化工学报 ›› 2025, Vol. 76 ›› Issue (3): 1323-1333.DOI: 10.11949/0438-1157.20240833
陈仲卿( ), 刘家旭, 王艳语, 井红权, 侯翠红(
), 刘家旭, 王艳语, 井红权, 侯翠红( ), 屈凌波
), 屈凌波
收稿日期:2024-07-23
修回日期:2024-10-11
出版日期:2025-03-25
发布日期:2025-03-28
通讯作者:
侯翠红
作者简介:陈仲卿(2000—),男,硕士研究生,czqzzu@163.com
基金资助:
Zhongqing CHEN( ), Jiaxu LIU, Yanyu WANG, Hongquan JING, Cuihong HOU(
), Jiaxu LIU, Yanyu WANG, Hongquan JING, Cuihong HOU( ), Lingbo QU
), Lingbo QU
Received:2024-07-23
Revised:2024-10-11
Online:2025-03-25
Published:2025-03-28
Contact:
Cuihong HOU
摘要:
为解决磷尾矿的堆积问题,以磷尾矿和低品位磷矿为主要原料,以B2O3、Al2O3、KCl等为助剂,通过热法加工实现磷尾矿及中低品位磷矿的活化并制备玻璃肥料。通过单因素和响应曲面实验探究了K-B-Al体系对磷尾矿体系熔融特性的影响,结合X射线衍射(XRD)、傅里叶红外光谱(FTIR)、X射线光电子能谱仪(XPS)探讨了助剂添加对熔融产物元素活化效果和渣相结构的影响。结果表明,在磷尾矿、低品位磷矿、蛇纹石、KCl、B2O3、Al2O3配比为55∶25∶20∶7∶10∶1.5时,体系熔融流动温度TF最低为1135℃,较常规体系降低335℃。在1350℃时熔融所得玻璃肥料P2O5、CaO、MgO活化率均达到95%左右。分析了熔融温度和助剂对物料玻璃网络结构的影响。在所选工艺条件下B2O3以断裂硅氧网络为主,Al2O3既参与网络组成,也破坏硅氧网络。
中图分类号:
陈仲卿, 刘家旭, 王艳语, 井红权, 侯翠红, 屈凌波. K-B-Al体系对磷矿熔融特性及玻璃结构的影响[J]. 化工学报, 2025, 76(3): 1323-1333.
Zhongqing CHEN, Jiaxu LIU, Yanyu WANG, Hongquan JING, Cuihong HOU, Lingbo QU. Effect of K-B-Al ternary system on the melting characteristics and glass structure of tailings[J]. CIESC Journal, 2025, 76(3): 1323-1333.
| 原料 | 元素组成/%(质量分数) | 灼烧失量/%(质量分数) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P2O5 | CaO | MgO | SiO2 | Fe2O3 | Al2O3 | K2O | B2O3 | ||
| 磷尾矿 | 9.22 | 26.81 | 8.67 | 5.74 | 0.62 | 0.80 | 0 | 0.65 | 44.73 |
| 低品位磷矿 | 18.33 | 43.05 | 0.95 | 6.51 | 0.35 | 0.58 | 0.07 | 0.29 | 29.60 |
| 蛇纹石 | 1.08 | 2.64 | 39.80 | 37.94 | 0 | 1.05 | 1.08 | 0 | 14.54 |
表1 原料矿主要元素组成
Table 1 Composition of main elements in raw ore
| 原料 | 元素组成/%(质量分数) | 灼烧失量/%(质量分数) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| P2O5 | CaO | MgO | SiO2 | Fe2O3 | Al2O3 | K2O | B2O3 | ||
| 磷尾矿 | 9.22 | 26.81 | 8.67 | 5.74 | 0.62 | 0.80 | 0 | 0.65 | 44.73 |
| 低品位磷矿 | 18.33 | 43.05 | 0.95 | 6.51 | 0.35 | 0.58 | 0.07 | 0.29 | 29.60 |
| 蛇纹石 | 1.08 | 2.64 | 39.80 | 37.94 | 0 | 1.05 | 1.08 | 0 | 14.54 |
| 元素种类 | 分析方法 |
|---|---|
| P2O5、SiO2、CaO、MgO | 《钙镁磷肥》(GB/T 20412—2021) |
