化工学报 ›› 2025, Vol. 76 ›› Issue (6): 2469-2482.DOI: 10.11949/0438-1157.20241244
杨盛华1,2( ), 孙阳杰1,2, 薛晓君1,2, 米杰1,2, 王建成2,3, 冯宇1,2(
), 孙阳杰1,2, 薛晓君1,2, 米杰1,2, 王建成2,3, 冯宇1,2( )
)
收稿日期:2024-11-02
修回日期:2024-12-16
出版日期:2025-06-25
发布日期:2025-07-09
通讯作者:
冯宇
作者简介:杨盛华(2001—),男,硕士研究生,1427143069@qq.com
基金资助:
Shenghua YANG1,2( ), Yangjie SUN1,2, Xiaojun XUE1,2, Jie MI1,2, Jiancheng WANG2,3, Yu FENG1,2(
), Yangjie SUN1,2, Xiaojun XUE1,2, Jie MI1,2, Jiancheng WANG2,3, Yu FENG1,2( )
)
Received:2024-11-02
Revised:2024-12-16
Online:2025-06-25
Published:2025-07-09
Contact:
Yu FENG
摘要:
金属氧化物因具有结构可调、易于改性、成本低廉等特性,广泛应用于气体净化领域。但未引入缺陷结构的金属氧化物对气体污染物的反应活性较低,且选择性仍有待提高。在金属氧化物中引入晶体缺陷后,能改变其晶体结构和物化性质,进而显著提高其对气体的吸附活性和选择性。但在气体脱除反应中,缺陷的促进作用机制极为复杂,这导致探究金属氧化物与气体反应中的缺陷作用机理变得十分困难,也使得将反应机理的研究成果应用于指导缺陷的策略性引入极具挑战性。首先概述了金属氧化物中的缺陷类型,其次对缺陷引入方法进行了分类概括,并总结了近年来缺陷型金属氧化物在气体净化领域的应用现状。最后,对金属氧化物缺陷工程的改进方法和未来研究方向进行了展望,以期为后续金属氧化物缺陷工程构筑及缺陷促进反应机制研究提供参考。
中图分类号:
杨盛华, 孙阳杰, 薛晓君, 米杰, 王建成, 冯宇. 缺陷型金属氧化物脱除气体污染物研究进展[J]. 化工学报, 2025, 76(6): 2469-2482.
Shenghua YANG, Yangjie SUN, Xiaojun XUE, Jie MI, Jiancheng WANG, Yu FENG. Research progress on gas pollutants removal by defective metal oxides[J]. CIESC Journal, 2025, 76(6): 2469-2482.
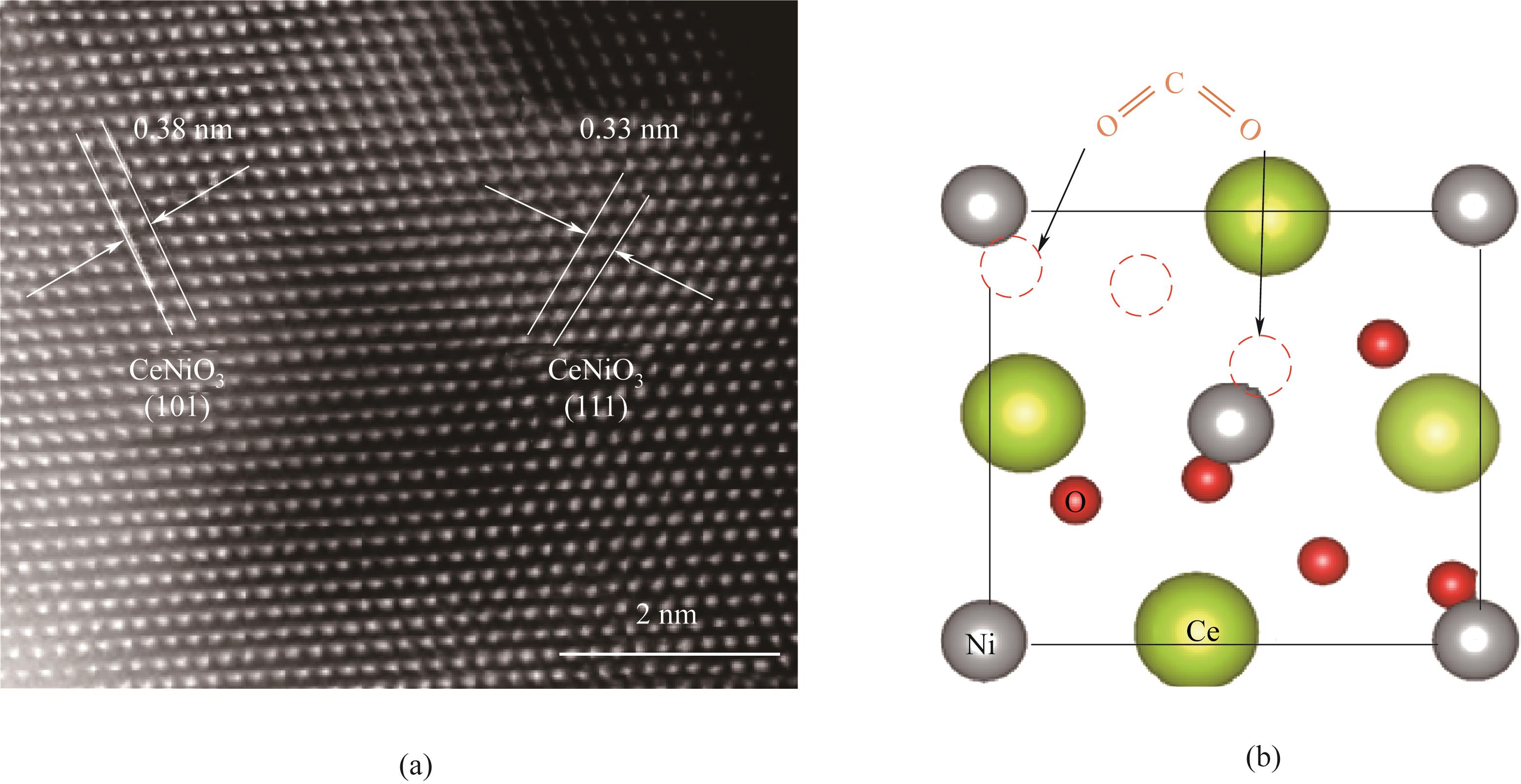
图2 CeNiO3-δ 的HAADF-扫描透射电子显微镜图(a)和CO2吸附模型示意图(b)[13]
Fig.2 HAADF-scanning transmission electron microscopy image (a) and schematic diagram of CO2 adsorption model (b) of CeNiO3-δ[13]

图3 Bi2MoO6(VMo)的高分辨率TEM图(a),原子分辨率HAADF-STEM图(b),晶体结构示意图(c)[17]
Fig.3 High-resolution TEM images (a), atomic resolution HAADF-STEM image and the contour line (b) and schematic crystal structure (c) of Bi2MoO6(VMo) [17]
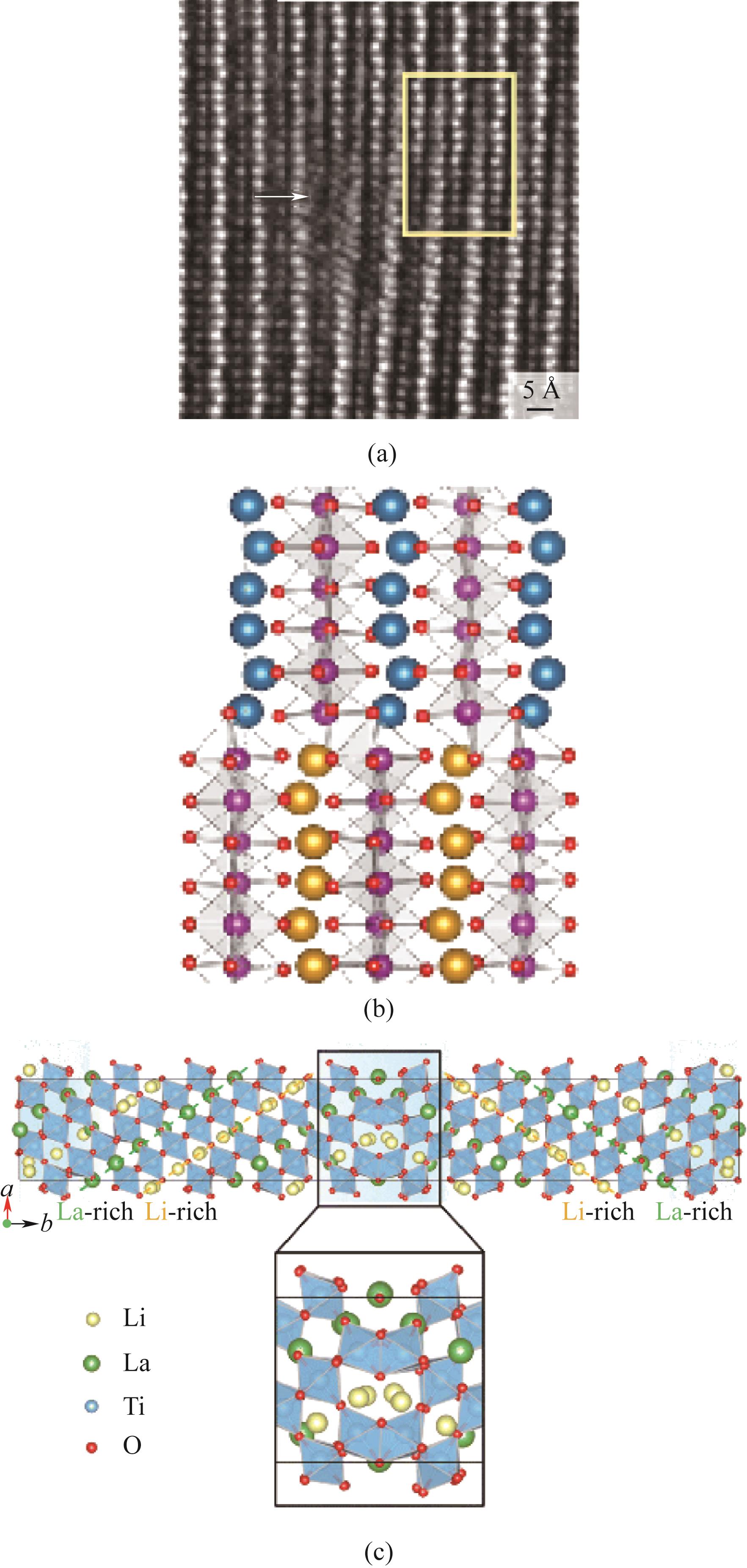
图4 (a)Er(Mn,Ti)O3的HAADF-STEM图(1 Å=0.1 nm);(b)模拟Er(Mn,Ti)O3的位错结构;(c)锂镧钛氧化物(LLTO)化学计量晶界的原子结构[19-20]
Fig.4 (a) HAADF-STEM map of Er(Mn,Ti)O3; (b) Simulated dislocation structure of Er(Mn,Ti)O3; (c) Atomic structure of lithium lanthanum titanium oxide (LLTO) stoichiometric grain boundaries[19-20]
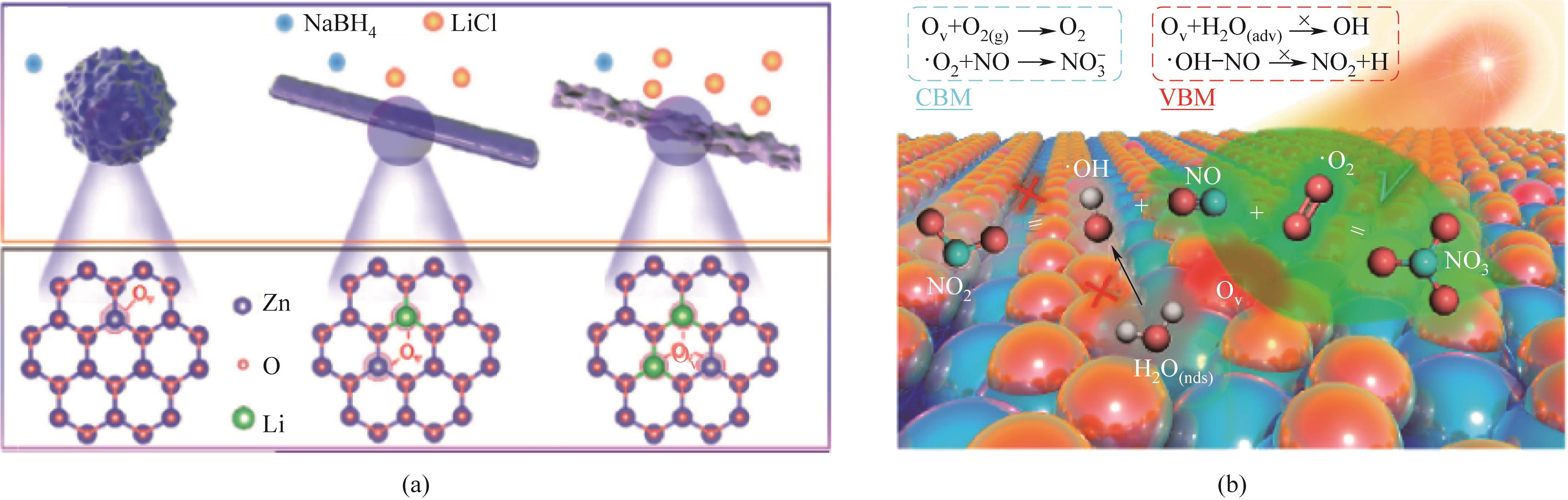
图6 (a)ZnO形成表面氧缺陷示意图;(b)含缺陷ZnO通过增强载流子和缺陷诱导的分子氧活化促进光催化NO转化[39]
Fig.6 (a) Schematic representation of ZnO forming surface oxygen defects; (b) Defect-containing ZnO promotes photocatalytic NO conversion by enhancing carrier and defect-induced molecular oxygen activation[39]
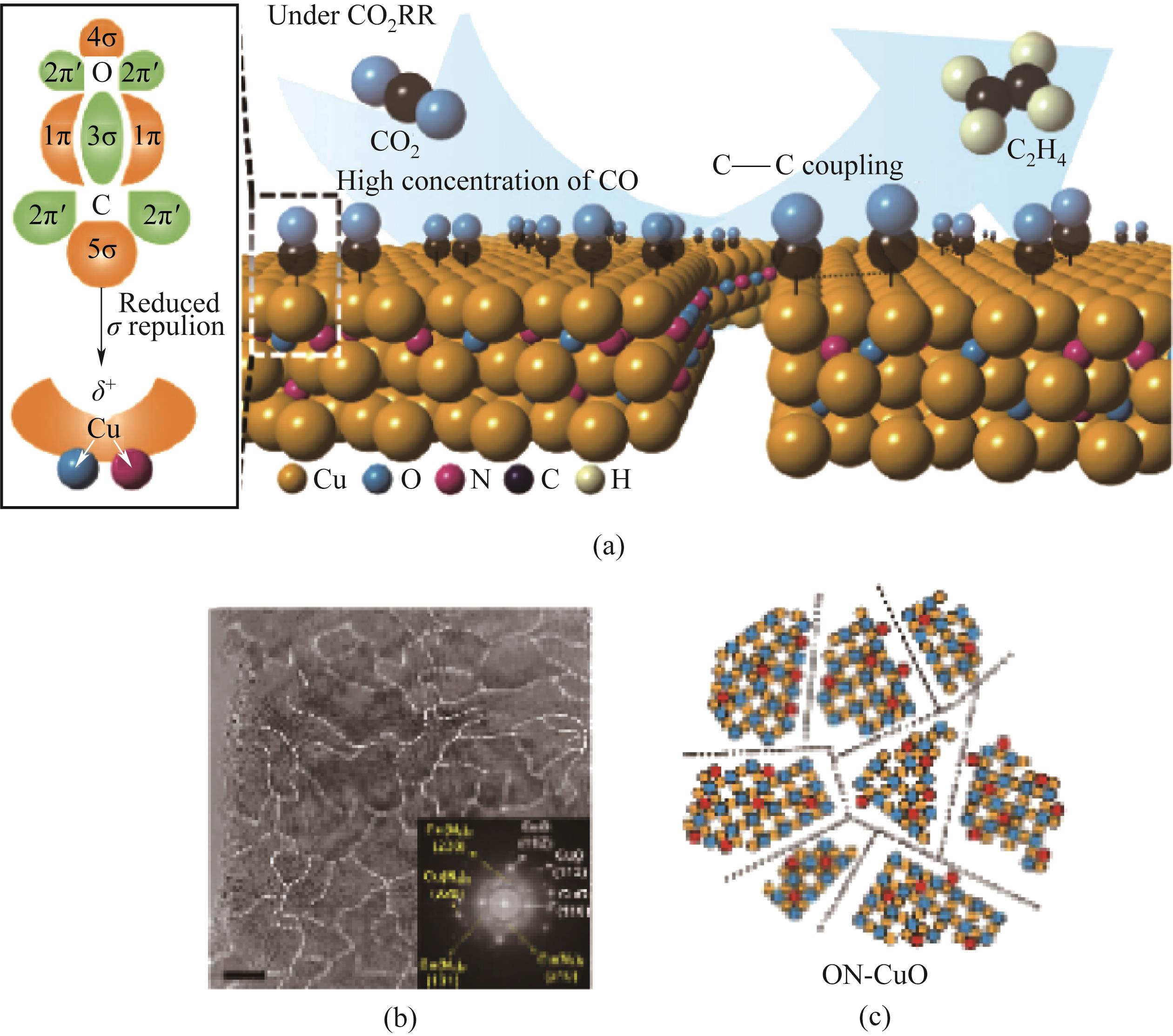
图7 含缺陷ON-CuO的二氧化碳还原反应示意图(a)、TEM图(b)和(c)模拟晶格结构(b)[44]
Fig.7 Schematic representation of the carbon dioxide reduction reaction (a), TEM image (b) and simulated lattice structures (c) of defective ON-CuO-containing[44]
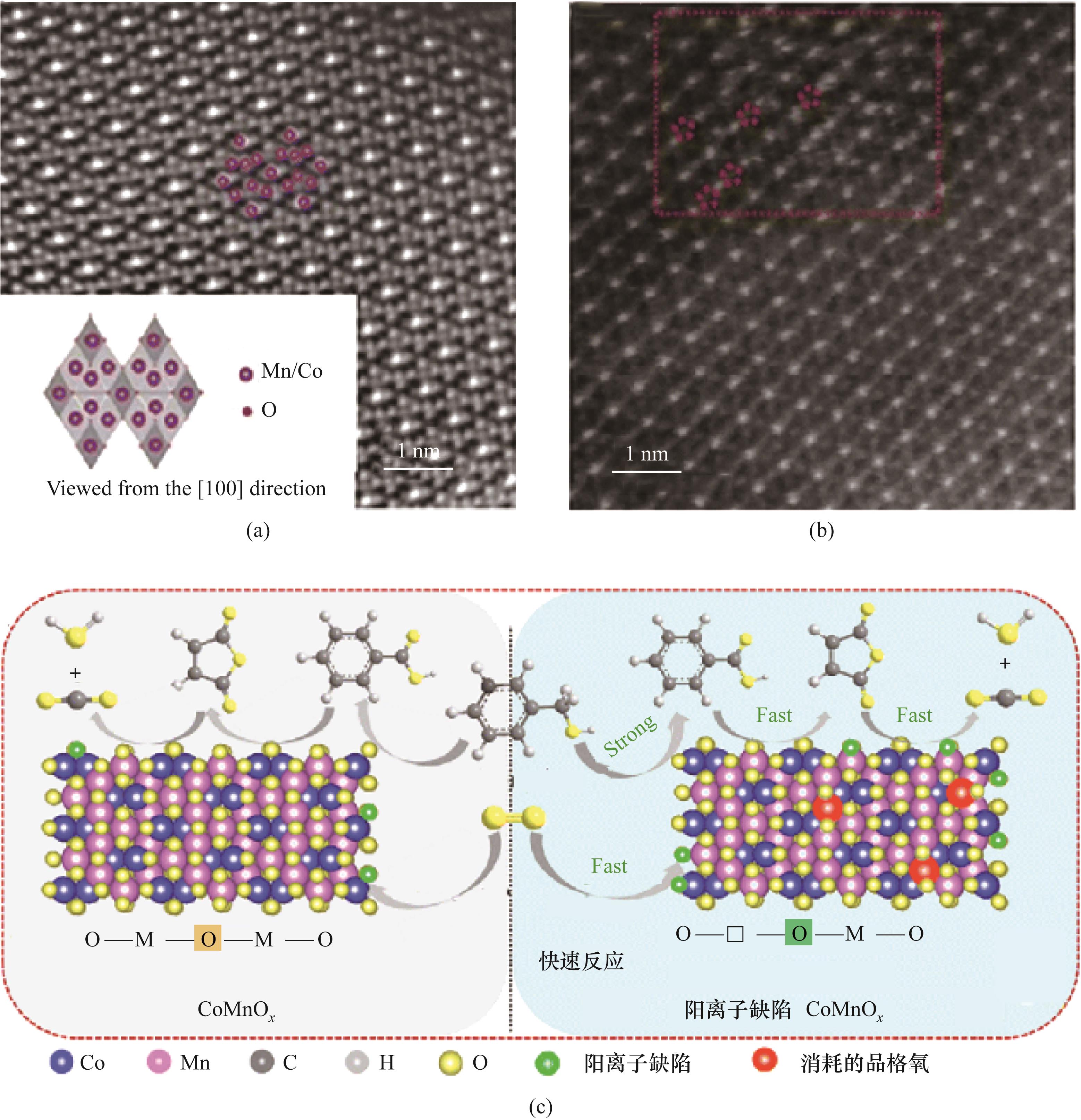
图8 (a)(Co,Mn)2O4原子分辨率球差校正高角度环形暗场扫描TEM图;(b)CoMnO x 原子分辨率球差校正高角度环形暗场扫描TEM图;(c)阳离子缺陷的CoMnO x 尖晶石的催化反应机理图[46]
