化工学报 ›› 2024, Vol. 75 ›› Issue (S1): 1-13.DOI: 10.11949/0438-1157.20240435
钟屹1( ), 周仕遇1, 纠连朝1, 李钰晓1, 吴豪江1, 周智勇2(
), 周仕遇1, 纠连朝1, 李钰晓1, 吴豪江1, 周智勇2( )
)
收稿日期:2024-04-22
修回日期:2024-05-11
出版日期:2024-12-25
发布日期:2024-12-17
通讯作者:
周智勇
作者简介:钟屹(2002—),男,本科生,1724439674@qq.com
基金资助:
Yi ZHONG1( ), Shiyu ZHOU1, Lianchao JIU1, Yuxiao LI1, Haojiang WU1, Zhiyong ZHOU2(
), Shiyu ZHOU1, Lianchao JIU1, Yuxiao LI1, Haojiang WU1, Zhiyong ZHOU2( )
)
Received:2024-04-22
Revised:2024-05-11
Online:2024-12-25
Published:2024-12-17
Contact:
Zhiyong ZHOU
摘要:
近年来,随着电动汽车的产量攀升,锂离子电池的消耗量急剧增加,大量报废电池带来了各种环境和资源问题,对废旧锂离子电池的处理和回收成为亟待解决的问题。磷酸铁锂(LFP)电池凭借高稳定性和高循环寿命等优点,成为目前主流应用于电动汽车的锂电池之一。但现有的LFP回收方法操作复杂、污染性大,且回收产物多为合金或金属盐,只能用作电池前体。相比较而言,废旧LFP正极材料直接修复再生具有流程短、方法简单、能耗低等优点,符合当前我国双碳目标。本文综述了废旧LFP正极材料直接修复再生的最新研究进展,包括固相烧结法、水热法、电化学法等方法的研究现状,分析比较了各种方法的优势与不足。最后,从多角度分析废旧LFP直接修复再生可能面临的应用挑战及发展前景,为废旧LFP高效回收研究提供参考与建议。
中图分类号:
钟屹, 周仕遇, 纠连朝, 李钰晓, 吴豪江, 周智勇. 废旧磷酸铁锂电池正极材料直接修复再生研究进展[J]. 化工学报, 2024, 75(S1): 1-13.
Yi ZHONG, Shiyu ZHOU, Lianchao JIU, Yuxiao LI, Haojiang WU, Zhiyong ZHOU. Research progress on direct remediation and regeneration of cathode materials from spent lithium iron phosphate batteries[J]. CIESC Journal, 2024, 75(S1): 1-13.
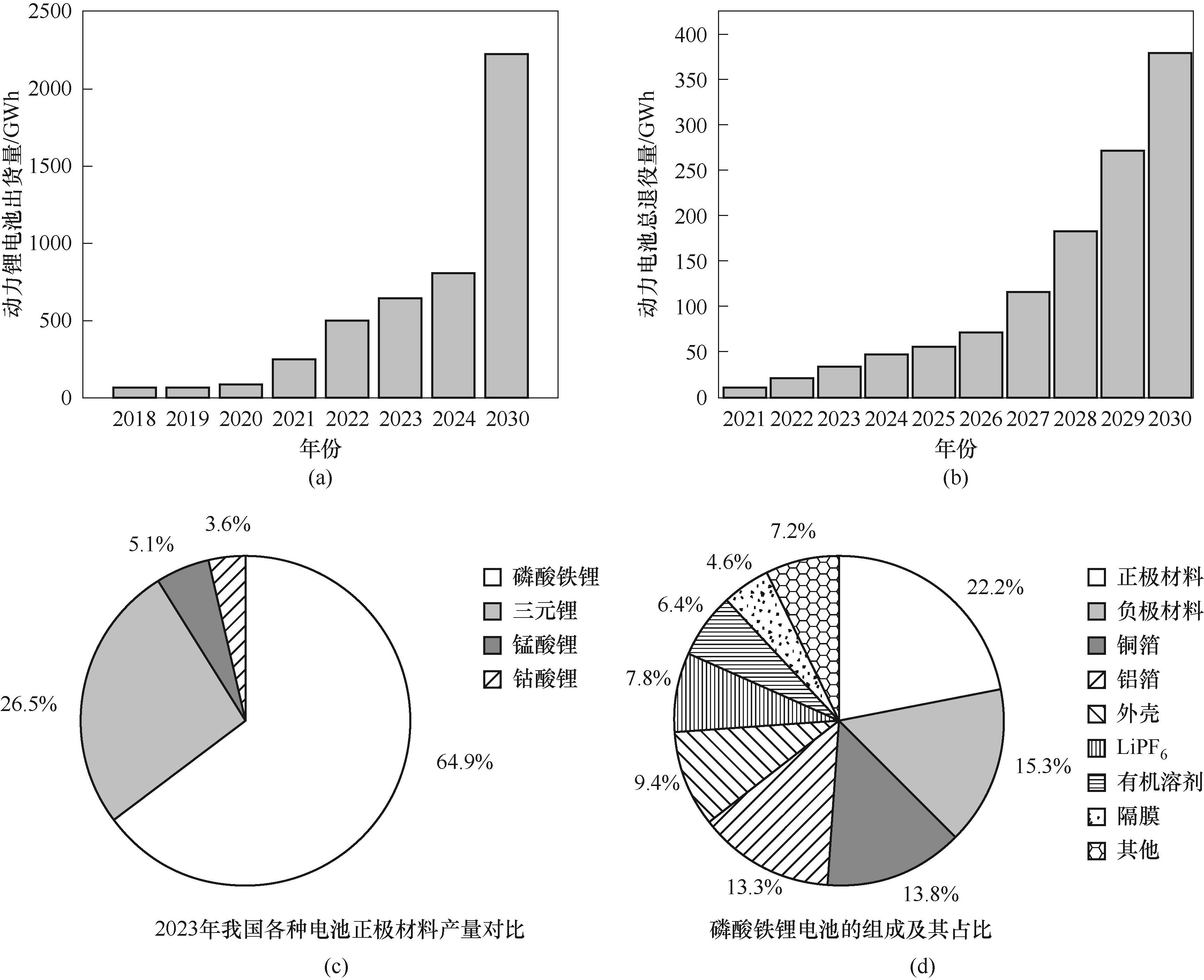
图1 动力锂电池出货量调查及预测图(a);动力锂电池总退役量调查及预测图(b); 2023年我国各种电池正极材料产量对比图(c);磷酸铁锂电池(LFP)组成及其占比(d)
Fig.1 Shipment survey and forecast chart of power lithium battery (a); Total decommissioning survey and forecast chart of power lithium battery (b); Comparison of the production of various battery anode materials in China in 2023 (c); Composition and percentage of lithium iron phosphate battery (d)
| 主要结构 | 主要组成材料 | 含量/% | 成本/% | 潜在环境污染 | |
|---|---|---|---|---|---|
| 电池壳 | 铝壳,铝塑复合膜 | 20~25 | 7~8 | 重金属污染 | |
| 电芯 | 正极 | 磷酸铁锂 | 25~30 | 76~78 | 重金属污染 |
| 负极 | 含碳石墨材料 | 14~19 | 7~8 | 粉尘污染 | |
| 隔膜 | 聚丙烯/聚乙烯 | 约5 | 约2 | 有机物污染 | |
| 电解液 | LiPF6溶液,碳酸乙烯酯,碳酸甲乙酯 | 10~15 | 约3 | 氟污染 | |
| 集流体 | 铝箔(正极) 铜箔(负极) | 10~16 | 3~4 | 重金属污染 | |
表1 磷酸铁锂电池主要组成与潜在环境污染[44-45]
Table1 Main components and potential environmental pollution of lithium iron phosphate battery[44-45]
| 主要结构 | 主要组成材料 | 含量/% | 成本/% | 潜在环境污染 | |
|---|---|---|---|---|---|
| 电池壳 | 铝壳,铝塑复合膜 | 20~25 | 7~8 | 重金属污染 | |
| 电芯 | 正极 | 磷酸铁锂 | 25~30 | 76~78 | 重金属污染 |
| 负极 | 含碳石墨材料 | 14~19 | 7~8 | 粉尘污染 | |
| 隔膜 | 聚丙烯/聚乙烯 | 约5 | 约2 | 有机物污染 | |
| 电解液 | LiPF6溶液,碳酸乙烯酯,碳酸甲乙酯 | 10~15 | 约3 | 氟污染 | |
| 集流体 | 铝箔(正极) 铜箔(负极) | 10~16 | 3~4 | 重金属污染 | |
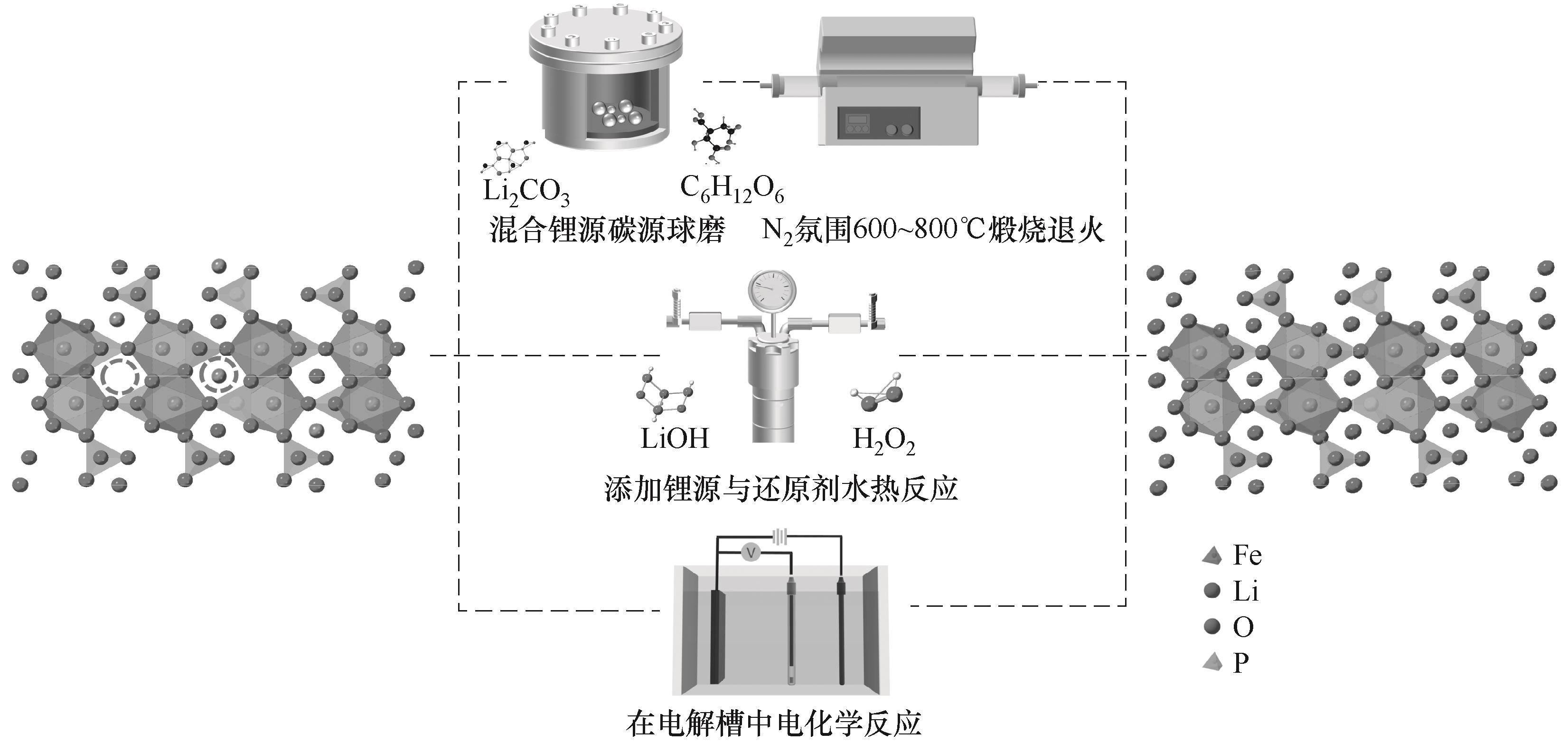
图2 废旧磷酸铁锂(LFP)正极材料直接修复再生方法示意图
Fig.2 Schematic diagram of direct remediation and regeneration method for spent lithium iron phosphate (LFP) cathode materials
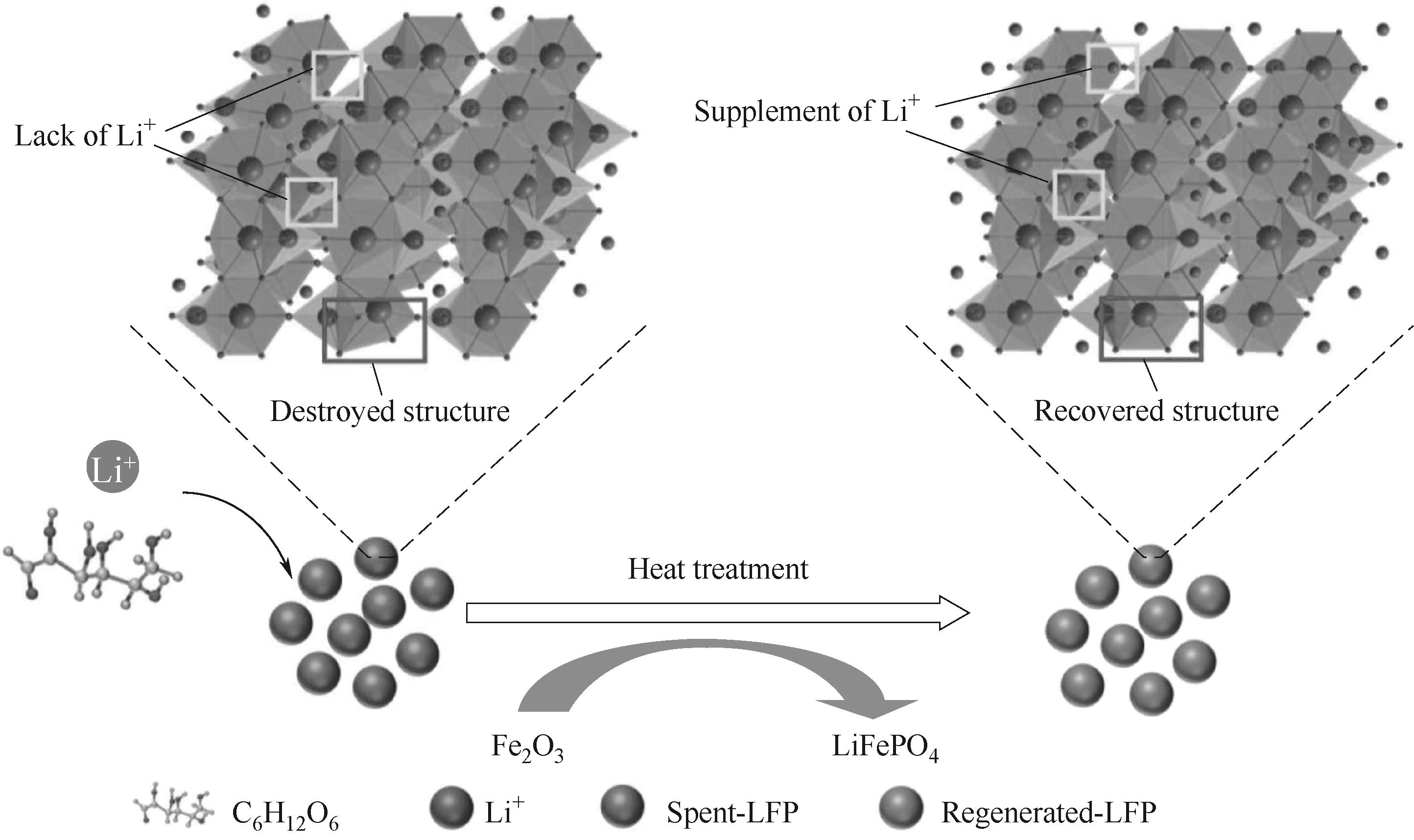
图3 固相烧结法直接修复再生废旧磷酸铁锂正极材料流程示意图
Fig.3 Flow diagram of solid phase sintering method for direct remediation and regeneration of spent lithium iron phosphate cathode materials
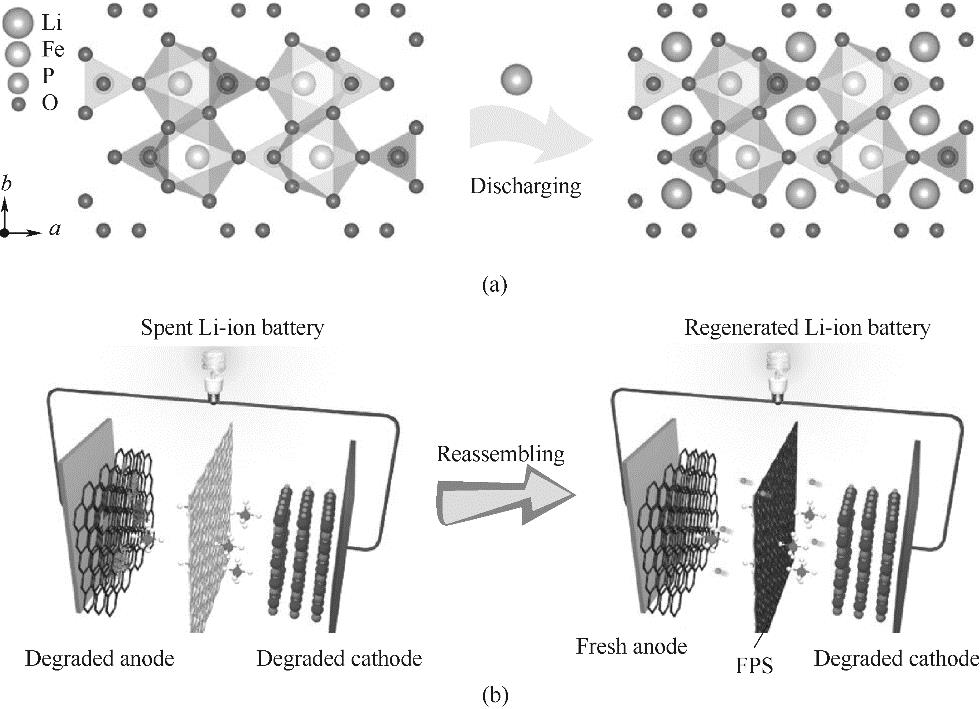
图5 磷酸铁锂电池正极电化学修复原理(a);功能预锂化隔膜法直接修复磷酸铁锂电池示意图(b)[72]
