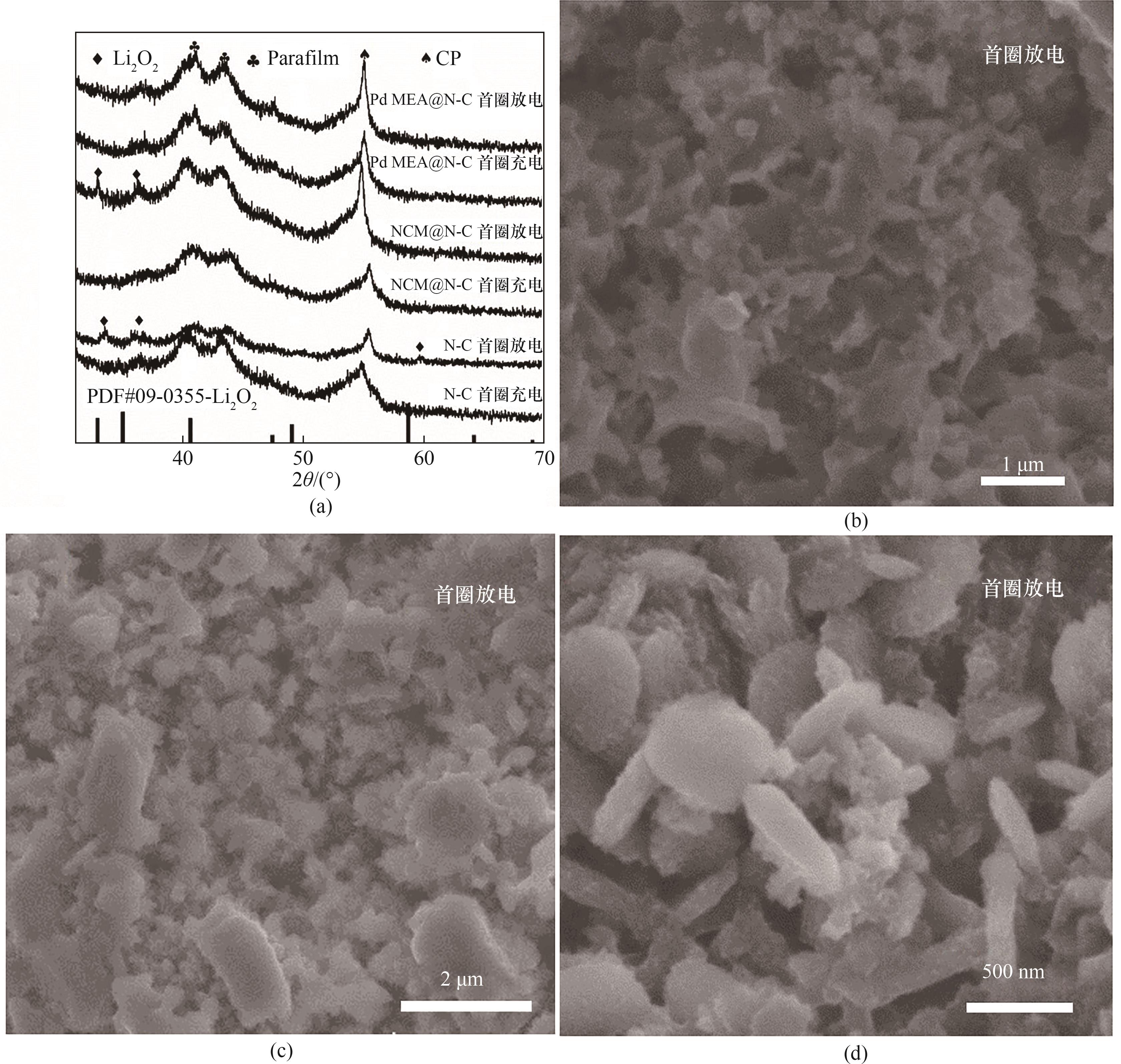
图9 (a) Pd MEA@N-C、NCM@N-C和N-C电极首圈充放电完成后的非原位XRD谱图;(b) Pd MEA@N-C、(c) NCM@N-C和(d) N-C电极放电1000 mAh·g-1的SEM图像
Fig.9 (a) Ex-situ XRD patterns of discharged and charged Pd MEA@N-C, NCM@N-C and N-C electrodes during the 1st cycle; SEM images after discharged to 1000 mAh·g-1 for (b) Pd MEA@N-C, (c) NCM@N-C and (d) N-C cathode
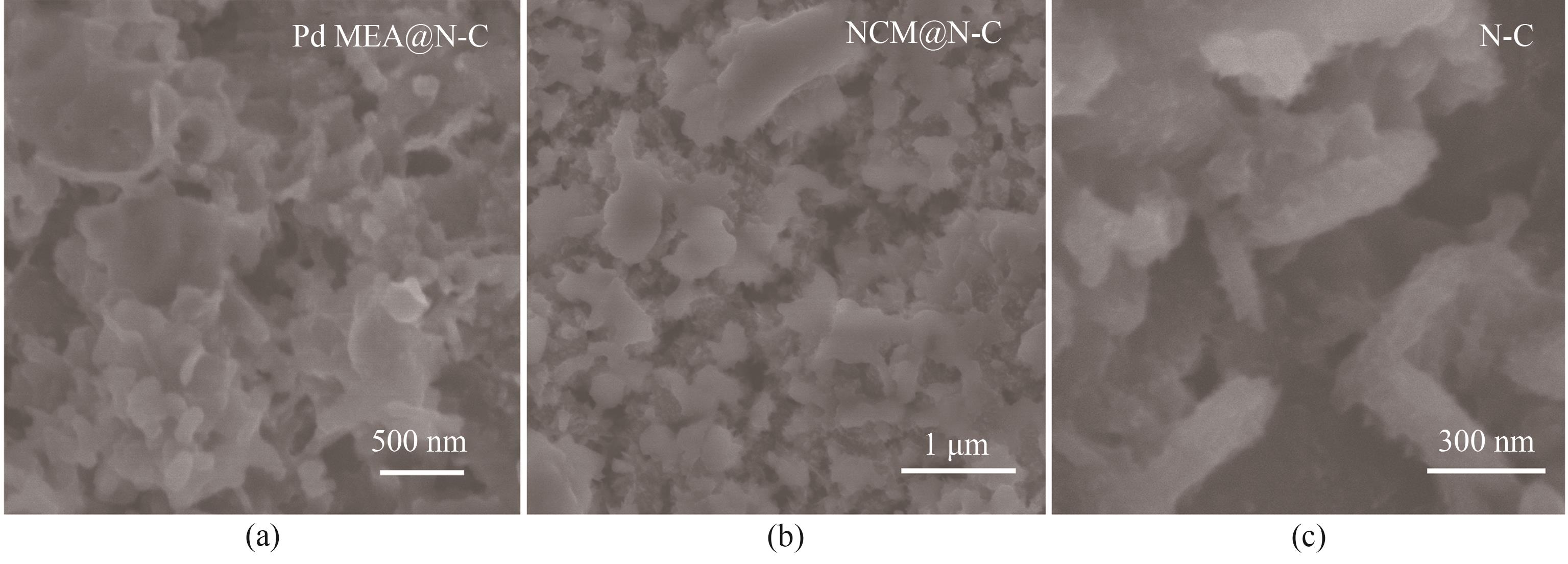
图10 (a) Pd MEA@N-C、(b) NCM@N-C和(c) N-C电极充电1000 mAh·g-1的SEM图像
Fig.10 SEM images after recharged to 1000 mAh·g-1 for (a) Pd MEA@N-C, (b) NCM@N-C and (c) N-C cathode
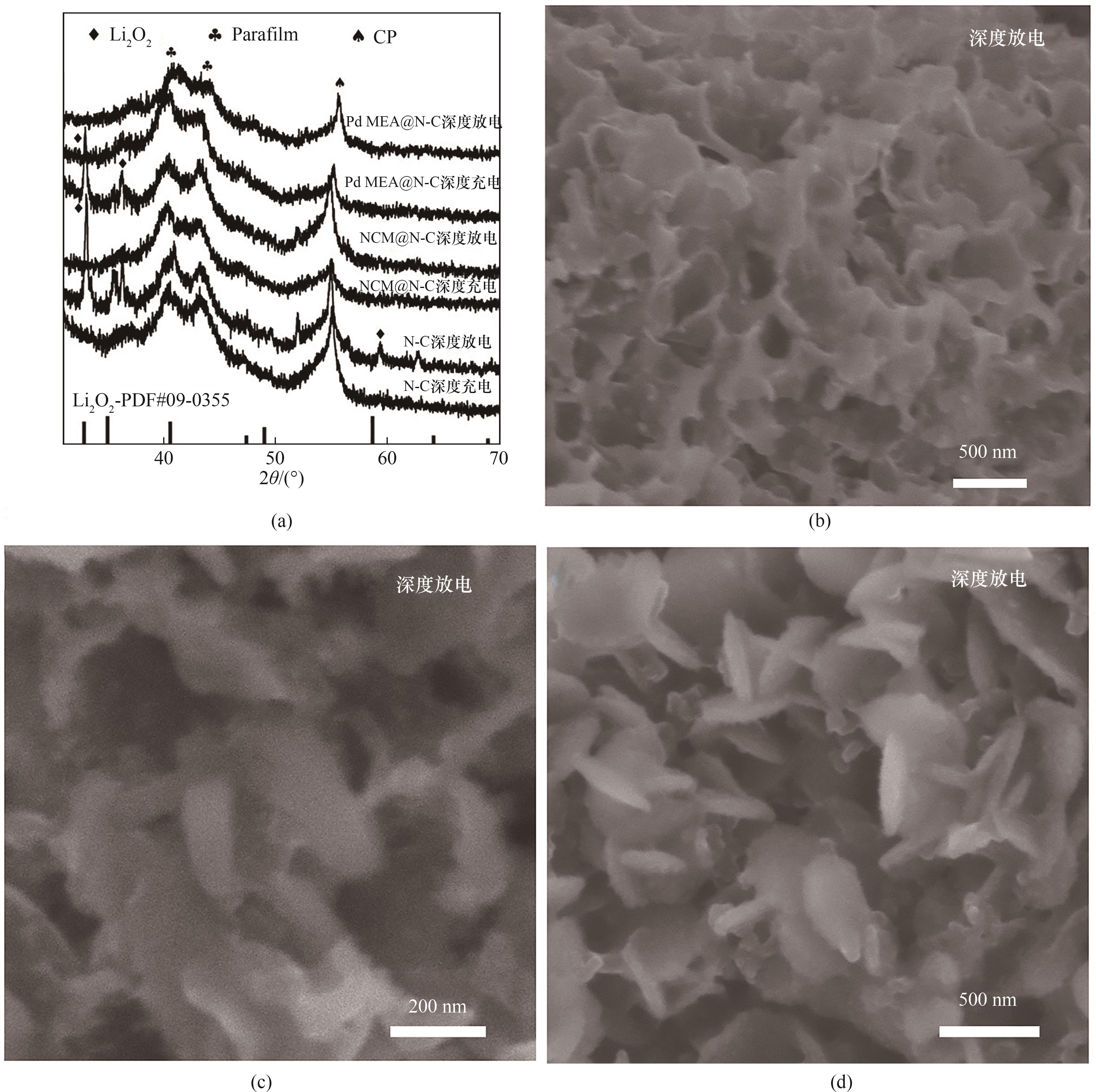
图11 (a) Pd MEA@N-C、NCM@N-C和N-C电极深度充放电完成后的非原位XRD谱图;(b) Pd MEA@N-C、(c) NCM@N-C和(d)N-C电极深度放电的SEM图像
Fig.11 (a) Ex-situ XRD patterns of the initial deep discharge-charge curves Pd MEA@N-C, NCM@N-C and N-C electrodes; SEM images after deep discharged for (b) Pd MEA@N-C, (c) NCM@N-C and (d) N-C cathode
| 1 | Lu Q, Zhou J L, Zhou X Y, et al. Evaluation of optimal waste lithium-ion battery recycling technology driven by multiple factors[J]. Journal of Energy Storage, 2024, 86: 111229. |
| 2 | Jiang W J, Tang T, Zhang Y, et al. Synergistic modulation of non-precious-metal electrocatalysts for advanced water splitting[J]. Accounts of Chemical Research, 2020, 53(6): 1111-1123. |
| 3 | Nie S, Cai G T, Huang Y P, et al. Deciphering stakeholder strategies in electric vehicle battery recycling: insights from a tripartite evolutionary game and system dynamics[J]. Journal of Cleaner Production, 2024, 452: 142174. |
