化工学报 ›› 2025, Vol. 76 ›› Issue (6): 2419-2433.DOI: 10.11949/0438-1157.20241449
姬海燕1,2( ), 刘家印3, 吴海军1,2, 何璟琳1,2, 靳紫恒2(
), 刘家印3, 吴海军1,2, 何璟琳1,2, 靳紫恒2( ), 魏钿航1,2, 江霞2
), 魏钿航1,2, 江霞2
收稿日期:2024-12-16
修回日期:2025-03-04
出版日期:2025-06-25
发布日期:2025-07-09
通讯作者:
靳紫恒
作者简介:姬海燕(1995—),女,博士研究生,2357863545@qq.com
基金资助:
Haiyan JI1,2( ), Jiayin LIU3, Haijun WU1,2, Jinglin HE1,2, Ziheng JIN2(
), Jiayin LIU3, Haijun WU1,2, Jinglin HE1,2, Ziheng JIN2( ), Dianhang WEI1,2, Xia JIANG2
), Dianhang WEI1,2, Xia JIANG2
Received:2024-12-16
Revised:2025-03-04
Online:2025-06-25
Published:2025-07-09
Contact:
Ziheng JIN
摘要:
氢气是未来能源中最有前途的能源载体之一。生物质气化制氢可实现废弃生物质资源化利用,减少环境污染,被认为是一种具有发展潜力和前景的技术。针对常规生物质气化制氢技术存在的氢气产率低、焦油产量大、反应不稳定等难题,低温等离子体高压放电产生高能电子和活性物质(·OH,·O,·CH等)可强化生物质焦油副产物的高效转化,协同催化剂重整可进一步延缓催化剂快速失活,同时大幅提高氢气产率。从克服传统生物质气化制氢技术瓶颈角度出发,梳理总结了低温等离子体反应器类型与应用、反应条件的优化、催化剂协同作用及反应路径等方面。低温等离子体生物质气化制氢技术的优势在于在较低温度下(<550℃)可实现生物质转化,提升反应物转化率及氢气选择性;在提高气化效率、降低成本等方面需要进一步研究和改进,推动低温等离子体在生物质气化制氢工业中的应用。
中图分类号:
姬海燕, 刘家印, 吴海军, 何璟琳, 靳紫恒, 魏钿航, 江霞. 低温等离子体在生物质气化制氢中的应用研究进展[J]. 化工学报, 2025, 76(6): 2419-2433.
Haiyan JI, Jiayin LIU, Haijun WU, Jinglin HE, Ziheng JIN, Dianhang WEI, Xia JIANG. Research progress on the application of low-temperature plasma in biomass gasification to produce hydrogen[J]. CIESC Journal, 2025, 76(6): 2419-2433.
| 反应类型 | 化学反应式 | 序号 |
|---|---|---|
| 氧化反应 | C+O2 CO2 CO2 | (1) |
2C+O2 2CO 2CO | (2) | |
2CO+O2 2CO2 2CO2 | (3) | |
2H2+O2 2H2O 2H2O | (4) | |
CH4+2O2 CO2+2H2O2 CO2+2H2O2 | (5) | |