Fig.8 (a) Atomic-resolution spherical aberration corrected high-angle annular dark-field scanning TEM images of (Co,Mn)2O4; (b) Atomic-resolution spherical aberration-corrected high-angle annular dark-field scanning TEM image of CoMnO x; (c) Mechanism of the catalytic reaction of cation-deficient CoMnO x spinel[46]
| 金属氧化物种类 | 缺陷类型 | 制备方法 | 应用 | 性能 | 文献 |
|---|---|---|---|---|---|
| UO2 | 氧空位 | 还原法 | 含硫气体 | — | [ |
| Ce1-x Zr x O2 | 氧空位 | 离子掺杂 | 含硫气体 | — | [ |
| Cu2O | 氧空位 | 还原法 | 含硫气体 | — | [ |
| Au/CeO2 | 氧空位 | 离子掺杂 | 含硫气体 | — | [ |
| NiO/ACF | 阳离子空位 | 热处理 | 含硫气体 | 23.65 mgs/g脱硫剂 | [ |
| Mn-TiO2 | 氧空位 | 还原法 | 含硫气体 | 0.40 ± 0.05(相对浓度) | [ |
| ZnO | 氧空位 | 热处理 | NO x | 36.7% | [ |
| TiO2 | 氧空位 | 热处理 | NO x | — | [ |
| Zn2SnO4 | 氧空位 | 离子掺杂 | NO x | 62% | [ |
| Ce-SnO2 | 氧空位 | 离子掺杂 | NO x | 5.001 μmol/(g·h) | [ |
| MnFeO x /TiO2 | 氧空位 | 还原法 | NO x | > 80% | [ |
| Bi2WO6 | 氧空位 | 离子掺杂 | NO x | 64% | [ |
| ZnO | 氧空位 | 热处理 | NO x | 55.45% | [ |
| Cu2O | 氧空位 | 还原法 | CO2 | 35.4%(法拉第效率) | [ |
| TiO2-Cu x O | 氧空位、阳离子空位 | 离子掺杂 | CO2 | — | [ |
| Bi4Ti3O12 | 氧空位、阳离子空位 | 还原法 | CO2 | 4.90 μmol/(g·h) | [ |
| ON-CuO | 氧空位、面缺陷 | 离子掺杂 | CO2 | 56%(法拉第效率) | [ |
| Cu/Co3O4 | 氧空位 | 离子掺杂 | CO2 | 99% | [ |
| TiO2 | 氧空位 | 热处理 | CO2 | 20.2% | [ |
| Cu2O | 线缺陷、面缺陷 | 还原法 | CO2 | 48.3%(法拉第效率) | [ |
| CeO2-MgO | 氧空位 | 热处理 | VOCs | 90% | [ |
| MnO x | 氧空位 | 热处理 | VOCs | T90 = 167℃ | [ |
| Ag-MnO x | 氧空位、阳离子空位 | 离子掺杂 | VOCs | T90 = 216℃ | [ |
| Fe-CeO2 | 氧空位 | 离子掺杂 | VOCs | > 98% | [ |
| Co3O4-ZrO2 | 氧空位 | 离子掺杂 | VOCs | 90% | [ |
| MnO x | 氧空位 | 热处理 | VOCs | 95% | [ |
表1 不同合成方法制备的含缺陷金属氧化物及其应用
Table 1 Defect-containing metal oxides prepared by different synthetic methods and their applications
| 金属氧化物种类 | 缺陷类型 | 制备方法 | 应用 | 性能 | 文献 |
|---|---|---|---|---|---|
| UO2 | 氧空位 | 还原法 | 含硫气体 | — | [ |
| Ce1-x Zr x O2 | 氧空位 | 离子掺杂 | 含硫气体 | — | [ |
| Cu2O | 氧空位 | 还原法 | 含硫气体 | — | [ |
| Au/CeO2 | 氧空位 | 离子掺杂 | 含硫气体 | — | [ |
| NiO/ACF | 阳离子空位 | 热处理 | 含硫气体 | 23.65 mgs/g脱硫剂 | [ |
| Mn-TiO2 | 氧空位 | 还原法 | 含硫气体 | 0.40 ± 0.05(相对浓度) | [ |
| ZnO | 氧空位 | 热处理 | NO x | 36.7% | [ |
| TiO2 | 氧空位 | 热处理 | NO x | — | [ |
| Zn2SnO4 | 氧空位 | 离子掺杂 | NO x | 62% | [ |
| Ce-SnO2 | 氧空位 | 离子掺杂 | NO x | 5.001 μmol/(g·h) | [ |
| MnFeO x /TiO2 | 氧空位 | 还原法 | NO x | > 80% | [ |
| Bi2WO6 | 氧空位 | 离子掺杂 | NO x | 64% | [ |
| ZnO | 氧空位 | 热处理 | NO x | 55.45% | [ |
| Cu2O | 氧空位 | 还原法 | CO2 | 35.4%(法拉第效率) | [ |
| TiO2-Cu x O | 氧空位、阳离子空位 | 离子掺杂 | CO2 | — | [ |
| Bi4Ti3O12 | 氧空位、阳离子空位 | 还原法 | CO2 | 4.90 μmol/(g·h) | [ |
| ON-CuO | 氧空位、面缺陷 | 离子掺杂 | CO2 | 56%(法拉第效率) | [ |
| Cu/Co3O4 | 氧空位 | 离子掺杂 | CO2 | 99% | [ |
| TiO2 | 氧空位 | 热处理 | CO2 | 20.2% | [ |
| Cu2O | 线缺陷、面缺陷 | 还原法 | CO2 | 48.3%(法拉第效率) | [ |
| CeO2-MgO | 氧空位 | 热处理 | VOCs | 90% | [ |
| MnO x | 氧空位 | 热处理 | VOCs | T90 = 167℃ | [ |
| Ag-MnO x | 氧空位、阳离子空位 | 离子掺杂 | VOCs | T90 = 216℃ | [ |
| Fe-CeO2 | 氧空位 | 离子掺杂 | VOCs | > 98% | [ |
