化工学报 ›› 2025, Vol. 76 ›› Issue (3): 1363-1374.DOI: 10.11949/0438-1157.20240820
• 材料化学工程与纳米技术 • 上一篇
马钟琛1,2,3( ), 魏子杰4, 朱明涛1,2, 叶恒棣1,2(
), 魏子杰4, 朱明涛1,2, 叶恒棣1,2( ), 郭学益3(
), 郭学益3( ), 谭磊4(
), 谭磊4( )
)
收稿日期:2024-07-18
修回日期:2024-10-12
出版日期:2025-03-25
发布日期:2025-03-28
通讯作者:
叶恒棣,郭学益,谭磊
作者简介:马钟琛(1990—),男,博士,高级工程师,mahuancontact@sina.com
基金资助:
Zhongchen MA1,2,3( ), Zijie WEI4, Mingtao ZHU1,2, Hengdi YE1,2(
), Zijie WEI4, Mingtao ZHU1,2, Hengdi YE1,2( ), Xueyi GUO3(
), Xueyi GUO3( ), Lei TAN4(
), Lei TAN4( )
)
Received:2024-07-18
Revised:2024-10-12
Online:2025-03-25
Published:2025-03-28
Contact:
Hengdi YE, Xueyi GUO, Lei TAN
摘要:
通过一步氧化制备Mn3O4,研究反应过程中温度、转速、氨锰摩尔比以及氨水流速变化对Mn3O4结构、形貌、粒径的影响。结果表明,升高温度会加快反应速率,降低体系过饱和度,从而增加产物的粒径;随着转速增加,溶液高速流动带来的剪切力会使Mn3O4的粒径趋于减小且分布更为集中;氨锰摩尔比的提高会直接降低体系的过饱和度并优化晶体的生长,使Mn3O4的粒径增加,球形度更好。其中,在搅拌转速900 r/min、氨锰摩尔比为2.0、温度为70℃的条件下获得的Mn3O4粒径D50为10.3 μm,振实密度高达2.65 g/cm3,比表面积仅为0.369 m2/g。在此基础上,通过反应前期调低氨水流速加快成核,在反应后期调高氨水流速从而促进颗粒生长的方式,将Mn3O4粒径D50降至5.34 μm,同时保证了2.51 g/cm3的高振实密度和0.400 m2/g的低比表面积。将粒径为10 μm级和5 μm级的Mn3O4作为前体混合Li2CO3烧制成LiMn2O4正极材料,结果表明10 μm的样品拥有更好的循环性能,在1 C的倍率下首圈放电比容量达到121.3 mAh/g,循环200圈后容量保持率为92.8%;5 μm样品具有更好的倍率性能,在3 C、5 C、10 C倍率下的比容量分别为102.6、93.4、77.6 mAh/g。
中图分类号:
马钟琛, 魏子杰, 朱明涛, 叶恒棣, 郭学益, 谭磊. 一步氧化法制备锰酸锂正极材料用电池级四氧化三锰[J]. 化工学报, 2025, 76(3): 1363-1374.
Zhongchen MA, Zijie WEI, Mingtao ZHU, Hengdi YE, Xueyi GUO, Lei TAN. Preparation of battery-grade manganese tetroxide for lithium manganate cathode material by one-step oxidation method[J]. CIESC Journal, 2025, 76(3): 1363-1374.