| 还原反应 | C+CO2 2CO 2CO | (6) |
H2O+C CO+H2 CO+H2 | (7) | |
2H2O+C CO2+2H2 CO2+2H2 | (8) | |
H2O+CO CO2+H2 CO2+H2 | (9) | |
3H2+CO CH4+H2O CH4+H2O | (10) | |
| Boudouard反应 | 2CO C+CO2 C+CO2 | (11) |
| 焦油分解 | C x H y (tar)  y/2H2+xC y/2H2+xC | (12) |
| 焦油水汽催化重整 | C x H y (tar)+xCO2 xCO+y/2H2 xCO+y/2H2 | (13) |
C x H y (tar)+xH2O xCO+(x+y/2)H2 xCO+(x+y/2)H2 | (14) | |
C x H y (tar)+2xH2O xCO2+(x+y/2)H2 xCO2+(x+y/2)H2 | (15) | |
| 水蒸气重整 | C x H y O z +(x-z)H2O xCO+(x+y/2-z)H2 xCO+(x+y/2-z)H2 | (16) |
| CO2重整 | C x H y O z +(x-z)CO2 (2x-z)CO+y/2H2 (2x-z)CO+y/2H2 | (17) |
| 部分氧化重整 | C x H y O z +(x/2-z/2)O2 xCO+y/2H2 xCO+y/2H2 | (18) |
表1 生物质气化水汽重整过程主要反应类型
Table 1 Main reaction types of biomass steam reforming process
| 反应类型 | 化学反应式 | 序号 |
|---|---|---|
| 氧化反应 | C+O2 CO2 CO2 | (1) |
2C+O2 2CO 2CO | (2) | |
2CO+O2 2CO2 2CO2 | (3) | |
2H2+O2 2H2O 2H2O | (4) | |
CH4+2O2 CO2+2H2O2 CO2+2H2O2 | (5) | |
| 还原反应 | C+CO2 2CO 2CO | (6) |
H2O+C CO+H2 CO+H2 | (7) | |
2H2O+C CO2+2H2 CO2+2H2 | (8) | |
H2O+CO CO2+H2 CO2+H2 | (9) | |
3H2+CO CH4+H2O CH4+H2O | (10) | |
| Boudouard反应 | 2CO C+CO2 C+CO2 | (11) |
| 焦油分解 | C x H y (tar)  y/2H2+xC y/2H2+xC | (12) |
| 焦油水汽催化重整 | C x H y (tar)+xCO2 xCO+y/2H2 xCO+y/2H2 | (13) |
C x H y (tar)+xH2O xCO+(x+y/2)H2 xCO+(x+y/2)H2 | (14) | |
C x H y (tar)+2xH2O xCO2+(x+y/2)H2 xCO2+(x+y/2)H2 | (15) | |
| 水蒸气重整 | C x H y O z +(x-z)H2O xCO+(x+y/2-z)H2 xCO+(x+y/2-z)H2 | (16) |