Fig.5 Electrochemical repair principle of lithium ion phosphate battery cathode materials (a); Schematic diagram of functional pre-lithiation diaphragm method for direct repair of lithium iron phosphate batteries (b)[72]
| 正极材料 | 添加物质 | 直接修复 再生方法 | 温度,时间 | 首次放电比容量/(mA·h/g) | 循环容量保持率 (测试条件) | 文献 |
|---|---|---|---|---|---|---|
| LFP | Li2CO3 | 固相烧结法 | 650℃,1 h | 140.4(0.2 C) | 95.32% (0.2 C,100次) | [ |
| LFP | 25%(质量分数)葡萄糖+10%(质量分数)Li2CO3 | 固相烧结法 | 350℃,2 h 650℃,12 h | 143(0.1 C) | 92.9% (0.1 C,100次) | [ |
| LFP | 5%(质量分数)CNTs+15%(质量分数)葡萄糖+5%(质量分数)Li2CO3 | 固相烧结法 | 350℃,2 h 650℃,12 h | 143.12(0.2 C) | 96.42% (0.2 C,100次) | [ |
| LFP | FC | 固相烧结法 | 350℃,2 h 600℃,8 h | 174.3(0.1 C) | 86.6% (10 C,1000次) | [ |
| LFP | CH3COOLi+15%(质量分数)蔗糖 | 固相烧结法 | 800℃,8 s | 152(0.1 C) | 108.6% (2 C,400次) | [ |
| LFP | Li2SO4+N2H4·H2O | 水热法 | 200℃,3 h 干燥10 h | 141.9(1 C) | 98.6% (1 C,200次) | [ |
| LFP | LiOH+H2O2+Li2CO3 | 水热法 固相烧结法 | 30℃,1 h 700℃,10 h | 146.3(1 C) | 84.9% (5 C,1000次) | [ |
| LFP | LiOH·H2O+DL-苹果酸 | 水热法 固相烧结法 | 100℃,6 h 650℃,3 h | 138.4(1 C) | 98.7% (1 C,200次) | [ |
| LFP | LiOH+柠檬酸+Li2CO3 | 水热法 固相烧结法 | 60℃,16 h 600℃,2 h | 162.0(0.2 C) | 100% (2 C,300次) | [ |
| LFP | LiOH·H2O+FeSO4·7H2O+H3PO4+C6H8O6+CNTs | 水热法 固相烧结法 | 200℃,6 h 600℃,10 h | 154.6(0.1C) | 90.9% (0.1 C,50次) | [ |
| LFP | LiOH·H2O+H3PO4+FeSO4·7H2O+[BMIM] BF4 | 水热法 固相烧结法 | 180℃,10 h 700℃,10 h | 162.2(0.1 C) 71.3(15 C) | 100% (0.1 C,40次) | [ |
| LFP | LiOH· H2O+H3PO4+FeSO4·7H2O+Ga | 水热法 固相烧结法 | 180℃,10 h 750℃,6 h | 154.5(1 C) | 98.77% (1 C,40次) | [ |
| LFP | Li2SO4 | 电化学法 | 室温 | 135.2(0.2 C) | 95.30% (1 C,500次) | [ |
| LFP | LiI | 电化学法 | 室温 | 126.6(0.1 C) | 65.5% (0.5 C,200次) | [ |
| LFP | Li2C2O4 | 电化学法 | 室温 | 152.0(0.05 C) | 90.7% (1 C,292次) | [ |
| LFP | Li2S/Co | 电化学法 | 室温 | 150.3(0.2 C) | 90.4% (0.2 C,200次) | [ |
表2 LFP直接修复再生方法汇总
Table 2 Summary of LFP direct repair regeneration methods
| 正极材料 | 添加物质 | 直接修复 再生方法 | 温度,时间 | 首次放电比容量/(mA·h/g) | 循环容量保持率 (测试条件) | 文献 |
|---|---|---|---|---|---|---|
| LFP | Li2CO3 | 固相烧结法 | 650℃,1 h | 140.4(0.2 C) | 95.32% (0.2 C,100次) | [ |
| LFP | 25%(质量分数)葡萄糖+10%(质量分数)Li2CO3 | 固相烧结法 | 350℃,2 h 650℃,12 h | 143(0.1 C) | 92.9% (0.1 C,100次) | [ |
| LFP | 5%(质量分数)CNTs+15%(质量分数)葡萄糖+5%(质量分数)Li2CO3 | 固相烧结法 | 350℃,2 h 650℃,12 h | 143.12(0.2 C) | 96.42% (0.2 C,100次) | [ |
| LFP | FC | 固相烧结法 | 350℃,2 h 600℃,8 h | 174.3(0.1 C) | 86.6% (10 C,1000次) | [ |
| LFP | CH3COOLi+15%(质量分数)蔗糖 | 固相烧结法 | 800℃,8 s | 152(0.1 C) | 108.6% (2 C,400次) | [ |
| LFP | Li2SO4+N2H4·H2O | 水热法 | 200℃,3 h 干燥10 h | 141.9(1 C) | 98.6% (1 C,200次) | [ |
| LFP | LiOH+H2O2+Li2CO3 | 水热法 固相烧结法 | 30℃,1 h 700℃,10 h | 146.3(1 C) | 84.9% (5 C,1000次) | [ |
| LFP | LiOH·H2O+DL-苹果酸 | 水热法 固相烧结法 | 100℃,6 h 650℃,3 h | 138.4(1 C) | 98.7% (1 C,200次) | [ |
| LFP | LiOH+柠檬酸+Li2CO3 | 水热法 固相烧结法 | 60℃,16 h 600℃,2 h | 162.0(0.2 C) | 100% (2 C,300次) | [ |
| LFP | LiOH·H2O+FeSO4·7H2O+H3PO4+C6H8O6+CNTs | 水热法 固相烧结法 | 200℃,6 h 600℃,10 h | 154.6(0.1C) | 90.9% (0.1 C,50次) | [ |