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-500 | 8.46 | 14.5 | 25.8 |
| Mn3O4-700 | 9.62 | 14.3 | 23.9 |
| Mn3O4-900 | 9.14 | 13.1 | 18.4 |
表1 不同转速下样品的粒径数据
Table 1 Particle size data of samples at different rotational speeds
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-500 | 8.46 | 14.5 | 25.8 |
| Mn3O4-700 | 9.62 | 14.3 | 23.9 |
| Mn3O4-900 | 9.14 | 13.1 | 18.4 |
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-1∶1 | 4.29 | 7.04 | 12.3 |
| Mn3O4-2∶1 | 6.7 | 10.3 | 15.7 |
| Mn3O4-2.5∶1 | 9.14 | 13.1 | 18.4 |
| Mn3O4-3∶1 | 10.9 | 16.8 | 25.5 |
表2 不同氨锰摩尔比的样品粒径数据
Table 2 Particle size data of samples with different ammonia-manganese ratios
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-1∶1 | 4.29 | 7.04 | 12.3 |
| Mn3O4-2∶1 | 6.7 | 10.3 | 15.7 |
| Mn3O4-2.5∶1 | 9.14 | 13.1 | 18.4 |
| Mn3O4-3∶1 | 10.9 | 16.8 | 25.5 |
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-50 | 5.54 | 8.08 | 11.7 |
| Mn3O4-60 | 4.94 | 8.66 | 14 |
| Mn3O4-70 | 6.7 | 10.3 | 15.7 |
| Mn3O4-80 | 6.95 | 11.4 | 17.4 |
表3 不同温度下的样品粒径数据
Table 3 Sample particle size data at different temperatures
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-50 | 5.54 | 8.08 | 11.7 |
| Mn3O4-60 | 4.94 | 8.66 | 14 |
| Mn3O4-70 | 6.7 | 10.3 | 15.7 |
| Mn3O4-80 | 6.95 | 11.4 | 17.4 |
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-0.3/1.4 | 3.49 | 5.34 | 9.33 |
| Mn3O4-0.5/1.3 | 3.01 | 6.34 | 10.40 |
| Mn3O4-0.7/1.25 | 5.31 | 7.11 | 9.55 |
表4 不同流速条件下的样品粒径数据
Table 4 Sample particle size data at different flow rates
| 样品 | D10/μm | D50/μm | D90/μm |
|---|---|---|---|
| Mn3O4-0.3/1.4 | 3.49 | 5.34 | 9.33 |
| Mn3O4-0.5/1.3 | 3.01 | 6.34 | 10.40 |
| Mn3O4-0.7/1.25 | 5.31 | 7.11 | 9.55 |
| 样品 | D50/μm | 振实密度/(g/cm3) | 比表面积/(m2/g) | Mn含量/% | 产率/% |
|---|---|---|---|---|---|
| Mn3O4-5 | 5.34 | 2.51 | 0.400 | 70.93 | 91.6 |
| Mn3O4-10 | 10.3 | 2.65 | 0.369 | 70.82 | 90.0 |
表5 不同流速条件下样品的部分参数
Table 5 Some parameters of samples at different flow rates
| 样品 | D50/μm | 振实密度/(g/cm3) | 比表面积/(m2/g) | Mn含量/% | 产率/% |