| K2O | 《钙镁磷钾肥》(HG/T 2598—1994) |
| Al2O3 | 《磷矿石和磷精矿中氧化铝含量的测定》(GB/T 1871.3—1995) |
| Fe2O3 | 《复混肥料中铜、铁、锰、锌、硼、钼含量的测定》(GB/T 14540—2003) |
| B2O3 | 《地下水质分析方法 第44部分:硼量的测定H酸-甲亚胺分光光度法》(DZ/T 0064.44—2021) |
表2 各元素含量分析方法
Table 2 Analysis method of each element content
| 元素种类 | 分析方法 |
|---|---|
| P2O5、SiO2、CaO、MgO | 《钙镁磷肥》(GB/T 20412—2021) |
| K2O | 《钙镁磷钾肥》(HG/T 2598—1994) |
| Al2O3 | 《磷矿石和磷精矿中氧化铝含量的测定》(GB/T 1871.3—1995) |
| Fe2O3 | 《复混肥料中铜、铁、锰、锌、硼、钼含量的测定》(GB/T 14540—2003) |
| B2O3 | 《地下水质分析方法 第44部分:硼量的测定H酸-甲亚胺分光光度法》(DZ/T 0064.44—2021) |
| CaO/SiO2 | 原料矿用量/%(质量分数) | TS/℃ | ||
|---|---|---|---|---|
| 磷尾矿 | 低品位磷矿 | 蛇纹石 | ||
| 1.8 | 80 | 0 | 20 | 1490 |
| 1.9 | 72 | 8 | 20 | 1482 |
| 2.0 | 64 | 16 | 20 | 1478 |
| 2.1 | 55 | 25 | 20 | 1455 |
| 2.2 | 47 | 33 | 20 | 1465 |
| 2.3 | 38 | 42 | 20 | 1467 |
| 2.4 | 30 | 50 | 20 | 1474 |
| 2.5 | 21 | 59 | 20 | 1482 |
| 2.6 | 12 | 68 | 20 | 1490 |
| 2.7 | 0 | 80 | 20 | 1494 |
表3 不同CaO/SiO2对原料矿流动温度TS的影响
Table 3 Effect of different CaO/SiO2 on TS of raw ore
| CaO/SiO2 | 原料矿用量/%(质量分数) | TS/℃ | ||
|---|---|---|---|---|
| 磷尾矿 | 低品位磷矿 | 蛇纹石 | ||
| 1.8 | 80 | 0 | 20 | 1490 |
| 1.9 | 72 | 8 | 20 | 1482 |
| 2.0 | 64 | 16 | 20 | 1478 |
| 2.1 | 55 | 25 | 20 | 1455 |
| 2.2 | 47 | 33 | 20 | 1465 |
| 2.3 | 38 | 42 | 20 | 1467 |
| 2.4 | 30 | 50 | 20 | 1474 |
| 2.5 | 21 | 59 | 20 | 1482 |
| 2.6 | 12 | 68 | 20 | 1490 |
| 2.7 | 0 | 80 | 20 | 1494 |
| 用量/%(质量分数) | 流动温度TF/℃ | ||
|---|---|---|---|
| KCl | B2O3 | Al2O3 | |
| 3 | 6 | 0 | 1286 |
| 7 | 6 | 0 | 1252 |
| 11 | 6 | 0 | 1221 |
| 15 | 6 | 0 | 1201 |
| 19 | 6 | 0 | 1210 |
| 11 | 2 | 0 | 1306 |
| 11 | 6 | 0 | 1221 |
| 11 | 10 | 0 | 1141 |
| 11 | 15 | 0 | 1143 |
| 11 | 8 | 0 | 1172 |
| 11 | 8 | 1.5 | 1163 |
| 11 | 8 | 3 | 1147 |
| 11 | 8 | 5 | 1144 |
表4 单因素实验结果
Table 4 Results of single factor experiment
| 用量/%(质量分数) | 流动温度TF/℃ | ||
|---|---|---|---|
| KCl | B2O3 | Al2O3 | |
| 3 | 6 | 0 | 1286 |
| 7 | 6 | 0 | 1252 |
| 11 | 6 | 0 | 1221 |
| 15 | 6 | 0 | 1201 |
| 19 | 6 | 0 | 1210 |
| 11 | 2 | 0 | 1306 |
| 11 | 6 | 0 | 1221 |
| 11 | 10 | 0 | 1141 |
| 11 | 15 | 0 | 1143 |