| LFP | LiOH·H2O+H3PO4+FeSO4·7H2O+[BMIM] BF4 | 水热法 固相烧结法 | 180℃,10 h 700℃,10 h | 162.2(0.1 C) 71.3(15 C) | 100% (0.1 C,40次) | [ |
| LFP | LiOH· H2O+H3PO4+FeSO4·7H2O+Ga | 水热法 固相烧结法 | 180℃,10 h 750℃,6 h | 154.5(1 C) | 98.77% (1 C,40次) | [ |
| LFP | Li2SO4 | 电化学法 | 室温 | 135.2(0.2 C) | 95.30% (1 C,500次) | [ |
| LFP | LiI | 电化学法 | 室温 | 126.6(0.1 C) | 65.5% (0.5 C,200次) | [ |
| LFP | Li2C2O4 | 电化学法 | 室温 | 152.0(0.05 C) | 90.7% (1 C,292次) | [ |
| LFP | Li2S/Co | 电化学法 | 室温 | 150.3(0.2 C) | 90.4% (0.2 C,200次) | [ |
| 1 | Bank M S, Swarzenski P W, Duarte C M, et al. Global plastic pollution observation system to aid policy[J]. Environmental Science & Technology, 2021, 55(12): 7770-7775. |
| 2 | Rogelj J, Geden O, Cowie A, et al. Net-zero emissions targets are vague: three ways to fix[J]. Nature, 2021, 591(7850): 365-368. |
| 3 | Rogelj J, Elzen D M, Höhne N, et al. Paris Agreement climate proposals need a boost to keep warming well below 2℃[J]. Nature, 2016, 534(7609): 631-639. |
| 4 | Wang F, Harindintwali J D, Yuan Z Z, et al. Technologies and perspectives for achieving carbon neutrality[J]. The Innovation, 2021, 2(4): 100180. |
| 5 | Nitta N, Wu F X, Lee J T, et al. Li-ion battery materials: present and future[J]. Materials Today, 2015, 18(5): 252-264. |
| 6 | Alfaro-Algaba M, Ramirez J. Techno-economic and environmental disassembly planning of lithium-ion electric vehicle battery packs for remanufacturing[J]. Resource, Conservation and Recycling, 2020, 154: 104461. |
| 7 | Harper G, Sommerville R, Kendirck E, et al. Recycling lithium-ion batteries from electric vehicles[J]. Nature, 2019, 575(7781): 75-86. |
| 8 | Schmuch R, Wagner R, Hörpel G, et al. Performance and cost of materials for lithium-based rechargeable automotive batteries[J]. Nature Energy, 2018, 3(4): 267-278. |
| 9 | Winter M, Barnett B, Xu K. Before Li ion batteries[J]. Chemical Reviews, 2018, 118(23): 11433-11456. |
| 10 | Ji G J, Wang J X, Liang Z. Direct regeneration of degraded lithium-ion battery cathodes with a multifunctional organic lithium salt[J]. Nature Communication, 2023, 14(1): 584. |
| 11 | Yao Q, Xiao F Y, Lin C Y. Regeneration of spent lithium manganate into cation-doped and oxygen-deficient MnO2 cathodes toward ultralong lifespan and wide-temperature-tolerant aqueous Zn-ion batteries[J]. Battery Energy, 2023, 13(9): 20220065. |
| 12 | Wang J X, Ma J, Zhuang Z F, et al. Toward direct regeneration of spent lithium-ion batteries: a next-generation recycling method[J]. Chemical Reviews, 2024, 124(5): 2839-2887. |
| 13 | Wang L, Shen Y H, Liu Y L, et al. Electrochemical restoration of battery materials guided by synchrotron radiation technology for sustainable lithium-ion batteries[J]. Small Methods, 2023, 7(9): 2201658. |
| 14 | Xiao J F, Li J, Xu Z M. Novel approach for in situ recovery of lithium carbonate from spent lithium ion batteries using vacuum metallurgy[J]. Environmental Science & Technology, 2017, 51(20): 11960-11966. |