| 4 | Guo R, He Y J, Tian X J, et al. New energy vehicle battery recycling strategy considering carbon emotion from a closed-loop supply chain perspective[J]. Scientific Reports, 2024, 14(1): 688. |
| 5 | Gerold E, Lerchbammer R, Antrekowitsch H. Recovery of cobalt, nickel, and lithium from spent lithium-ion batteries with gluconic acid leaching process: kinetics study[J]. Batteries, 2024, 10(4): 120. |
| 6 | Kang S Y, Ou J T, Wang X, et al. Effect analysis on recycling of cathode material from spent ternary lithium-ion batteries via supercritical water oxidation and acid-leaching[J]. The Journal of Supercritical Fluids, 2024, 211: 106297. |
| 7 | Li C Y, Dai G F, Liu R Y, et al. Separation and recovery of nickel cobalt manganese lithium from waste ternary lithium-ion batteries[J]. Separation and Purification Technology, 2023, 306: 122559. |
| 8 | Sun C Y, Cui X H, Xiao F L, et al. Modulating the d-band center of RuO2 via Ni incorporation for efficient and durable Li-O2 batteries[J]. Small, 2024, 20(32): e2400010. |
| 9 | Su L W, Zhang L, Zhan X Y, et al. Oxygen defect regulation, catalytic mechanism, and modification of HfO2 as a novel catalyst for lithium-oxygen batteries[J]. Journal of Materials Chemistry A, 2024, 12(2): 1176-1184. |
| 10 | Xiao F L, Bao Q S, Sun C Y, et al. d-band center regulation for durable catalysts and constructing a robust hybrid layer on Li anode enable long-life Li-O2 batteries[J]. Advanced Energy Materials, 2024, 14(15): 2303766. |