| 11 | 8 | 0 | 1172 |
| 11 | 8 | 1.5 | 1163 |
| 11 | 8 | 3 | 1147 |
| 11 | 8 | 5 | 1144 |
| 自变量 | 单位 | 编码变量和级别 | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| KCl | %(质量分数) | 7 | 11 | 15 |
| B2O3 | %(质量分数) | 6 | 8 | 10 |
| Al2O3 | %(质量分数) | 0 | 1.5 | 3 |
表5 参数编码和取值
Table 5 Parameter coding and value selection
| 自变量 | 单位 | 编码变量和级别 | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| KCl | %(质量分数) | 7 | 11 | 15 |
| B2O3 | %(质量分数) | 6 | 8 | 10 |
| Al2O3 | %(质量分数) | 0 | 1.5 | 3 |
| KCl/% (质量分数) | B2O3/% (质量分数) | Al2O3/% (质量分数) | TF/℃ |
|---|---|---|---|
| 11 | 10 | 3.0 | 1129 |
| 11 | 8 | 1.5 | 1160 |
| 7 | 10 | 1.5 | 1135 |
| 11 | 8 | 1.5 | 1162 |
| 15 | 8 | 0 | 1167 |
| 15 | 10 | 1.5 | 1144 |
| 7 | 8 | 3.0 | 1176 |
| 15 | 6 | 1.5 | 1222 |
| 11 | 6 | 0 | 1221 |
| 11 | 10 | 0 | 1141 |
| 11 | 8 | 1.5 | 1169 |
| 11 | 8 | 1.5 | 1162 |
| 7 | 8 | 0 | 1180 |
| 15 | 8 | 3.0 | 1146 |
| 11 | 8 | 1.5 | 1160 |
| 11 | 6 | 3.0 | 1189 |
| 7 | 6 | 1.5 | 1232 |
表6 不同助剂添加量对流动温度的影响
Table 6 Effect of different flux addition on flow temperature
| KCl/% (质量分数) | B2O3/% (质量分数) | Al2O3/% (质量分数) | TF/℃ |
|---|---|---|---|
| 11 | 10 | 3.0 | 1129 |
| 11 | 8 | 1.5 | 1160 |
| 7 | 10 | 1.5 | 1135 |
| 11 | 8 | 1.5 | 1162 |
| 15 | 8 | 0 | 1167 |
| 15 | 10 | 1.5 | 1144 |
| 7 | 8 | 3.0 | 1176 |
| 15 | 6 | 1.5 | 1222 |
| 11 | 6 | 0 | 1221 |
| 11 | 10 | 0 | 1141 |
| 11 | 8 | 1.5 | 1169 |
| 11 | 8 | 1.5 | 1162 |
| 7 | 8 | 0 | 1180 |
| 15 | 8 | 3.0 | 1146 |
| 11 | 8 | 1.5 | 1160 |
| 11 | 6 | 3.0 | 1189 |
| 7 | 6 | 1.5 | 1232 |
| Source | Sum of squares | F-value | P-value |
|---|---|---|---|
| model | 14507.58 | 23.81 | 0.0002 |
| A-KCl | 242.00 | 3.57 | 0.1006 |
| B-B2O3 | 12403.12 | 183.19 | <0.0001 |
| C-Al2O3 | 595.13 | 8.79 | 0.0210 |
| AB | 90.25 | 1.33 | 0.2862 |
| AC | 72.25 | 1.07 | 0.3360 |
| BC | 100.00 | 1.48 | 0.2636 |
| A2 | 337.27 | 4.98 | 0.0608 |
| B2 | 576.38 | 8.51 | 0.0224 |
| C2 | 77.85 | 1.15 | 0.3192 |
| residual | 473.95 | — | — |
| lack of fit | 418.75 | 10.11 | 0.0244 |
| pure error | 55.20 | — | — |
| cor total | 14981.53 | — | — |