| 15 | Swain B. Recovery and recycling of lithium: a review[J]. Separation and Purification Technology, 2017, 172: 388-403. |
| 16 | Meshram P, Mishra A, Sahu R. Environmental impact of spent lithium ion batteries and green recycling perspectives by organic acids—A review[J]. Chemosphere, 2020, 242: 125291. |
| 17 | Xiao J F, Li J, Xu Z M. Challenges to future development of spent lithium ion batteries recovery from environmental and technological perspectives[J]. Environmental Science & Technology, 2020, 54(1): 9-25. |
| 18 | 王崇国, 刘广龙, 金小容, 等. 锂离子电池正极材料的研究进展[J]. 当代化工研究, 2023, 9: 12-14. |
| Wang C G, Liu G L, Jin X R, et al. Research progress of lithium-ion battery cathode material[J]. Modern Chemical Research, 2023, 9: 12-14. | |
| 19 | 王薇薇, 吴华珠, 赵斐, 等. 锂离子电池正极材料国内外专利分布及关键技术解析[J]. 中国科技信息, 2024, 4: 32-36. |
| Wang W W, Wu H Z, Zhao F, et al. Analysis of domestic and international patent distribution and key technologies of anode materials for lithium-ion batteries[J]. China Science and Technology Information, 2024, 4: 32-36. | |
| 20 | 梅洋, 张强. 锂离子电池磷酸铁锂正极材料的研究进展及预测[J]. 化工科技. DOI: 10.16664/j.cnki.issn1008-0511. 20240403.002 . |
| Mei Y, Zhang Q. Research progress and prediction of lithium iron phosphate cathode materials for lithium-ion batteries[J]. Science & Technology in Chemical Industry. DOI: 10.16664/j.cnki.issn1008-0511.20240403.002 . | |
| 21 | 李玉婷. 碳中和背景下锂离子电池正极材料的发展趋势及应对措施[J]. 化学与生物工程, 2022, 39(9): 7-10. |
| Li Y T. Development trend and countermeasures of lithium ion battery cathode materials under background of carbon neutralization[J]. Chemistry & Bioengineering, 2022, 39(9): 7-10. | |
| 22 | 兰凯惠, 吴利萍, 丁若兰, 等. 锂离子电池正极材料浅析[J]. 电工材料, 2023, 3: 49-52. |
| Lan K H, Wu L P, Ding R L, et al. A review of cathode materials for rechargeable lithium-ion batteries[J]. Electrical Engineering Materials, 2023, 3: 49-52. | |
| 23 | 周弋惟, 陈卓, 徐建鸿. 湿法冶金回收废旧锂电池正极材料的研究进展[J]. 化工学报, 2022, 73(1): 85-96. |
| Zhou Y W, Chen Z, Xu J H. Progress and prospect of recycling spent lithium battery cathode materials by hydrometallurgy[J]. CIESC Journal, 2022, 73(1): 85-96. | |
| 24 | 王振华, 彭代冲, 孙克宁. 锂离子电池隔膜材料研究进展[J]. 化工学报, 2018, 69(1): 282-294. |
| Wang Z H, Peng D C, Sun K N. Research progress of separator materials for lithium ion batteries[J]. CIESC Journal, 2018, 69(1): 282-294. | |
| 25 | Sheng O W, Hu H L, Liu T F, et al. Interfacial and ionic modulation of poly(ethylene oxide) electrolyte via localized iodization to enable dendrite-free lithium metal batteries[J]. Advanced Functional Materials, 2022, 32(14): 2111026. |
| 26 | Yan W, Fan K, Zheng L M, et al. Cluster-bridging-coordinated bimetallic metal-organic framework as high-performance anode material for lithium-ion storage[J]. Small Structures, 2021, 2(12): 2100122. |
| 27 | Zhao T Y, Li W L, Traversy M, et al. A review on the recycling of spent lithium iron phosphate batteries[J]. Journal of Environmental Management, 2024, 351: 119670. |