| Co3O4-ZrO2 | 氧空位 | 离子掺杂 | VOCs | 90% | [ |
| MnO x | 氧空位 | 热处理 | VOCs | 95% | [ |
| [1] | Zewdie D T, Bizualem Y D, Nurie A G. A review on removal CO2, SO2, and H2S from flue gases using zeolite based adsorbents[J]. Discover Applied Sciences, 2024, 6(7): 331. |
| [2] | Ning H Y, Tang R J, Li C M, et al. Recent advances in process and materials for dry desulfurization of industrial flue gas: an overview[J]. Separation and Purification Technology, 2025, 353: 128425. |
| [3] | Chen R M, Li J Y, Wang H, et al. Photocatalytic reaction mechanisms at a gas-solid interface for typical air pollutant decomposition[J]. Journal of Materials Chemistry A, 2021, 9(36): 20184-20210. |
| [4] | 李振山, 蔡宁生. 气固反应动力学速率方程理论[J]. 清华大学学报(自然科学版), 2022, 62(4): 704-721. |
| Li Z S, Cai N S. Rate equation theory for gas-solid reaction kinetics[J]. Journal of Tsinghua University (Science and Technology), 2022, 62(4): 704-721. | |
| [5] | Xie X W, Li Y, Liu Z Q, et al. Low-temperature oxidation of CO catalysed by Co3O4 nanorods[J]. Nature, 2009, 458: 746-749. |
| [6] | Sun X H, Wu D X, Zou L F, et al. Dislocation-induced stop-and-go kinetics of interfacial transformations[J]. Nature, 2022, 607(7920): 708-713. |
| [7] | She X J, Zhu X W, Yang J M, et al. Grain-boundary surface terminations incorporating oxygen vacancies for selectively boosting CO2 photoreduction activity[J]. Nano Energy, 2021, 84: 105869. |
| [8] | Zhang C, Liu G F, Geng X, et al. Metal oxide semiconductors with highly concentrated oxygen vacancies for gas sensing materials: a review[J]. Sensors and Actuators A: Physical, 2020, 309: 112026. |
| [9] | Sun Y F, Gao S, Lei F C, et al. Atomically-thin two-dimensional sheets for understanding active sites in catalysis[J]. Chemical Society Reviews, 2015, 44(3): 623-636. |
| [10] | Pei D N, Gong L, Zhang A Y, et al. Defective titanium dioxide single crystals exposed by high-energy {001} facets for efficient oxygen reduction[J]. Nature Communications, 2015, 6: 8696. |
| [11] | An W, Wu X J, Zeng X C. Adsorption of O2, H2, CO, NH3, and NO2 on ZnO nanotube: a density functional theory study[J]. The Journal of Physical Chemistry C, 2008, 112(15): 5747-5755. |
| [12] | Sun Y F, Liu Q H, Gao S, et al. Pits confined in ultrathin cerium ( Ⅳ ) oxide for studying catalytic centers in carbon monoxide oxidation[J]. Nature Communications, 2013, 4: 2899. |
| [13] | Du P P, Deng G Q, Li Z H, et al. Effective CO2 activation of enriched oxygen vacancies for photothermal CO2 methanation[J]. Journal of Materials Science & Technology, 2024, 189: 203-210. |
| [14] | 郑跃楠, 杨佳奇, 乔振安. 凝聚态化学视角下的多孔材料缺陷工程[J]. 化学进展, 2023, 35(6): 954-967. |
| Zheng Y N, Yang J Q, Qiao Z A. Condensed matter chemistry: the defect engineering of porous materials[J]. Progress in Chemistry, 2023, 35(6): 954-967. | |