表7 K-B-Al对流动温度影响的方差分析结果
Table 7 ANOVA results of influence of K-B-Al on flowing temperature
| Source | Sum of squares | F-value | P-value |
|---|---|---|---|
| model | 14507.58 | 23.81 | 0.0002 |
| A-KCl | 242.00 | 3.57 | 0.1006 |
| B-B2O3 | 12403.12 | 183.19 | <0.0001 |
| C-Al2O3 | 595.13 | 8.79 | 0.0210 |
| AB | 90.25 | 1.33 | 0.2862 |
| AC | 72.25 | 1.07 | 0.3360 |
| BC | 100.00 | 1.48 | 0.2636 |
| A2 | 337.27 | 4.98 | 0.0608 |
| B2 | 576.38 | 8.51 | 0.0224 |
| C2 | 77.85 | 1.15 | 0.3192 |
| residual | 473.95 | — | — |
| lack of fit | 418.75 | 10.11 | 0.0244 |
| pure error | 55.20 | — | — |
| cor total | 14981.53 | — | — |

图3 熔融时间对四种配料磷活化率的影响GF1—K∶B∶Al=11∶10∶3; GF2—K∶B∶Al=11∶10∶0; GF3—K∶B∶Al=7∶10∶1.5; GF4—K∶B∶Al=15∶10∶1.5
Fig.3 Effect of melting time on phosphorus activation rate of four schemes
| 结构单元 | Q n | 振动峰位置/cm-1 |
|---|---|---|
| [SiO4] | Q0 | 850~875 |
| [Si2O7] | Q1 | 920~950 |
| [Si2O6] | Q2 | 980~1020 |
| [Si2O5] | Q3 | 1050~1080 |
表8 四种硅氧四面体Q n 在红外光谱图中对应峰位[29]
Table 8 Peak positions and shapes of four silicon-oxygen tetrahedrons Q n in infrared spectra[29]
| 结构单元 | Q n | 振动峰位置/cm-1 |
|---|---|---|
| [SiO4] | Q0 | 850~875 |
| [Si2O7] | Q1 | 920~950 |
| [Si2O6] | Q2 | 980~1020 |
| [Si2O5] | Q3 | 1050~1080 |
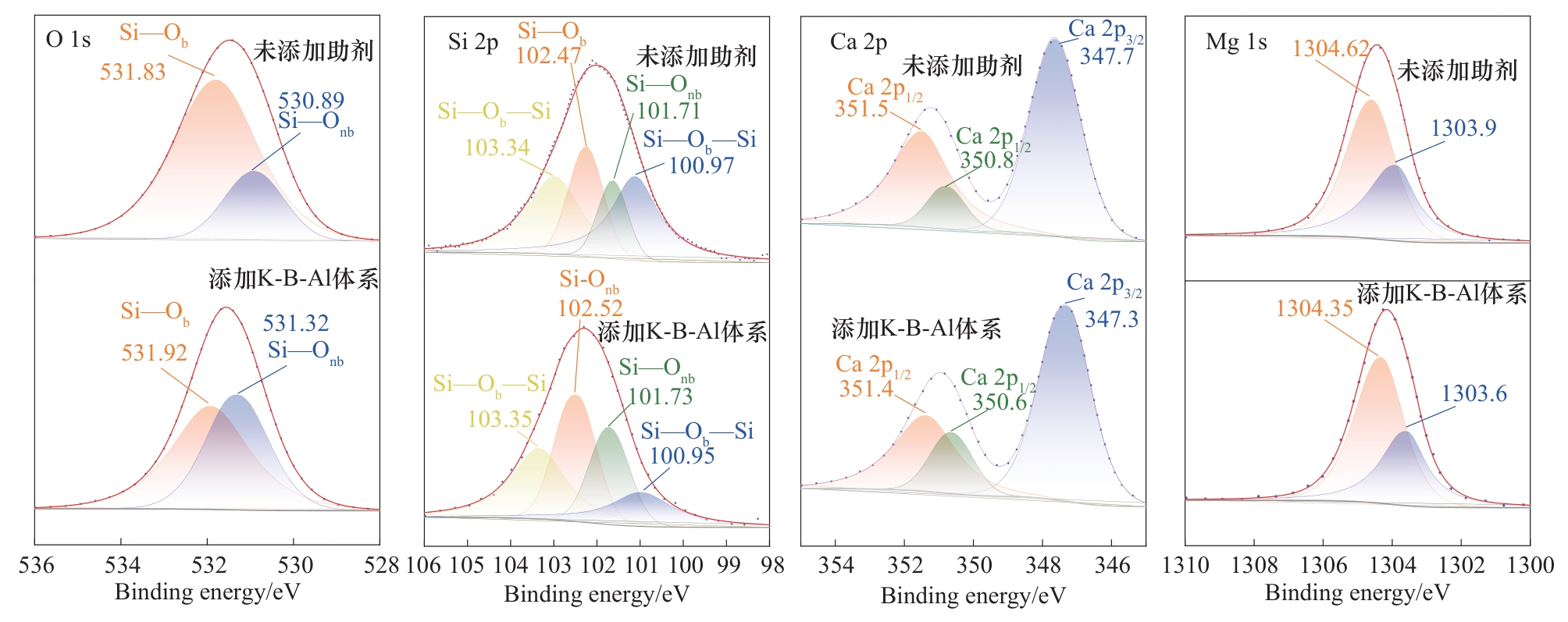
图9 K-B-Al体系添加前后活化产物的元素摩尔含量和XPS图谱比较
Fig.9 Comparison of elemental molar content and XPS spectra of activation products before and after addition of K-B-Al system
| 1 | 林升鉴, 饶峰, 郑艳金, 等. 磷尾矿、磷石膏和黄磷渣的地质聚合反应资源化利用研究进展[J]. 矿产保护与利用, 2021, 41(4): 150-156. |
| Lin S J, Rao F, Zheng Y J, et al. Research progress on resource utilization of phosphorus tailings, phosphogypsum and yellow phosphorous slag by geological polymerization[J]. Conservation and Utilization of Mineral Resources, 2021, 41(4): 150-156. | |
| 2 | 王保明, 王兴龙, 杨英, 等. 磷尾矿综合利用现状及研究进展[J]. 无机盐工业, 2024, 56(10): 1-11. |
| Wang B M, Wang X L, Yang Y, et al. Current status and research progress of comprehensive utilization of phosphorus tailings[J]. Inorganic Chemicals Industry, 2024, 56(10): 1-11. | |