|---|---|---|---|---|---|
| Mn3O4-5 | 5.34 | 2.51 | 0.400 | 70.93 | 91.6 |
| Mn3O4-10 | 10.3 | 2.65 | 0.369 | 70.82 | 90.0 |
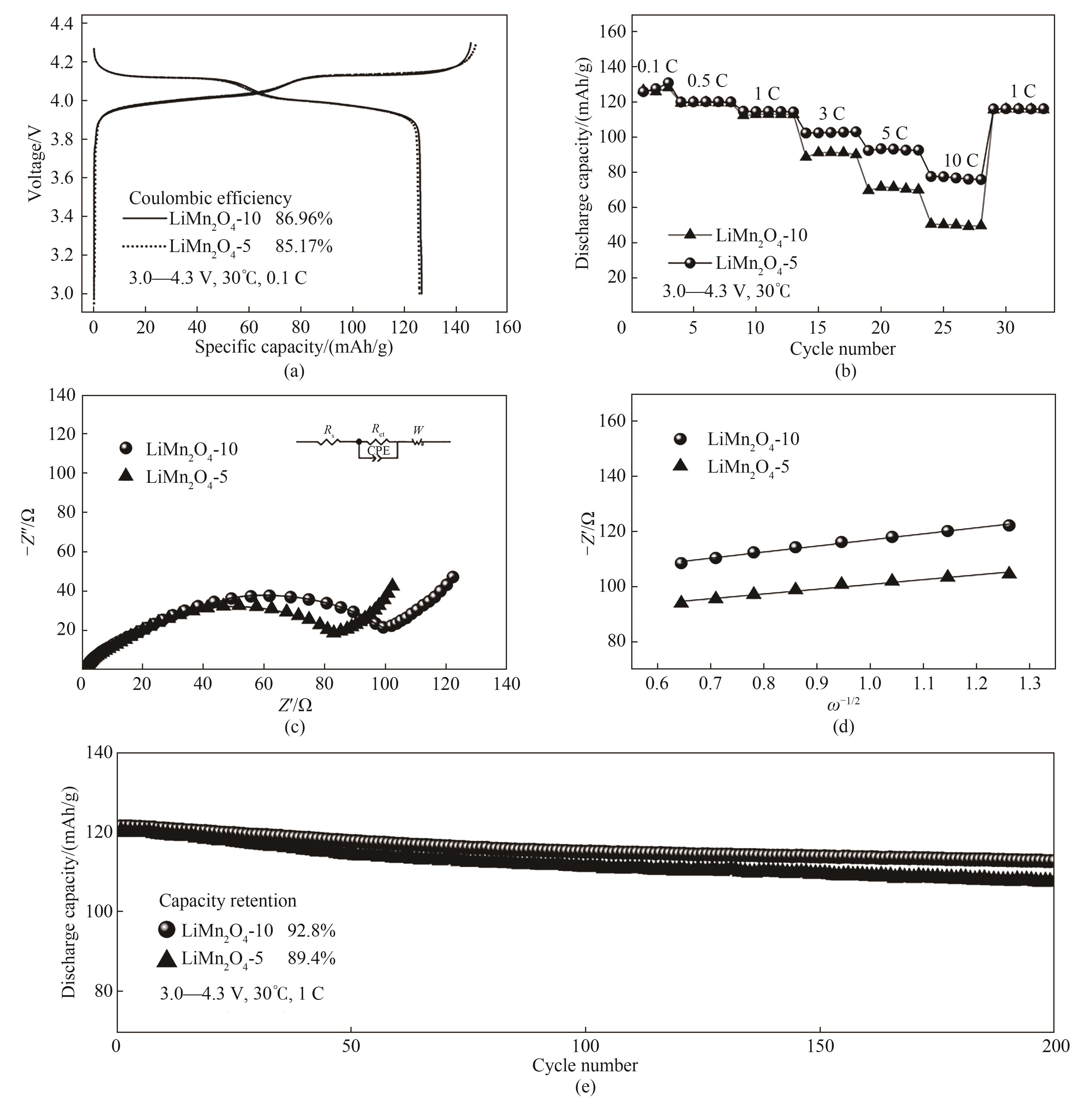
图18 LiMn2O4-5和LiMn2O4-10材料的电化学性能:(a) LiMn2O4的首圈充放电曲线;(b) LiMn2O4正极材料的倍率性能图;(c) Nyquist图;(d) Z’和ω-1/2关系示意图;(e) LiMn2O4正极材料的循环曲线
Fig.18 Electrochemical properties of LiMn2O4-5 and LiMn2O4-10: (a) Charge-discharge curves of LiMn2O4; (b) Rate performance curves of LiMn2O4 cathode materials; (c) Nyquist plot; (d) Schematic diagram of the relationship between Z' and ω-1/2; (e) Cycle curves of LiMn2O4 cathode material
| 样品 | 电压区间/V | 1 C比容量/(mAh/g) | 5 C比容量/(mAh/g) | 10 C比容量/(mAh/g) | 容量保持率/% |
|---|---|---|---|---|---|
| 文献样品1[ | 3~4.3 | 109.2 | 91.0 | 77.9 | 72.1(1 C, 100圈) |
| 文献样品2[ | 3~4.3 | 108.4 | 96.5 | 85.1 | 91.3(0.2 C, 100圈) |
| 文献样品3[ | 3~4.3 | 121.0 | 90.6 | 80.5 | 88.1(1 C, 100圈) |
| 文献样品4[ | 3~4.4 | 117.0 | 49.0 | — | 65.8(1 C, 100圈) |
| 文献样品5[ | 3.2~4.3 | 102.0 | 85.0 | 69.0 | 41.1(2 C, 200圈) |
| LiMn2O4-10 | 3~4.3 | 121.3 | 71.6 | 50.6 | 92.8(1 C, 200圈) |
| LiMn2O4-5 | 3~4.3 | 120.2 | 93.4 | 77.6 | 89.4(1 C, 200圈) |
表6 样品LiMn2O4-10和LiMn2O4-5与文献样品的电化学性能对比
Table 6 Comparison of the electrochemical properties of LiMn2O4-10 and LiMn2O4-5 samples with those in the literatures
| 样品 | 电压区间/V | 1 C比容量/(mAh/g) | 5 C比容量/(mAh/g) | 10 C比容量/(mAh/g) | 容量保持率/% |
|---|---|---|---|---|---|
| 文献样品1[ | 3~4.3 | 109.2 | 91.0 | 77.9 | 72.1(1 C, 100圈) |
| 文献样品2[ | 3~4.3 | 108.4 | 96.5 | 85.1 | 91.3(0.2 C, 100圈) |
| 文献样品3[ | 3~4.3 | 121.0 | 90.6 | 80.5 | 88.1(1 C, 100圈) |
| 文献样品4[ | 3~4.4 | 117.0 | 49.0 | — | 65.8(1 C, 100圈) |
| 文献样品5[ | 3.2~4.3 | 102.0 | 85.0 | 69.0 | 41.1(2 C, 200圈) |
| LiMn2O4-10 | 3~4.3 | 121.3 | 71.6 | 50.6 | 92.8(1 C, 200圈) |
| LiMn2O4-5 | 3~4.3 | 120.2 | 93.4 | 77.6 | 89.4(1 C, 200圈) |
| 1 | Xu G L, Liu Q, Lau K K S, et al. Building ultraconformal protective layers on both secondary and primary particles of layered lithium transition metal oxide cathodes[J]. Nature Energy, 2019, 4: 484-494. |