| 28 | Liao H Y, Zhao S W, Cai M Z, et al. Direct conversion of waste battery cathodes to high-volumetric-capacity anodes with assembled secondary‐particle morphology[J]. Advanced Energy Materials, 2023, 13(22): 2300596. |
| 29 | Yang T Z, Luo D, Yu A P, et al. Enabling future closed-loop recycling of spent lithium-ion batteries: direct cathode regeneration[J]. Advanced Material, 2023, 35(36): 2203218. |
| 30 | Zhu X H, Li Y J, Gong M Q, et al. Recycling valuable metals from spent lithium‐ion batteries using carbothermal shock method[J]. Angewandte Chemie International Edition, 2023, 62(15): e202300074. |
| 31 | Lan Y Q, LI X K, Zhou G M, et al. Direct regenerating cathode materials from spent lithium-ion batteries[J]. Advanced Science, 2024, 11(1): 2304425. |
| 32 | Mrozik W, Rajaeifar M A, Heidrich O, et al. Environmental impacts, pollution sources and pathways of spent lithium-ion batteries[J]. Energy Environmental Science, 2021, 14(12): 6099-6121. |
| 33 | He Y Q, Yuan X, Zhang G W, et al. A critical review of current technologies for the liberation of electrode materials from foils in the recycling process of spent lithium-ion batteries[J]. Science of Total Environment, 2021, 766: 142382. |
| 34 | Xu P P, Yang Z Z, Yu X L, et al. Design and optimization of the direct recycling of spent Li-ion battery cathode materials[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(12): 4543-4553. |
| 35 | Xu P P, Yu X L, Chen Z. A materials perspective on direct recycling of lithium‐ion batteries: principles, challenges and opportunities[J]. Advanced Functional Materials, 2023, 33(14): 2213168. |
| 36 | Li J L, Lu Y Q, Yang T R, et al. Water-based electrode manufacturing and direct recycling of lithium-ion battery electrodes—a green and sustainable manufacturing system[J]. iScience, 2020, 23(5): 101081. |
| 37 | Shin Y, Kim S, Park S, et al. A comprehensive review on the recovery of cathode active materials via direct recycling from spent Li-ion batteries[J]. Renewable and Sustainable Energy Reviews, 2023, 187: 113693. |
| 38 | Wang T, Luo H M, Bai Y C, et al. Direct recycling of spent NCM cathodes through ionothermal lithiation[J]. Advanced Energy Materials, 2020, 10(30): 2001204. |
| 39 | Shi Y, Chen G, Chen Z. Effective regeneration of LiCoO2 from spent lithium-ion batteries: a direct approach towards high-performance active particles[J]. Green Chemistry, 2018, 20(4): 851-862. |
| 40 | Fan M, Chang X, Guo Y J, et al. Increased residual lithium compounds guided design for green recycling of spent lithium-ion cathodes[J]. Energy and Environmental Science, 2021, 14(3): 1461-1468. |
| 41 | Li Y K, Lv W G, Huang H L, et al. Recycling of spent lithium-ion batteries in view of green chemistry[J]. Green Chemistry, 2021, 23(17): 6139-6171. |
| 42 | 王猛, 张家靓, 陈永强, 等. 退役磷酸铁锂电池回收技术综述[J]. 有色金属(冶炼部分), 2023, (5): 100-110. |
| Wang M, Zhang J L, Chen Y Q, et al. Review on recycling technology of retired LiFePO4 batteries[J]. Non-Ferrous Metals (Smelting Part), 2023, (5): 100-110. | |