| CO2重整 | C x H y O z +(x-z)CO2 (2x-z)CO+y/2H2 (2x-z)CO+y/2H2 | (17) |
| 部分氧化重整 | C x H y O z +(x/2-z/2)O2 xCO+y/2H2 xCO+y/2H2 | (18) |
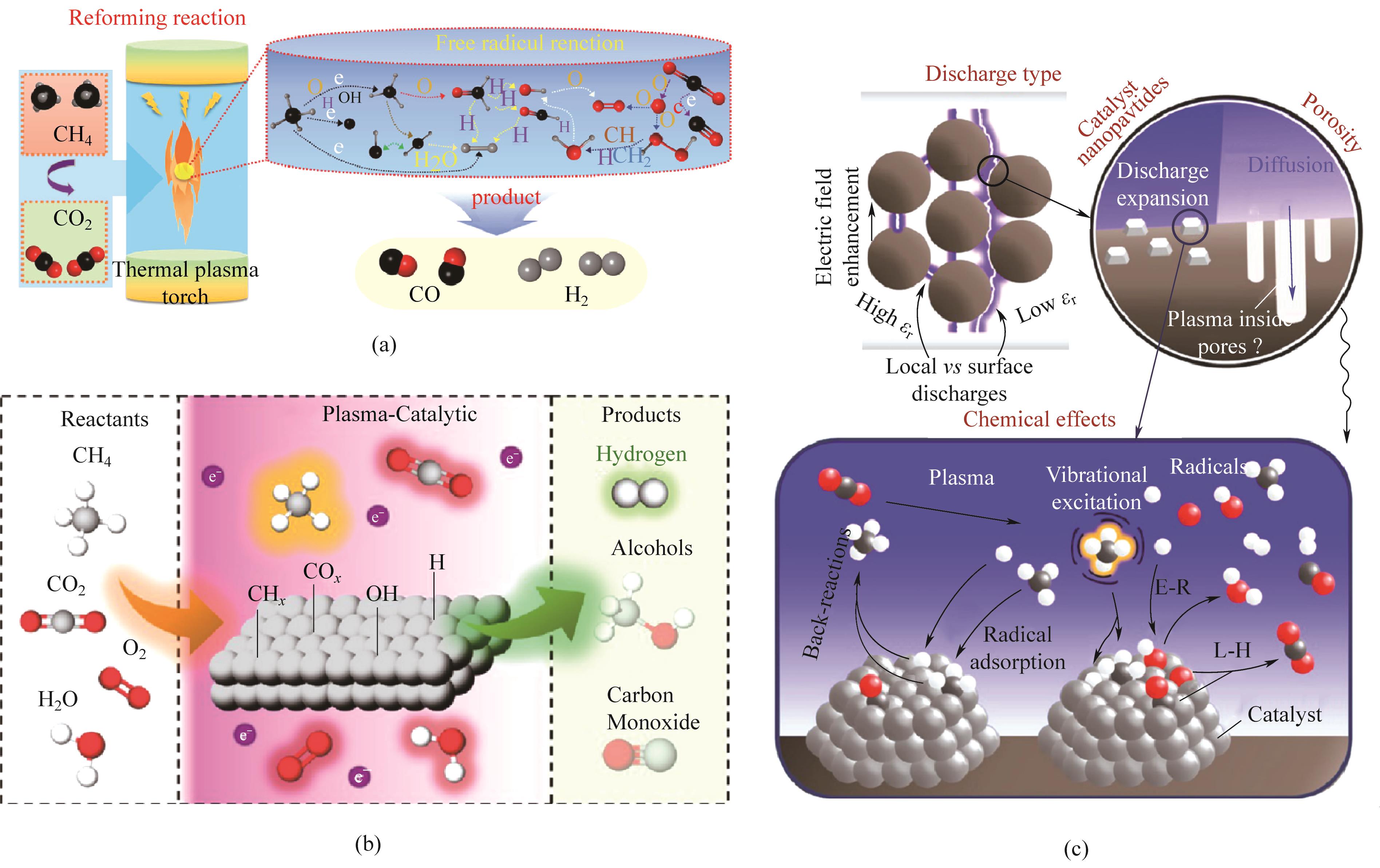
图3 (a) 非催化条件下热等离子体诱导甲烷干重整[59];(b) 等离子体催化甲烷氧化重整过程示意图;(c) 低温等离子体协同催化反应路径[29]
Fig.3 (a) Dry reforming of methane induced by thermal plasma under non-catalytic conditions[59]; (b) Schematic diagram of plasma-catalytic assisted oxidative methane reforming processes; (c) Plasma-catalyst synergy and reaction pathways in plasma catalysis[29]
| 原料 | 处理工艺 | 重整温度/℃ | 催化剂/助剂 | 产气量/(mmol/g) | 合成气/%(体积) | 文献 | ||
|---|---|---|---|---|---|---|---|---|
| Syngas | H2 | H2 | CO | |||||
| 木材/城市生活垃圾 | MSW等离子体气化 | 2227~2527 | 甘油 | 63.00 | 29.10 | 29.1~45.2 | 21~26.3 | [ |
| 不同藻类 | MSW等离子体气化 | 2227~2527 | — | — | 25.3 | — | — | [ |
| 纤维素 | 热解-DBD | 550,250 | Ni-Co/Al2O3 | 54.65 | 26.60 | 55.71 | 42.85 | [ |
| 化石燃料 | DBD催化 | 400 | Fe2O3/ LaSrFeO3 | — | — | 9.42 | — | [ |
| CH4 | DBD催化 | 400 | Fe2O3 | — | 10.00 | 68.00 | 8.00 | [ |
| SrFeO3-δ | — | 5.00 | 37.00 | 6.00 | ||||
| NiO/Fe2O3 | — | 54.00 | 84.00 | 16.00 | ||||
| NiO/SrFeO3- δ | — | 28.00 | 53.00 | 10.00 | ||||
| 甲苯 | DBD催化 | 约200 | NiFe/(Mg, Al)O x | — | — | — | — | [ |
| 甲苯 | DBD催化 | 600~800 | Mn-MOF-74 | — | — | — | — | [ |
| CeO2/13X | — | — | — | — | [ | |||
表2 低温等离子体协同催化应用及产物区别
Table 2 Low temperature plasma coordination catalytic applications and product differences
| 原料 | 处理工艺 | 重整温度/℃ | 催化剂/助剂 | 产气量/(mmol/g) | 合成气/%(体积) | 文献 | ||