| 2 | Xu S L, Zhu Y, Li X Y, et al. PVA-regulated construction of 3D rGO-hosted Na3V2(PO4)2F3 for fast and stable sodium storage[J]. Journal of Energy Chemistry, 2024, 99: 100-109. |
| 3 | 王崇国, 刘广龙, 金小容, 等. 锂离子电池正极材料的研究进展[J]. 当代化工研究, 2023(9): 12-14. |
| Wang C G, Liu G L, Jin X R, et al. Research progress of lithium-ion battery cathode material[J]. Modern Chemical Research, 2023(9): 12-14. | |
| 4 | 木宗云, 许定胜, 李庆波, 等. 锰系氧化物在LiMn2O4中的应用[J]. 中国锰业, 2020, 38(5): 1-5. |
| Mu Z Y, Xu D S, Li Q B, et al. An application of manganese series oxides in LiMn2O4 [J]. China's Manganese Industry, 2020, 38(5): 1-5. | |
| 5 | Cui X L, Du S L, Zhu K L, et al. Elevated electrochemical property of LiMn2O4 originated from nano-sized Mn3O4 [J]. Ionics, 2018, 24(3): 697-706. |
| 6 | Park S H, Ahn H S, Park G J, et al. Cycle mechanism and electrochemical properties of lithium manganese oxide prepared using different Mn sources[J]. Materials Chemistry and Physics, 2008, 112(2): 696-701. |
| 7 | Chen F L, Lin J N, Chen Y F, et al. Enhancement of electrochemical performance in lithium-ion battery via tantalum oxide coated nickel-rich cathode materials[J]. Chinese Physics B, 2022, 31(5): 058101. |
| 8 | An Q, Liu Q, Wang S M, et al. Oxygen vacancies with localized electrons direct a functionalized separator toward dendrite-free and high loading LiFePO4 for lithium metal batteries[J]. Journal of Energy Chemistry, 2022, 75: 38-45. |
| 9 | Zawrah M F, El Fadaly E A, Khattab R M, et al. Synthesis and characterization of nano Mn3O4 and LiMn2O4 spinel from manganese ore and pure materials[J]. Ceramics International, 2020, 46(11): 17514-17522. |
| 10 | 杨洋, 王以存, 王志鹏, 等. 电解锰悬浮液氧化法制备电池级四氧化三锰[J]. 中国锰业, 2021, 39(4): 65-68. |
| Yang Y, Wang Y C, Wang Z P, et al. Battery grade manganese tetroxide synthesized by oxidation of manganese suspension[J]. China Manganese Industry, 2021, 39(4): 65-68. | |
| 11 | 杨洋, 何克杰, 罗炎, 皮露, 等. 硫酸锰溶液碱化沉淀—焙烧法制备Mn3O4 [J]. 湿法冶金, 2015, 34(3): 213-217. |
| He K J, Luo Y, Pi L, et al. Preparation of Mn3O4 by alkaline precipitation-calcination process using MnSO4 solution[J]. Hydrometallurgy of China, 2015, 34(3): 213-217. | |