| 11 | Zhang E H, Dong A Q, Yin K, et al. Electron localization in rationally designed Pt1Pd single-atom alloy catalyst enables high-performance Li-O2 batteries[J]. Journal of the American Chemical Society, 2024, 146(4): 2339-2344. |
| 12 | Yan W, Wang X, Liu M M, et al. PCTS-controlled synthesis of L10/L12-typed Pt-Mn intermetallics for electrocatalytic oxygen reduction[J]. Advanced Functional Materials, 2024, 34(6): 2310487. |
| 13 | Liao Y T, Zhu R T, Zhang W J, et al. Transient synthesis of carbon-supported high-entropy alloy sulfide nanoparticles via flash Joule heating for efficient electrocatalytic hydrogen evolution[J]. Nano Research, 2024, 17(4): 3379-3389. |
| 14 | Ma S Y, Lou A Q, Yao K, et al. Epitaxial growth of Li2O2 achieved by a rational designed Fe3O4@N-doped carbon nanoflower structure for improving the performance of Li-O2 batteries[J]. ChemNanoMat, 2024, 10(1): e202300447. |
| 15 | Zhao Y J, Meng K, Luo T, et al. Electronic structure engineering of RuCo nanoalloys supported on nanoporous carbon for Li-O2 batteries[J]. Journal of Power Sources, 2024, 597: 234130. |
| 16 | Zhang T, He Z, Yin L, et al. CoNi alloy nanoparticles confined by N-doped carbon matrix with tailored d-band center for electrocatalytic hydrogen evolution[J]. Fuel, 2024, 365: 131176. |
| 17 | Zhang F, Sun S W, Ge X H, et al. Synthesizing Pd-based high entropy alloy nanoclusters for enhanced oxygen reduction[J]. Chemical Communications, 2024, 60(26): 3591-3594. |
| 18 | Li Z, Tian Z L, Cheng H, et al. Engineering d-band center of FeN4 moieties for efficient oxygen reduction reaction electrocatalysts[J]. Energy Storage Materials, 2023, 59: 102764. |