| [15] | Koketsu T, Ma J W, Morgan B J, et al. Reversible magnesium and aluminium ions insertion in cation-deficient anatase TiO2 [J]. Nature Materials, 2017, 16(11): 1142-1148. |
| [16] | Liu Z S, Xu H M, Fan Y R, et al. Cation concavities induced d-band electronic modulation on Co/FeO x nanostructure to activate molecular and interfacial oxygen for CO oxidation[J]. Environmental Science & Technology, 2023, 57(50): 21272-21283. |
| [17] | Li S G, Chen F, Chu S Q, et al. Synergy-compensation effect of ferroelectric polarization and cationic vacancy collaboratively promoting CO2 photoreduction[J]. Small, 2023, 19(5): 2203559. |
| [18] | Szot K, Rodenbücher C, Bihlmayer G, et al. Influence of dislocations in transition metal oxides on selected physical and chemical properties[J]. Crystals, 2018, 8(6): 241. |
| [19] | Evans D M, Småbråten D R, Holstad T S, et al. Observation of electric-field-induced structural dislocations in a ferroelectric oxide[J]. Nano Letters, 2021, 21(8): 3386-3392. |
| [20] | Kim H, Conlin P, Bergschneider M, et al. First principles study on Li metallic phase nucleation at grain boundaries in a lithium lanthanum titanium oxide (LLTO) solid electrolyte[J]. Journal of Materials Chemistry A, 2023, 11(6): 2889-2898. |
| [21] | Senthamizhan A, Balusamy B, Aytac Z, et al. Grain boundary engineering in electrospun ZnO nanostructures as promising photocatalysts[J]. CrystEngComm, 2016, 18(34): 6341-6351. |
| [22] | Shamjitha C, Vargeese A A. Copper-cobalt oxide nanoparticles with tailored cobalt oxidation state and lattice oxygen vacancy for low-temperature ignition of ammonium dinitramide monopropellants[J]. ACS Applied Nano Materials, 2024, 7(8): 8557-8566. |
| [23] | Lian S T, Sun C L, Xu W N, et al. Built-in oriented electric field facilitating durable Zn-MnO2 battery[J]. Nano Energy, 2019, 62: 79-84. |
| [24] | Ghosh S, Khan G G, Das B, et al. Vacancy-induced intrinsic d ferromagnetism and photoluminescence in potassium doped ZnO nanowires[J]. Journal of Applied Physics, 2011, 109(12): 123927. |
| [25] | van Dao D, Jung H D, Nguyen T T D, et al. Defect-rich N-doped CeO2 supported by N-doped graphene as a metal-free plasmonic hydrogen evolution photocatalyst[J]. Journal of Materials Chemistry A, 2021, 9(16): 10217-10230. |
| [26] | Xu J, Xie Y B. Dual-defects induced band edge reconstruction of tin dioxide via cobalt and nitrogen co-doping for wearable supercapacitor application[J]. Journal of Power Sources, 2021, 493: 229685. |
| [27] | Badola S, Shah J, Gaur A, et al. Strategic enhancement of oxygen defects in ZnO from ZnS for water splitting to generate green electricity by hydroelectric cell[J]. Applied Materials Today, 2023, 34: 101904. |