| 43 | 卫寿平, 孙杰, 周添, 等. 废旧锂离子电池中金属材料回收研究进展[J]. 储能科学与技术, 2017, 6(6): 1196-1207. |
| Wei S P, Sun J, Zhou T, et al. Research development of metals recovery from spent lithium-ion batteries[J]. Energy Storage Science and Technology, 2017, 6(6): 1196-1207. | |
| 44 | 李洪枚, 姜亢. 废旧锂离子电池对环境污染的分析与对策[J]. 上海环境科学, 2004, 23(5): 201-203. |
| Li H M, Jiang K. An analysis of waste lithium-ion battery contamination to environment and its countermeasures[J]. Shanghai Environment Science, 2004, 23(5): 201-203. | |
| 45 | 任国庆. 废旧锂离子电池直接还原熔炼高效分离回收有价金属研究[D]. 长沙: 长沙矿冶研究院, 2014. |
| Ren G Q. Research of valuable metals separation and recovery from spent lithium-ion battery by reduction smelting[D]. Changsha: Changsha Research Institute of Mining and Metallurgy, 2014. | |
| 46 | Tiaan P, Steven M B, Guven A. The efficiency of black mass preparation by discharge and alkaline leaching for LIB recycling[J]. Minerals, 2022, 12(6): 753. |
| 47 | 蒋良兴, 郑文军, 张刚, 等. 废旧锂离子电池预处理的绿色放电技术研究[J]. 中南大学学报(自然科学版), 2023, 54(2): 684-693. |
| Jiang L X, Zheng W J, Zhang G, et al. Research on pretreatment green discharge technology of spent lithium-ion batteries[J]. Journal of Central South University (Natural Science Edition), 2023, 54(2): 684-693. | |
| 48 | 郭宇, 于刚强, 陈标华. 废锂离子电池的冶金回收工艺研究进展[J]. 北京工业大学学报, 2024, 50(2): 230-245. |
| Guo Y, Yu G Q, Chen B H. Research progress on metallurgical recovery process of waste lithium batteries[J]. Journal of Beijing University of Technology, 2024, 50(2): 230-245. | |
| 49 | 康飞, 孙峙, 卢雄辉. 面向分选的退役锂电池拆解设备与工艺研究[J]. 有色金属(选矿部分), 2023(2): 124-132. |
| Kang F, Sun Z, Lu X H. Research on decommissioned lithium battery disassembly equipment and technology for separation [J]. Non-Ferrous Metals (Mineral Processing Part), 2023(2): 124-132. | |
| 50 | Wang X, Gaustad G, Babbitt C W. Targeting high value metals in lithium⁃ion battery recycling via shredding and size-based separation[J]. Waste Management, 2016, 51: 204⁃213. |
| 51 | Sovacool B K, Kester J, Noel L, et al. Contested visions and sociotechnical expectations of electric mobility and vehicle⁃to⁃grid innovation in five Nordic countries[J]. Environmental Innovation and Societal Transitions, 2019, 31: 170⁃183. |
| 52 | Chen X P, Li S Z, Wang Y, et al. Recycling of LiFePO4 cathode materials from spent lithium-ion batteries through ultrasound-assisted Fenton reaction and lithium compensation[J]. Waste Management, 2021, 136: 67-75. |
| 53 | Sun L, Qiu K Q. Organic oxalate as leachant and precipitant for the recovery of valuable metals from spent lithium-ion batteries[J]. Waste Management, 2012, 32: 1575-1582. |
| 54 | Jin Y C, Zhang T, Zhang M D. Advancesin intelligent regeneration of cathode materials for sustainable lithium-ion batteries[J]. Advanced Energy Materials, 2022, 12(36): 2201526. |
| 55 | Li X L, Zhang J, Song D W, et al. Direct regeneration of recycled cathode material mixture from scrapped LiFePO4 batteries[J]. Journal of Power Sources, 2017, 345: 78-84. |