| 19 | Li D Y, Zhao L L, Wang J, et al. Tailoring the d-band center over isomorphism pyrite catalyst for optimized intrinsic affinity to intermediates in lithium-oxygen batteries[J]. Advanced Energy Materials, 2023, 13(15): 2204057. |
| 20 | Sun L, Yuwono J A, Zhang S L, et al. High entropy alloys enable durable and efficient lithium-mediated CO2 redox reactions[J]. Advanced Materials, 2024, 36(25): 2401288. |
| 21 | Li T F, Zhang L P, Zhang L, et al. Tailoring the chemisorption manner of Fe d-band center with La2O3 for enhanced oxygen reduction in anion exchange membrane fuel cells[J]. Advanced Functional Materials, 2024, 34(9): 2309886. |
| 22 | Zhang P, Hui X B, Nie Y J, et al. New conceptual catalyst on spatial high-entropy alloy heterostructures for high-performance Li-O2 batteries[J]. Small, 2023, 19(15): e2206742. |
| 23 | Tian J M, Rao Y, Shi W H, et al. Sabatier relations in electrocatalysts based on high-entropy alloys with wide-distributed d-band centers for Li-O2 batteries[J]. Angewandte Chemie International Edition, 2023, 62(44): e202310894. |
| 24 | Han X, Zhao L L, Wang J, et al. Delocalized electronic engineering of Ni5P4 nanoroses for durable Li-O2 batteries[J]. Advanced Materials, 2023, 35(35): 2301897. |
| 25 | 杨阳. ZIF-67衍生物的合成及其对锂氧电池催化性能的影响[D]. 南京: 南京邮电大学, 2019. |
| Yang Y. Synthesis of ZIF-67 derivatives and their effects on catalytic performance of lithium-oxygen batteries[D]. Nanjing: Nanjing University of Posts and Telecommunications, 2019. | |
| 26 | 张萌. 锂氧电池正极材料的设计及性能研究[D]. 天津: 天津工业大学, 2020. |