| [28] | Ding W M, Hai Y, Li X M, et al. Modulation of cationic vacancies in Co3O4 for promoted photocatalytic nitrogen fixation and electrocatalytic nitrate reduction to ammonia[J]. Journal of Environmental Chemical Engineering, 2024, 12(4): 113141. |
| [29] | Li Y X, Lv G C, Liu H, et al. Improvement of magnetite adsorption performance for P b ( Ⅱ ) by introducing defects[J]. Frontiers in Chemistry, 2023, 11: 1137246. |
| [30] | Ou G, Xu Y S, Wen B, et al. Tuning defects in oxides at room temperature by lithium reduction[J]. Nature Communications, 2018, 9(1): 1302. |
| [31] | Zhang Y M, Feng L X, Zhan W T, et al. Co3O4 hollow porous nanospheres with oxygen vacancies for enhanced Li-O2 batteries[J]. ACS Applied Energy Materials, 2020, 3(4): 4014-4022. |
| [32] | Zhang Y, Sun D, Wang Y X, et al. Facile electrochemically induced vacancy modulation of NiCo2O4 cathode toward high-performance aqueous Zn-based battery[J]. Chemical Engineering Journal, 2023, 453: 139736. |
| [33] | Chung C, Pottimurthy Y, Xu M Y, et al. Fate of sulfur in coal-direct chemical looping systems[J]. Applied Energy, 2017, 208: 678-690. |
| [34] | Awasthi M K, Amobonye A, Bhagwat P, et al. Biochemical engineering for elemental sulfur from flue gases through multi-enzymatic based approaches—a review[J]. Science of the Total Environment, 2024, 914: 169857. |
| [35] | Ling L X, Song J J, Zhao S P, et al. DFT study on the effects of defect and metal-doping on the decomposition of H2S on the α-Fe2O3(0001) surface[J]. RSC Advances, 2014, 4: 22411-22418. |
| [36] | Wang B, Li X, Liang S S, et al. Adsorption and oxidation of SO2 on the surface of TiO2 nanoparticles: the role of terminal hydroxyl and oxygen vacancy-Ti3+ states[J]. Physical Chemistry Chemical Physics, 2020, 22(18): 9943-9953. |
| [37] | Lin B L, Chen H X, Wei W Y, et al. Enriched oxygen vacancies of copper(Ⅰ) oxide particles for enhanced removal of hydrogen sulfide at room temperature[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110113. |
| [38] | Mohammadi A, Thurner C W, Haug L, et al. How defects in lanthanum iron manganite perovskite structures promote the catalytic reduction of NO by CO[J]. Materials Today Chemistry, 2024, 35: 101910. |
| [39] | Hailili R, Ji H W, Wang K W, et al. ZnO with controllable oxygen vacancies for photocatalytic nitrogen oxide removal[J]. ACS Catalysis, 2022, 12(16): 10004-10017. |
| [40] | Spencer M J S, Yarovsky I. ZnO nanostructures for gas sensing: interaction of NO2, NO, O, and N with the ZnO(101̅0) surface[J]. The Journal of Physical Chemistry C, 2010, 114(24): 10881-10893. |
| [41] | Wei W Q, Wei Z, Li R Z, et al. Subsurface oxygen defects electronically interacting with active sites on In2O3 for enhanced photothermocatalytic CO2 reduction[J]. Nature Communications, 2022, 13(1): 3199. |