| 56 | Qi C, Wang S H, Zhu X K, et al. Environmental-friendly low-cost direct regeneration of cathode material from spent LiFePO4 [J]. Journal of Alloys and Compounds, 2022, 924: 166612. |
| 57 | Song L, Qi C, Wang S H, et al. Direct regeneration of waste LiFePO4 cathode materials with a solid-phase method promoted by activated CNTs[J]. Waste Management, 2023, 157: 141-148. |
| 58 | Wang X F, Feng Z J, Hou X L, et al. Fluorine doped carbon coating of LiFePO4 as a cathode material for lithium-ion batteries[J]. Chemical Engineering Journal, 2020, 379: 122371. |
| 59 | Zheng S H, Wang X T, Gu Z Y, et al. Direct and rapid regeneration of spent LiFePO4 cathodes via a high-temperature shock strategy[J]. Journal of Power Sources, 2023, 587: 233697. |
| 60 | Gao H P, Tran D, Chen Z. Seeking direct cathode regeneration for more efficient lithium-ion battery recycling[J]. Current Opinion in Electrochemistry, 2022, 31: 100875. |
| 61 | Jin H, Zhang J L, Wang D D, et al. Facile and efficient recovery of lithium from spent LiFePO4 batteries via air oxidation-water leaching at room temperature[J]. Green Chemistry, 2022, 24: 152-162. |
| 62 | Macarena K, Samuel A H, James N O, et al. Lithium iron phosphate/carbon (LFP/C) composite using nanocellulose as a reducing agent and carbon source[J]. Polymers, 2023, 15(12): 2628. |
| 63 | Christian H, Thomas L, Jan D, et al. Recycling of lithium-ion batteries: a novel method to separate coating and foil of electrodes[J]. Journal of Cleaner Production, 2015, 108: 301-311. |
| 64 | Xu Y L, Zhang B C, Ge Z F, et al. Direct recovery of degraded LiFePO4 cathode via mild chemical relithiation strategy[J]. Chemical Engineering Journal, 2023, 477: 147201. |
| 65 | Gupta V, Yu X L, Gao H P, et al. Scalable direct recycling of cathode black mass from spent lithium‐ion batteries[J]. Advanced Energy Materials, 2023, 13(6): 2203093. |
| 66 | Yang J Y, Zhou K, Gong R, et al. Direct regeneration of spent LiFePO4 materials via a green and economical one-step hydrothermal process[J]. Journal of Environmental Management, 2023, 348:119384. |
| 67 | Xu P P, Dai Q, Gao H P, et al. Efficient direct recycling of lithium-ion battery cathodes by targeted healing[J]. Joule, 2020, 4(12): 2609-2626. |
| 68 | Feng W J, Cao Yue, Zhao X, et al. Effect of carbon nanotubes on the electrochemical performance of LiFePO4 particles in lithium ion batteries[J]. International Journal of Electrochemical Science, 2017, 12(6): 5199-5207. |
| 69 | Meng Y S, Li Y Z, Xia J, et al. F-doped LiFePO4@N/B/F-doped carbon as high performance cathode materials for Li-ion batteries[J]. Applied Surface Science, 2019, 476: 761-768. |
| 70 | Yi D W, Cui X M, Li N L, et al. Enhancement of electrochemical performance of LiFePO4@C by Ga coating[J]. ACS Omega, 2020, 5(17): 9752-9758. |