| Zhang M. Design and performance study of cathode materials for lithium-oxygen batteries[D]. Tianjin: Tianjin Polytechnic University, 2020. | |
| 27 | Feng J J, Wang H C, Guo L, et al. Stacking surface derived catalytic capability and by-product prevention for high efficient two dimensional Bi2Te3 cathode catalyst in Li-oxygen batteries[J]. Applied Catalysis B: Environmental, 2022, 318: 121844. |
| 28 | Li S S, Liu Y S, Wu X Y, et al. Tailoring the growth and morphology of lithium peroxide: nickel sulfide/nickel phosphate nanotubes with optimized electronic structure for lithium-oxygen batteries[J]. Small, 2023, 19(52): e2304435. |
| 29 | Li M, Wu J X, You Z C, et al. Crown ether electrolyte induced Li2O2 amorphization for low polarization and long lifespan Li-O2 batteries[J]. Angewandte Chemie International Edition, 2024, 63(27): e202403521. |
| 30 | Yan H, Wang W W, Wu T R, et al. Morphology-dictated mechanism of efficient reaction sites for Li2O2 decomposition[J]. Journal of the American Chemical Society, 2023, 145(22): 11959-11968. |
| 31 | Zhang Y, Zhang S T, Ma J G, et al. Single-atom-mediated spinel octahedral structures for elevated performances of Li-oxygen batteries[J]. Angewandte Chemie International Edition, 2023, 62(15): e202218926. |
| 32 | Zhang Y, Zhang S T, Li H N, et al. Tunable oxygen vacancies of cobalt oxides in lithium-oxygen batteries: morphology control of discharge product[J]. Nano Letters, 2023, 23(19): 9119-9125. |