| 12 | 陈丽鹃, 彭天剑, 田梅, 等. 锂二次电池正极材料用四氧化三锰的制备研究[J]. 应用化工, 2012, 41(3): 473-475, 479. |
| Chen L J, Peng T J, Tian M, et al. Preparation of trimanganese tetraoxide used as anode material in lithium secondary battery[J]. Applied Chemical Industry, 2012, 41(3): 473-475, 479. | |
| 13 | Du K, Hu G R, Peng Z D, et al. Synthesis of spinel LiMn2O4 with manganese carbonate prepared by micro-emulsion method[J]. Electrochimica Acta, 2010, 55(5): 1733-1739. |
| 14 | Zhu H L, Chen Z Y, Ji S, et al. Influence of different morphologies on electrochemical performance of spinel LiMn2O4 [J]. Solid State Ionics, 2008, 179(27/28/29/30/31/32): 1788-1793. |
| 15 | 邹兴, 朱鸿民. 硫酸锰在氨性介质中制备四氧化三锰的研究[J]. 中国锰业, 2009, 27(4): 4-6, 19. |
| Zou X, Zhu H M. A study on preparation of Mn3O4 by oxidizing hydrolysis of manganese sulfate with air in ammonia alkalescence media[J]. China's Manganese Industry, 2009, 27(4): 4-6, 19. | |
| 16 | 骆艳华, 陈思学, 王凡, 等. 硫酸锰直接制备四氧化三锰的研究[J]. 矿业快报, 2008, 24(2): 17-19. |
| Luo Y H, Chen S X, Wang F, et al. Research on direct preparation of Mn3O4 by MnSO4 [J]. Express Information of Mining Industry, 2008, 24(2): 17-19. | |
| 17 | 莫燕娇. 络合沉淀法制备高密度四氧化三锰[J]. 化学与生物工程, 2022, 39(6): 33-36. |
| Mo Y J. Preparation of manganese tetroxide with high density by complex precipitation method[J]. Chemistry & Bioengineering, 2022, 39(6): 33-36. | |
| 18 | 蔡启果, 王海峰, 王家伟, 等. 硫酸锰液制取四氧化三锰的工艺条件研究[J]. 金属矿山, 2018(5): 80-83. |
| Cai Q G, Wang H F, Wang J W, et al. Study on technological conditions for preparation of manganese oxide from manganese sulfate solution[J]. Metal Mine, 2018(5): 80-83. | |
| 19 | 薛娟琴, 王成刚, 刘小勇. 以硫酸锰为原料制备四氧化三锰的理论分析[J]. 西安建筑科技大学学报(自然科学版), 2000, 32(3): 297-299. |
| Xue J Q, Wang C G, Liu X Y. Study on technology in preparation of Mn3O4 from MnSO4 [J]. Journal of Xi’an University of Architecture & Technology(Natural Science Edition), 2000, 32(3): 297-299. | |
| 20 | Jiang J B, Du K, Cao Y B, et al. Syntheses of spherical LiMn2O4 with Mn3O4 and its electrochemistry performance[J]. Journal of Alloys and Compounds, 2013, 577: 138-142. |