| 71 | Ganter, Matthew J, Brian J, et al. Cathode refunctionalization as a lithium ion battery recycling alternative[J]. Journal of Power Sources, 2014, 256: 274-280. |
| 72 | Fan M, Meng Q H, Chang X, et al. In situ electrochemical regeneration of degraded LiFePO4 electrode with functionalized prelithiation separator[J]. Advanced Energy Materials, 2022, 12(18): 2103630. |
| 73 | Peng D Z, Wang X W, Wang S B, et al. Efficient regeneration of retired LiFePO4 cathode by combining spontaneous and electrically driven processes[J]. Green Chemistry, 2022, 24(11): 4544-4556. |
| 74 | Wang T, Yu X S, Fan M, et al. Direct regeneration of spent LiFePO4 via a graphite prelithiation strategy[J]. Chemical Communications, 2020, 56(2): 245-248. |
| 75 | Rao Z X, Wu J Y, He B, et al. A prelithiation separator for compensating the initial capacity loss of lithium-ion batteries[J]. Applied Materials & Interfaces, 2021, 13(32): 38194-38201. |
| 76 | Sun J, Jiang Z Y, Li S, et al. A sustainable revival process for defective LiFePO4 cathodes through the synergy of defect-targeted healing and in-situ construction of 3D-interconnected porous carbon networks[J]. Waste Management, 2023, 158: 125-135. |
| 77 | Wu C, Jiang J Y, He B, et al. Direct regeneration of spent Li-ion battery cathodes via chemical relithiation reaction[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(48): 16384-16393. |
| 78 | Zhao X X, Wang X T, Guo J Z, et al. Dynamic Li+ capture through ligand-chain interaction for the regeneration of depleted LiFePO4 cathode[J]. Advanced Materials, 2024, 36(14): e2308927. |
| [1] | 赵昂然, 韩永强, 王志鹏, 李鹏飞, 许亚伟, 佟会玲. 常温条件下赤泥同时脱硫脱硝实验研究[J]. 化工学报, 2024, 75(S1): 276-282. |
| [2] | 胡俭, 姜静华, 范生军, 刘建浩, 邹海江, 蔡皖龙, 王沣浩. 中深层U型地埋管换热器取热特性研究[J]. 化工学报, 2024, 75(S1): 76-84. |
| [3] | 胡术刚, 田国庆, 刘文娟, 徐广飞, 刘华清, 张建, 王艳龙. 纳米零价铁的制备及氧化还原技术的应用进展[J]. 化工学报, 2024, 75(9): 3041-3055. |
| [4] | 高文芳, 崔晗, 孙一冉, 彭佳晴, 朱睿, 夏然, 张馨予, 李佳奇, 王学良, 孙峙, 吕龙义. 典型金属生产过程的环境影响评价研究进展[J]. 化工学报, 2024, 75(9): 3056-3073. |
| [5] | 黄晓峰, 刘朝晖, 杨帆. 高密度碳氢燃料JP-10流动换热及热裂解结焦实验研究[J]. 化工学报, 2024, 75(8): 2917-2928. |
| [6] | 郑晓园, 蔡炎嶙, 应芝, 王波, 豆斌林. 污水污泥磷形态亚临界水热转化研究[J]. 化工学报, 2024, 75(8): 2970-2982. |
| [7] | 李洪瑞, 黄纯西, 洪小东, 廖祖维, 王靖岱, 阳永荣. 基于自适应变步长同伦法的循环流程收敛算法[J]. 化工学报, 2024, 75(7): 2604-2612. |
| [8] | 马君霞, 李林涛, 熊伟丽. 基于Tri-training GPR的半监督软测量建模方法[J]. 化工学报, 2024, 75(7): 2613-2623. |
| [9] | 张晗, 张淑宁, 刘珂, 邓冠龙. 基于慢特征分析与最小二乘支持向量回归集成的草酸钴合成过程粒度预报[J]. 化工学报, 2024, 75(6): 2313-2321. |
| [10] | 江洋, 彭长宏, 陈伟, 周豪, 马忠彬, 李洪博, 邱在容, 张国鹏, 周康根. 废旧磷酸铁锂粉料综合回收中试研究[J]. 化工学报, 2024, 75(6): 2353-2361. |
| [11] | 吴立盛, 刘杰, 王添添, 罗正鸿, 周寅宁. 开环易位烯烃聚合物的动态交联改性研究进展[J]. 化工学报, 2024, 75(4): 1118-1136. |
| [12] | 王宝凤, 王术高, 程芳琴. 固废基硫掺杂多孔炭材料制备及其对CO2吸附性能研究进展[J]. 化工学报, 2024, 75(2): 395-411. |
| [13] | 刘昌会, 肖桐, 刘庆祎, 耿龙, 赵佳腾. 多孔二氧化钛强化的相变材料储热机理研究[J]. 化工学报, 2024, 75(2): 706-714. |
| [14] | 尹玉华, 方灿, 易清风, 李广. 不同碳导电剂对铁-空气电池性能的影响[J]. 化工学报, 2024, 75(2): 685-694. |
| [15] | 孙琼, 杨富鑫, 谭厚章, 王晓坡. 低共熔溶剂捕集烟气CO2模拟研究[J]. 化工学报, 2024, 75(10): 3705-3717. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号