| 33 | Zhang X Q, Zhang G L, Yang R N, et al. Lattice-dependent activation of highly efficient SnTe cathode catalyst for Li-air batteries[J]. Energy Storage Materials, 2024, 69: 103392. |
| [1] | 徐子易, 席阳, 宋泽文, 周海骏. 碳纳米材料在锌离子电池中的应用研究进展[J]. 化工学报, 2025, 76(1): 40-52. |
| [2] | 纪之骄, 张晓方, 甘汶, 薛云鹏. 载体对单原子电催化剂合成氨性能的影响与调控策略[J]. 化工学报, 2025, 76(1): 18-39. |
| [3] | 邹吉军, 刘宝宏, 史成香, 潘伦, 张香文. 综纤维素衍生物转化合成生物航空燃料的非均相催化剂研究进展[J]. 化工学报, 2025, 76(1): 1-17. |
| [4] | 杨晨, 毛伟, 董兴宗, 田松, 赵锋伟, 吕剑. 选择性加氢脱氯合成烯烃研究进展[J]. 化工学报, 2025, 76(1): 53-70. |
| [5] | 石美琳, 赵连达, 邓行健, 王静松, 左海滨, 薛庆国. 催化甲烷重整工艺的研究进展[J]. 化工学报, 2024, 75(S1): 25-39. |
| [6] | 吴德威, 汪郑鹏, 周玥, 李晓宁, 陈招, 李卓, 刘成伟, 李学刚, 肖文德. 固定床法制备锂离子电池硅碳负极材料及其储锂性能研究[J]. 化工学报, 2024, 75(S1): 300-308. |
| [7] | 赵焕娟, 包颖昕, 于康, 刘婧, 钱新明. 多元组分爆轰不稳定性定量实验研究[J]. 化工学报, 2024, 75(S1): 339-348. |
| [8] | 王冉, 王焕, 熊晓云, 关慧敏, 郑云锋, 陈彩琳, 秦玉才, 宋丽娟. FCC催化剂传质强化活性位利用效率的可视化分析[J]. 化工学报, 2024, 75(9): 3198-3209. |
| [9] | 王树振, 王玉婷, 马梦茜, 张巍, 向江南, 鲁海莹, 王琰, 范彬彬, 郑家军, 代卫炯, 李瑞丰. 两步晶化合成ZSM-22分子筛及其临氢异构反应性能[J]. 化工学报, 2024, 75(9): 3176-3187. |
| [10] | 王舒英, 左涛, 石志伟, 范小明, 张卫新. 阳离子交换树脂基介孔石墨化碳合成与储钠性能[J]. 化工学报, 2024, 75(9): 3338-3347. |
| [11] | 罗欣怡, 徐强, 佘永璐, 聂腾飞, 郭烈锦. 光电分解水制氢气泡动力学特性及其传质机理研究[J]. 化工学报, 2024, 75(9): 3083-3093. |
| [12] | 刘亚超, 谭晓杰, 李旭东, 王瑞, 王慧, 韩璇, 赵青山. DES合成高活性CoCO3纳米片及析氧反应性能研究[J]. 化工学报, 2024, 75(9): 3320-3328. |
| [13] | 张梦婷, 王书林, 桑熙, 元兴昊, 徐刚. 人工Cu-TM1459金属酶催化不对称迈克尔加成反应[J]. 化工学报, 2024, 75(9): 3255-3265. |
| [14] | 彭丹, 卢俊杰, 倪文静, 杨媛, 汪靖伦. 高电压钴酸锂电池电解液研究进展[J]. 化工学报, 2024, 75(9): 3028-3040. |
| [15] | 刘旭升, 李泽洋, 杨宇森, 卫敏. 电催化二氧化碳还原制备气态产物的研究进展[J]. 化工学报, 2024, 75(7): 2385-2408. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号