| 21 | 何佳, 张昭. 硫酸锰为原料沉淀-氧化法制备四氧化三锰的研究[J]. 无机盐工业, 2014, 46(3): 45-49. |
| He J, Zhang Z. Synthesis of Mn3O4 powder from manganese sulfate by precipitation-oxidation process[J]. Inorganic Chemicals Industry, 2014, 46(3): 45-49. | |
| 22 | 陈飞宇, 梅光贵, 谭柱中. 硫酸锰溶液制备电子级四氧化三锰的研究[J]. 中国锰业, 2003, 21(3): 14-16. |
| Chen F Y, Mei G G, Tan Z Z. Study on producing mangano-manganic oxide from manganous sulphate solution[J]. China's Manganese Industry, 2003, 21(3): 14-16. | |
| 23 | Wang H, Wang J W, Wang H F, et al. Study and property characterization of LiMn2O4 synthesized from octahedral Mn3O4 [J]. Sustainability, 2023, 15(18): 13858. |
| 24 | 李春流, 农艳莉, 闫冠杰, 等. 一步氧化法制备类球形四氧化三锰[J]. 湿法冶金, 2020, 39(3): 232-236. |
| Li C L, Nong Y L, Yan G J, et al. Preparation of near spherical manganese tetroxide by one-step oxidation[J]. Hydrometallurgy of China, 2020, 39(3): 232-236. | |
| 25 | 董昌文, 叶华, 杨光辉. 四氧化三锰的制备方法[J]. 广东化工, 2016, 43(12): 92-93. |
| Dong C W, Ye H, Yang G H. The manufacturing methods of manganous-manganic oxide(Mn3O4)[J]. Guangdong Chemical Industry, 2016, 43(12): 92-93. | |
| 26 | 粟海锋, 高家利, 文衍宣, 等. MnSO4·H2O热解制备四氧化三锰反应动力学[J]. 化工学报, 2008, 59(2): 359-365. |
| Su H F, Gao J L, Wen Y X, et al. Kinetics of hausmannite preparation by thermal decomposition of manganese sulfate[J]. Journal of Chemical Industry and Engineering (China), 2008, 59(2): 359-365. | |
| 27 | LaMer V K, Dinegar R H. Theory, production and mechanism of formation of monodispersed hydrosols[J]. Journal of the American Chemical Society, 1950, 72(11): 4847-4854. |
| 28 | Wang B, Yu J, Lu Q F, et al. Preparation of Mn3O4 microspheres via glow discharge electrolysis plasma as a high-capacitance supercapacitor electrode material[J]. Journal of Alloys and Compounds, 2022, 926: 166775. |
| 29 | Zhang S, Chen H Y, Chen J, et al. Atomically interlocked chemistry activated by interstitial sites in LiMn2O4 cathode[J]. Advanced Functional Materials, 2023, 33(5): 2210731. |