| [42] | Jia T B, Wang L L, Zhu Z H, et al. Modulating the degree of O vacancy defects to achieve selective control of electrochemical CO2 reduction products[J]. Chinese Chemical Letters, 2024, 35(5): 108692. |
| [43] | Liu L Z, Hu J C, Lei B, et al. Cation vacancy activating surface neighboring sites for efficient CO2 photoreduction on Bi4Ti3O12 nanosheets[J]. Journal of Materials Chemistry A, 2022, 10(38): 20396-20401. |
| [44] | Park D G, Choi J W, Chun H, et al. Increasing CO binding energy and defects by preserving Cu oxidation state via O2-plasma-assisted N doping on CuO enables high C2+ selectivity and long-term stability in electrochemical CO2 reduction[J]. ACS Catalysis, 2023, 13(13): 9222-9233. |
| [45] | Sun L, Zhao S Z, Tang X L, et al. Recent advances in catalytic oxidation of VOCs by two-dimensional ultra-thin nanomaterials[J]. Science of the Total Environment, 2024, 920: 170748. |
| [46] | Wang W, Huang Y, Rao Y F, et al. The role of cationic defects in boosted lattice oxygen activation during toluene total oxidation over nano-structured CoMnO x spinel[J]. Environmental Science: Nano, 2023, 10(3): 812-823. |
| [47] | Feng C, Gao Q Q, Xiong G Y, et al. Defect engineering technique for the fabrication of LaCoO3 perovskite catalyst via urea treatment for total oxidation of propane[J]. Applied Catalysis B: Environmental, 2022, 304: 121005. |
| [48] | Schlereth T W, Hedhili M N, Yakshinskiy B V, et al. Adsorption and reaction of SO2 with a polycrystalline UO2 film: promotion of S—O bond cleavage by creation of O-defects and Na or Ca coadsorption[J]. The Journal of Physical Chemistry B, 2005, 109(44): 20895-20905. |
| [49] | Liu G, Rodriguez J A, Chang Z P, et al. Adsorption and reaction of SO2 on model Ce1- x Zr x O2(111) catalysts[J]. The Journal of Physical Chemistry B, 2004, 108(9): 2931-2938. |
| [50] | Rodriguez J A, Pérez M, Evans J, et al. Reaction of SO2 with AuCeO2(111): importance of O vacancies in the activation of gold[J]. The Journal of Chemical Physics, 2005, 122(24): 241101. |
| [51] | Li K L, Wang C, Ning P, et al. Surface characterization of metal oxides-supported activated carbon fiber catalysts for simultaneous catalytic hydrolysis of carbonyl sulfide and carbon disulfide[J]. Journal of Environmental Sciences, 2020, 96: 44-54. |
| [52] | Ke J C R, Thomas A G, Peake J, et al. The effect of Mn doping and Ti3+ defects at TiO2 surfaces in NO and SO2 gas capture investigated using near-ambient pressure X-ray photoelectron spectroscopy[J]. Surfaces, 2024, 7(1): 26-43. |
| [53] | Zhu P F, Yin X H, Gao X H, et al. Enhanced photocatalytic NO removal and toxic NO2 production inhibition over ZIF-8-derived ZnO nanoparticles with controllable amount of oxygen vacancies[J]. Chinese Journal of Catalysis, 2021, 42(1): 175-183. |