| 3 | Sarswat P K, Singh R S, Pathapati S V S H. Real time electronic-waste classification algorithms using the computer vision based on convolutional neural network (CNN): enhanced environmental incentives[J]. Resources, Conservation and Recycling, 2024, 207: 107651. |
| 4 | Zeng L, Li X M, Liu J D. Adsorptive removal of phosphate from aqueous solutions using iron oxide tailings[J]. Water Research, 2004, 38(5): 1318-1326. |
| 5 | Cross A T, Lambers H. Young calcareous soil chronosequences as a model for ecological restoration on alkaline mine tailings[J]. Science of the Total Environment, 2017, 607: 168-175. |
| 6 | 张亚丽, 侯翠红, 籍婷婷, 等. 热法制备含磷钾中微量元素肥料的实验研究[J]. 矿产综合利用, 2021(2): 185-191. |
| Zhang Y L, Hou C H, Ji T T, et al. Experimental study on thermal process of preparing phosphorus potassium fertilizers containing medium and trace elements[J]. Multipurpose Utilization of Mineral Resources, 2021(2): 185-191. | |
| 7 | Tang J W, Wang H B, Xu X C, et al. Glass structure of aluminosilicate containing phosphate with low chemical stability and complex component: development of glass structure model of aluminosilicate containing phosphate and manufacture of glass fertilizer[J]. Scientia Sinica Chimica, 2010, 40(7): 922-926. |
| 8 | Kiwsakunkran N, Chanthima N, Kaewkhao J, et al. Composition and structural studies of glass fertilizer[J]. Journal of Physics: Conference Series, 2018, 1120: 012016. |
| 9 | 丁洪, 张玉树, 郑祥洲, 等. 玻璃肥料的研究与应用进展[J]. 现代化工, 2017, 37(3): 14-19, 21. |
| Ding H, Zhang Y S, Zheng X Z, et al. A review on development and application of glassy fertilizer[J]. Modern Chemical Industry, 2017, 37(3): 14-19, 21. | |
| 10 | Labbilta T, Ait-El-Mokhtar M, Abouliatim Y, et al. Innovative formulations of phosphate glasses as controlled-release fertilizers to improve tomato crop growth, yield and fruit quality[J]. Molecules, 2021, 26(13): 3928. |
| 11 | Zeng J L, Zhu H Z, Ding Y, et al. Effects of Al2O3 and B2O3 on the structural features of iron phosphate glasses[J]. Journal of Molecular Structure, 2022, 1261: 132928. |