| 30 | Jiang S J, Wang S, Li Y J, et al. Structure and interface modification via Gd boosting excellent high-temperature electrochemical performance of LiMn2O4 [J]. Materials Today Energy, 2022, 29: 101096. |
| 31 | Guo Z X, Jiang H R, Sun X Y, et al. Ultrafast non-equilibrium phase transition induced twin boundaries of spinel lithium manganate[J]. Advanced Energy Materials, 2024, 14(5): 2302484. |
| 32 | Zhang S, Fang S S, Chen J, et al. Engineering d-p orbital hybridization for high-stable lithium manganate cathode[J]. Chemical Engineering Journal, 2023, 451: 138511. |
| [1] | 肖志华, 房浩楠, 郑方植, 孙冬, 陶丽达, 李永峰, 徐春明, 马新龙. NaCl辅助构筑高性能沥青基硬炭负极材料[J]. 化工学报, 2025, 76(2): 846-857. |
| [2] | 江洋, 彭长宏, 陈伟, 周豪, 马忠彬, 李洪博, 邱在容, 张国鹏, 周康根. 废旧磷酸铁锂粉料综合回收中试研究[J]. 化工学报, 2024, 75(6): 2353-2361. |
| [3] | 吴吉昊, 陈涛, 刘思宇, 刘梦柯, 杨卷. 双功能活化制备沥青基硬炭用于钠离子电池负极[J]. 化工学报, 2024, 75(3): 1019-1027. |
| [4] | 刘宇喆, 李成才, 李琳, 王少辉, 刘培慧, 王同华. 活性炭的微结构与超级电容器性能的构效关系[J]. 化工学报, 2022, 73(4): 1807-1816. |
| [5] | 任博阳, 车晓刚, 刘思宇, 王满, 韩兴华, 董婷, 杨卷. 熔融盐法制备煤基多孔碳纳米片用于钠离子电池负极[J]. 化工学报, 2022, 73(10): 4745-4753. |
| [6] | 胡涛, 张熊, 安亚斌, 李晨, 马衍伟. 锂离子电容器碳正极材料的研究进展[J]. 化工学报, 2020, 71(6): 2530-2546. |
| [7] | 韩世昌, 邹正光, 吕婷婷, 吴星宇, 杨倩. Ag掺杂VO2(B)正极材料的合成及其电化学性能[J]. 化工学报, 2018, 69(4): 1741-1748. |
| [8] | 李巧乐, 燕映霖, 杨蓉, 陈利萍, 秦海超, 史忙忙, 魏一奇. 锂硫电池用玉米苞叶基活性炭/硫复合正极材料的电化学性能[J]. 化工学报, 2017, 68(11): 4376-4382. |
| [9] | 陈南雄, 谢罗生, 舒建成, 李华成, 王春飞, 刘作华. 氨缓冲体系强化高纯重质碳酸锰的制备[J]. 化工学报, 2016, 67(7): 3078-3083. |
| [10] | 杨蓉, 王黎晴, 吕梦妮, 邓坤发, 燕映霖, 任冰, 李兰. 锂硫电池石墨烯/纳米硫复合正极材料的制备及电化学性能[J]. 化工学报, 2016, 67(10): 4363-4369. |
| [11] | 张睿, 吴元欣, 何云蔚, 艾常春. Li3PO4掺杂的Li(Ni0.5Co0.2Mn0.3)O2锂离子电池正极材料的流变相法合成及电化学性能表征[J]. 化工学报, 2015, 66(8): 3177-3182. |
| [12] | 江浩, 李春忠. 表面化学反应控制制备多级结构电极材料及性能[J]. 化工学报, 2015, 66(8): 2872-2877. |
| [13] | 李吉, 魏彤, 闫俊, 龙从来, 范壮军. 石墨烯纳米片/CoS2复合材料的制备及其在超级电容器中的应用[J]. 化工学报, 2014, 65(7): 2849-2854. |
| [14] | 万厚钊, 缪灵, 徐葵, 亓同, 江建军. MnO2基超级电容器电极材料[J]. 化工学报, 2013, 64(3): 801-813. |
| [15] | 胡素琴,杨改,蔡飞鹏,蒋波. 离子液体基锂离子电池电解液的应用与性能改进[J]. 化工学报, 2011, 62(S2): 1-6. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号