| [54] | Shen X L, Dong G H, Wang L, et al. Enhancing photocatalytic activity of NO removal through an in situ control of oxygen vacancies in growth of TiO2 [J]. Advanced Materials Interfaces, 2019, 6(19): 1901032. |
| [55] | He Z J, Chen B F, Li Y H, et al. Deep NO oxidation in Zn2SnO4 by dual-anionic-defects engineering[J]. Separation and Purification Technology, 2023, 320: 123886. |
| [56] | Song X J, Qin G D, Cheng G, et al. Oxygen defect-induced NO-intermediates promoting NO deep oxidation over Ce doped SnO2 under visible light[J]. Applied Catalysis B: Environmental, 2021, 284: 119761. |
| [57] | Lin L Y, Hsieh T T, Hsu J C, et al. Insight into the enhanced catalytic activity and H2O/SO2 resistance of MnFeO x /defect-engineered TiO2 for low-temperature selective catalytic reduction of NO with NH3 [J]. Applied Surface Science, 2023, 614: 156139. |
| [58] | Yang X L, Wang S Y, Chen T, et al. Chloridion-induced dual tunable fabrication of oxygen-deficient Bi2WO6 atomic layers for deep oxidation of NO[J]. Chinese Journal of Catalysis, 2021, 42(6): 1013-1023. |
| [59] | Hailili R, Reyimu X, Li Z L, et al. Tuning the microstructures of ZnO to enhance photocatalytic NO removal performances[J]. ACS Applied Materials & Interfaces, 2023, 15(19): 23185-23198. |
| [60] | Liu B Q, Yao X, Zhang Z J, et al. Synthesis of Cu2O nanostructures with tunable crystal facets for electrochemical CO2 reduction to alcohols[J]. ACS Applied Materials & Interfaces, 2021, 13(33): 39165-39177. |
| [61] | Savchuk T P, Kytina E V, Konstantinova E A, et al. Photocatalytic CO2 conversion using anodic TiO2 nanotube-Cu x O composites[J]. Catalysts, 2022, 12(9): 1011. |
| [62] | Wang Y X, Qiu C H, Xie Y J, et al. Intentionally introducing oxygen vacancies and Ti3+ defects on the surface of Bi4Ti3O12 nanosheets for promoting the photoreduction of CO2 to CH3OH[J]. ACS Applied Nano Materials, 2024, 7(3): 3012-3023. |
| [63] | Zhou J M, Yang S W, Wan W H, et al. Synergistic catalysis of mesoporous Cu/Co3O4 and surface oxygen vacancy for CO2 fixation to carbamates[J]. Journal of Catalysis, 2023, 418: 178-189. |
| [64] | Liang L, Ling P Q, Li Y H, et al. Atmospheric CO2 capture and photofixation to near-unity CO by Ti3+-Vo-Ti3+ sites confined in TiO2 ultrathin layers[J]. Science China Chemistry, 2021, 64(6): 953-958. |
| [65] | Fu W L, Liu Z, Wang T Y, et al. Promoting C2+ production from electrochemical CO2 reduction on shape-controlled cuprous oxide nanocrystals with high-index facets[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(40): 15223-15229. |
| [66] | Hao Y J, Zhang X, Zhang H, et al. Contributions of surface oxygen species and photoinduced holes on photothermocatalytic toluene oxidation over CeO2-MgO[J]. ACS Applied Nano Materials, 2023, 6(11): 9385-9396. |