| 12 | Sharmin N, Gu F, Ahmed I, et al. Compositional dependency on dissolution rate and cytocompatibility of phosphate-based glasses: effect of B2O3 and Fe2O3 addition[J]. Journal of Tissue Engineering, 2017, 8: 2041731417744454. |
| 13 | Sun L B, Liu C F, Fang J, et al. Crystallization behavior and thermal properties of B2O3-containing MgO-Al2O3-SiO2-Li2O glass-ceramic and its wettability on Si3N4 ceramic[J]. Journal of the European Ceramic Society, 2019, 39(4): 1532-1539. |
| 14 | 徐美君, 杜念娟. 磷酸盐玻璃国内外发展概况[J]. 建材世界, 2009, 30(3): 53-57. |
| Xu M J, Du N J. Phosphate glass development at home and abroad[J]. The World of Building Materials, 2009, 30(3): 53-57. | |
| 15 | Ni H. Compositional dependence of alkali diffusivity in silicate melts: mixed alkali effect and pseudo-alkali effect[J]. American Mineralogist, 2012, 97(1): 70-79. |
| 16 | 张璐. 化学组成对高碱铝硅酸盐玻璃微观结构影响作用规律研究[D]. 北京:北京工业大学, 2018. |
| Zhang L. Effect of chemical composition on the microstructure of high alkali aluminosilicate glass[D]. Beijing: Beijing University of Technology, 2018. | |
| 17 | 元田委, 袁坚, 郑伟宏, 等. 碱金属氧化物引入量对MgO-Al2O3-SiO2微晶玻璃结构及其化学增强效果的影响[J]. 硅酸盐通报, 2019, 38(5): 1522-1526, 1555. |
| Yuan T W, Yuan J, Zheng W H, et al. Effect of the introduction of alkali metal oxides on the structure and chemical enhancement process of MgO-Al2O3-SiO2 glass-ceramics[J]. Bulletin of the Chinese Ceramic Society, 2019, 38(5): 1522-1526, 1555. | |
| 18 | Noritake F, Naito S. Mechanism of mixed alkali effect in silicate glass/liquid: pathway and network analysis[J]. Journal of Non-Crystalline Solids, 2023, 610: 122321. |
| 19 | Hou Y, Zhang G H, Chou K C. Mixed alkali effect in SiO2-CaO-Al2O3-TiO2-R2O (R = Li, Na) glass ceramics[J]. Journal of Alloys and Compounds, 2021, 856: 158239. |
| 20 | 范钧翔. 磷尾矿制备矿山复垦基质土及高温活化研究[D]. 郑州: 郑州大学, 2022. |
| Fan J X. Study on preparation of mine reclamation matrix soil from phosphorus tailings and its high temperature activation[D]. Zhengzhou: Zhengzhou University, 2022. | |
| 21 | Li L Y, Yao Y, Hou C H, et al. Preparation of multifunctional fused magnesium phosphate fertilizer from low-grade phosphate ores[M]//The Minerals, Metals & Materials Series. Cham: Springer International Publishing, 2021: 111-120. |
| 22 | Atta Mends E, Manka Tita A, Hussaini S, et al. Investigation of leaching of nickel sulfide flotation tailings to recover valuable metals[J]. Minerals Engineering, 2024, 212: 108716. |
| 23 | 赵玥琪, 王亚琴, 张晨, 等. 铁尾矿/粉煤灰基地质聚合物的制备及微观结构分析[J]. 化工进展, 2024, 43(12): 7067-7077. |
| Zhao Y Q, Wang Y Q, Zhang C, et al, Preparation and microstructure analysis of iron tailings/fly ash based geopolymers[J]. Chemical Industry and Engineering Progress, 2024, 43(12): 7067-7077. | |
| 24 | Grice J V, Montgomery D C. Design and analysis of experiments[J]. Technometrics, 2000, 42(2): 208. |
| 25 | 王义江, 孙莉, 刘梦涵, 等. 基于响应面法的矿用翅片管空冷器参数优化[J]. 化工学报, 2024, 75(1): 279-291. |
| Wang Y J, Sun L, Liu M H, et al. Optimization on parameter of plate-fin-and-tube air cooler in mines based on response surface method[J]. CIESC Journal, 2024, 75(1): 279-291. | |
| 26 | Jia R D, Deng L B, Yun F, et al. Effects of SiO2/CaO ratio on viscosity, structure, and mechanical properties of blast furnace slag glass ceramics[J]. Materials Chemistry and Physics, 2019, 233: 155-162. |
| 27 | 倪怀玮. 硅酸盐熔体的物理化学性质研究进展及其应用[J]. 科学通报, 2013, 58(10): 865-890. |
| Ni H W. Advances and application in physicochemical properties of silicate melts[J]. Chinese Science Bulletin, 2013, 58(10): 865-890. | |
| 28 | Bi Z S, Li K J, Jiang C H, et al. Effects of amphoteric oxide (Al2O3 and B2O3) on the structure and properties of SiO2-CaO melts by molecular dynamics simulation[J]. Journal of Non-Crystalline Solids, 2021, 559: 120687. |
| 29 | He D F, Gao C, Pan J T, et al. Preparation of glass-ceramics with diopside as the main crystalline phase from low and medium titanium-bearing blast furnace slag[J]. Ceramics International, 2018, 44(2): 1384-1393. |
| 30 | 曾麟, 黄守佳, 林鸿剑, 等. 混合碱效应对Li2O-Al2O3-SiO2系玻璃结构和热膨胀性能的影响[J]. 硅酸盐通报, 2021, 40(11): 3813-3821. |
| Zeng L, Huang S J, Lin H J, et al. Influence of mixed alkali effect on structure and thermal expansion properties of Li2O-Al2O3-SiO2 glass[J]. Bulletin of the Chinese Ceramic Society, 2021, 40(11): 3813-3821. | |