| [67] | Zheng Y F, Liu Q L, Shan C P, et al. Defective ultrafine MnO x nanoparticles confined within a carbon matrix for low-temperature oxidation of volatile organic compounds[J]. Environmental Science & Technology, 2021, 55(8): 5403-5411. |
| [68] | Deng H, Kang S Y, Ma J Z, et al. Role of structural defects in MnO x promoted by Ag doping in the catalytic combustion of volatile organic compounds and ambient decomposition of O3 [J]. Environmental Science & Technology, 2019, 53(18): 10871-10879. |
| [69] | Jiang C L, Wang H, Wang Y Q, et al. Modifying defect states in CeO2 by Fe doping: a strategy for low-temperature catalytic oxidation of toluene with sunlight[J]. Journal of Hazardous Materials, 2020, 390: 122182. |
| [70] | He C L, Ao C C, Ruan S S, et al. Catalytic combustion of propane over Zr-modified Co3O4 catalysts: an experimental and theoretical study[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2022, 641: 128617. |
| [71] | Lu Y Q, Deng H, Pan T T, et al. Thermal annealing induced surface oxygen vacancy clusters in α - M n O 2 nanowires for catalytic ozonation of VOCs at ambient temperature[J]. ACS Applied Materials & Interfaces, 2023, 15(7): 9362-9372. |
| [1] | 裴星亮, 叶翠平, 裴赢丽, 李文英. 碱改性MIL-53(Cr)选择性吸附分离二甲苯异构体[J]. 化工学报, 2025, 76(S1): 258-267. |
| [2] | 吴梓航, 徐震原, 游锦方, 潘权稳, 王如竹. 基于吸附式储冷技术的深井钻探设备冷却系统[J]. 化工学报, 2025, 76(S1): 309-317. |
| [3] | 黄国瑞, 赵耀, 谢明熹, 陈尔健, 代彦军. 一种新型数据中心余热回收系统实验与分析[J]. 化工学报, 2025, 76(S1): 409-417. |
| [4] | 彭新艳, 刘云鸿, 陈凌宇, 韦跃兰, 陈淑琴, 胡柱东. 小分子外交联法制备超高交联聚苯乙烯血液灌流吸附剂[J]. 化工学报, 2025, 76(6): 3093-3103. |
| [5] | 张涵川, 尚超, 吕文祥, 黄德先, 张亚宁. 基于无监督时序聚类的催化裂化装置工况划分识别与产率预测方法[J]. 化工学报, 2025, 76(6): 2781-2790. |
| [6] | 何军, 李勇, 赵楠, 何孝军. 碳负载硒掺杂硫化钴在锂硫电池中的性能研究[J]. 化工学报, 2025, 76(6): 2995-3008. |
| [7] | 宋粉红, 王文光, 郭亮, 范晶. C元素修饰g-C3N4对TiO2的调控及复合材料光催化产氢性能研究[J]. 化工学报, 2025, 76(6): 2983-2994. |
| [8] | 姬海燕, 刘家印, 吴海军, 何璟琳, 靳紫恒, 魏钿航, 江霞. 低温等离子体在生物质气化制氢中的应用研究进展[J]. 化工学报, 2025, 76(6): 2419-2433. |
| [9] | 彭健, 沈鲁恺, 王立坤, 忻利宏, 刘涌, 赵高凌, 马赛男, 韩高荣. 钨酸盐纳米材料的制备及其在电致变色领域的研究进展[J]. 化工学报, 2025, 76(6): 2451-2468. |
| [10] | 李愽龙, 蒋雨希, 任傲天, 秦雯琪, 傅杰, 吕秀阳. TS-1/In-TS-1催化果糖一步法醇解制备乳酸甲酯连续化试验[J]. 化工学报, 2025, 76(6): 2678-2686. |
| [11] | 卢丽丽, 李晨, 陈柳云, 谢新玲, 罗轩, 苏通明, 秦祖赠, 纪红兵. BiOBr的形貌调控及其光催化CO2还原性能的研究[J]. 化工学报, 2025, 76(6): 2687-2700. |
| [12] | 龚丽芳, 任美慧, 蒋吉春, 郭光召, 胡红云, 黄永达, 姚洪. 垃圾焚烧烟气中芳香烃化合物在线监测和选择性催化还原脱除研究[J]. 化工学报, 2025, 76(6): 3018-3028. |
| [13] | 王一非, 任婧杰, 毕明树, 叶昊天. 基于本质安全与经济性的环己烷氧化工艺参数多目标优化研究[J]. 化工学报, 2025, 76(6): 2722-2732. |
| [14] | 朱迪, 高守建, 方望熹, 靳健. 水蒸气诱导相分离构筑海绵孔结构超亲水聚醚砜膜及其油/水乳液分离性能研究[J]. 化工学报, 2025, 76(5): 2397-2409. |
| [15] | 王金月, 谢恩泽, 马翰泽, 袁晟, 何光伟, 姜忠义. 单原子层分离膜:进展与展望[J]. 化工学报, 2025, 76(5): 1943-1959. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号