| 31 | 王柯晴, 徐劼, 沈芷璇, 等. LaCoO3钙钛矿活化过一硫酸盐降解萘普生[J]. 化工学报, 2020, 71(3): 1326-1334. |
| Wang K Q, Xu J, Shen Z X, et al. Degradation of naproxen by peroxymonosulfate activated with LaCoO3 [J]. CIESC Journal, 2020, 71(3): 1326-1334. | |
| 32 | 孙巾茹, 宋傲磊, 赵明新, 等. 沉淀剂对Co3O4催化分解N2O的性能影响[J]. 化工进展, 2024, 43(4): 1823-1831. |
| Sun J R, Song A L, Zhao M X, et al. Effect of precipitating agent on the performance of Co3O4-catalyzed decomposition of N2O[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 1823-1831. |
| [1] | 杨端康慧, 周文晋, 刘琳琳. 考虑压缩机分组级联布置的氢网络综合[J]. 化工学报, 2025, 76(3): 1102-1110. |
| [2] | 高越, 李丁, 高玉苗. 有机污染场地土壤催化氧化修复技术研究[J]. 化工学报, 2025, 76(3): 1297-1304. |
| [3] | 殷梦凡, 王倩, 郑涛, 姬奎, 王绍贵, 郭辉, 林志强, 张睿, 孙晖, 刘海燕, 刘植昌, 徐春明, 孟祥海, 王月平. 可再生能源电解水制氢-低温低压合成氨万吨级工业示范流程设计[J]. 化工学报, 2025, 76(2): 825-834. |
| [4] | 张俊杰, 陈源, 李运堂, 李孝禄, 王冰清, 彭旭东. 超椭圆织构浮动坝箔片端面气膜密封动态性能分析与优化[J]. 化工学报, 2025, 76(1): 296-310. |
| [5] | 刘萍, 邱雨生, 李世婧, 孙瑞奇, 申晨. 微通道内纳米流体传热流动特性[J]. 化工学报, 2025, 76(1): 184-197. |
| [6] | 杨晔, 卢建刚. 基于融合Transformer的门尼系数预测建模研究[J]. 化工学报, 2025, 76(1): 266-282. |
| [7] | 李雨诗, 陈源, 李运堂, 彭旭东, 王冰清, 李孝禄. 新型柔性坝箔片端面气膜密封变形协调分析及性能智能优化[J]. 化工学报, 2025, 76(1): 324-334. |
| [8] | 李海东, 张奇琪, 杨路, AKRAM Naeem, 常承林, 莫文龙, 申威峰. 采用智能进化算法的管壳式换热器详细设计[J]. 化工学报, 2025, 76(1): 241-255. |
| [9] | 赵昂然, 韩永强, 王志鹏, 李鹏飞, 许亚伟, 佟会玲. 常温条件下赤泥同时脱硫脱硝实验研究[J]. 化工学报, 2024, 75(S1): 276-282. |
| [10] | 钟屹, 周仕遇, 纠连朝, 李钰晓, 吴豪江, 周智勇. 废旧磷酸铁锂电池正极材料直接修复再生研究进展[J]. 化工学报, 2024, 75(S1): 1-13. |
| [11] | 高文芳, 崔晗, 孙一冉, 彭佳晴, 朱睿, 夏然, 张馨予, 李佳奇, 王学良, 孙峙, 吕龙义. 典型金属生产过程的环境影响评价研究进展[J]. 化工学报, 2024, 75(9): 3056-3073. |
| [12] | 张佳颖, 王聪, 王雅君. CNT-Co/Bi2O3催化剂光催化协同过硫酸盐活化高效降解四环素[J]. 化工学报, 2024, 75(9): 3163-3175. |
| [13] | 李彦熹, 王晔春, 谢向东, 王进芝, 王江, 周煜, 潘盈秀, 丁文涛, 郭烈锦. 蜗壳式多通道气液旋流分离器结构优化及分离特性研究[J]. 化工学报, 2024, 75(8): 2875-2885. |
| [14] | 郑晓园, 蔡炎嶙, 应芝, 王波, 豆斌林. 污水污泥磷形态亚临界水热转化研究[J]. 化工学报, 2024, 75(8): 2970-2982. |
| [15] | 黄志鸿, 周利, 柴士阳, 吉旭. 耦合加氢装置优化的多周期氢网络集成[J]. 化工学报, 2024, 75(5): 1951-1965. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号