|---|---|---|---|---|---|---|---|---|
| Syngas | H2 | H2 | CO | |||||
| 木材/城市生活垃圾 | MSW等离子体气化 | 2227~2527 | 甘油 | 63.00 | 29.10 | 29.1~45.2 | 21~26.3 | [ |
| 不同藻类 | MSW等离子体气化 | 2227~2527 | — | — | 25.3 | — | — | [ |
| 纤维素 | 热解-DBD | 550,250 | Ni-Co/Al2O3 | 54.65 | 26.60 | 55.71 | 42.85 | [ |
| 化石燃料 | DBD催化 | 400 | Fe2O3/ LaSrFeO3 | — | — | 9.42 | — | [ |
| CH4 | DBD催化 | 400 | Fe2O3 | — | 10.00 | 68.00 | 8.00 | [ |
| SrFeO3-δ | — | 5.00 | 37.00 | 6.00 | ||||
| NiO/Fe2O3 | — | 54.00 | 84.00 | 16.00 | ||||
| NiO/SrFeO3- δ | — | 28.00 | 53.00 | 10.00 | ||||
| 甲苯 | DBD催化 | 约200 | NiFe/(Mg, Al)O x | — | — | — | — | [ |
| 甲苯 | DBD催化 | 600~800 | Mn-MOF-74 | — | — | — | — | [ |
| CeO2/13X | — | — | — | — | [ | |||

图5 (a) 低温等离子体与催化剂的相互作用[70]; (b) 非热等离子体单独和非热等离子体协同催化苯蒸汽重整的反应机理[71]
Fig.5 (a) Interaction between low temperature plasma and catalysts[70]; (b) Supposed reaction mechanism of benzene steam reforming over NTP alone and NTP-catalytic system[71]
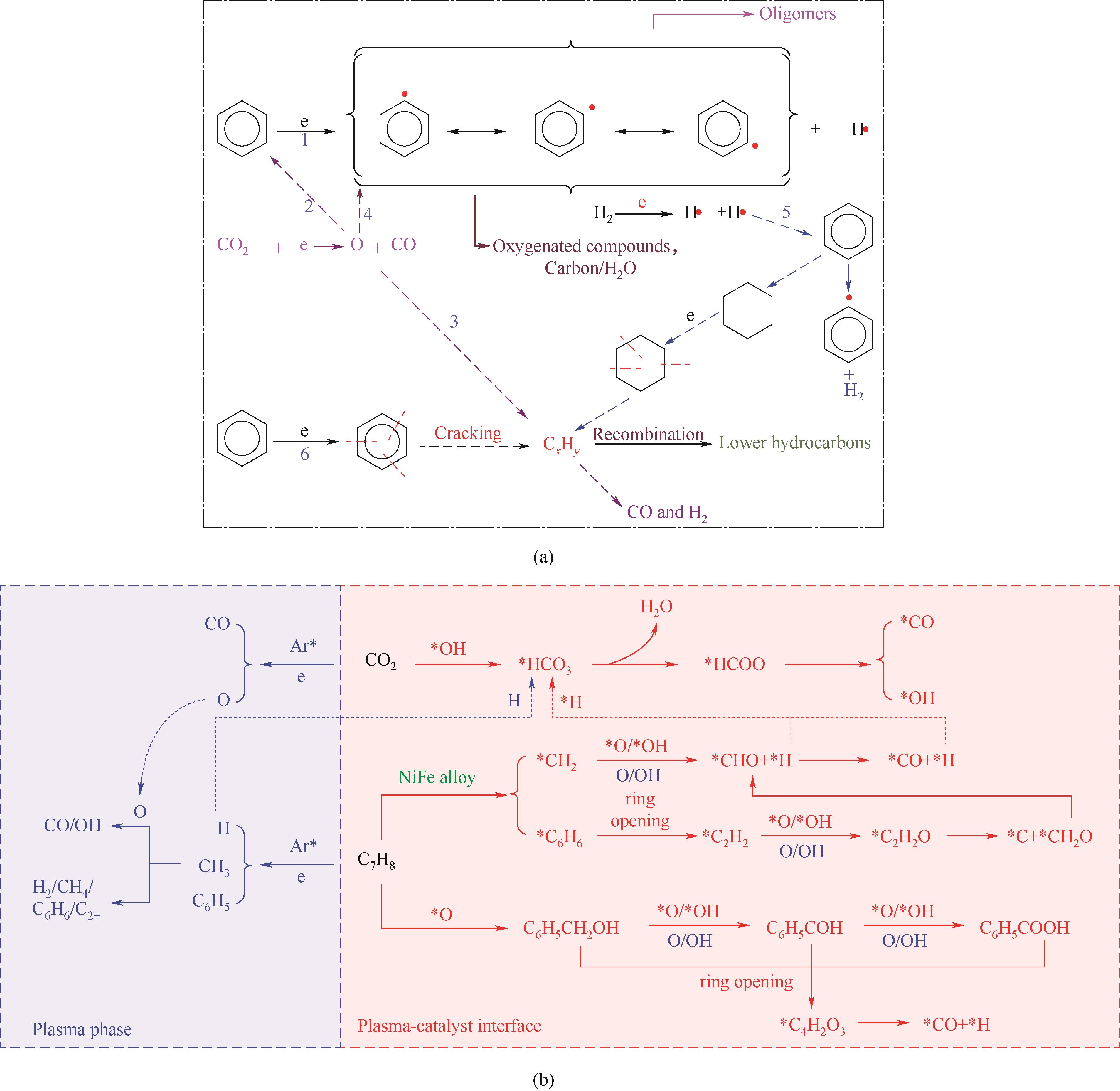
图6 (a)苯降解路径[67]; (b)等离子体协同Ni-Fe/(Mg, Al)O x 催化CO2重整甲苯反应路径[27]
Fig.6 (a) General mechanism for benzene decomposition[67]; (b) Proposed reaction pathway of plasma-catalytic CO2 reforming of toluene reaction over LDH-derived Ni-Fe/(Mg, Al)O x[27]
| 序号 | 国家和地区 | 项目方 | 技术原理 | 垃圾种类 | 产氢效率 | 进展及规模 |
|---|---|---|---|---|---|---|
| 1 | 加拿大 | PyroGenesis 公司 | 等离子体制氢 | 垃圾 | — | 技术研发 |
| 2 | 加拿大渥太华 | OMNI C | 气化和等离子体精炼系统 | 垃圾 | 产生约5000 t负碳氢/a | 200 t/d(67000 t/a) |
| 3 | 美国 | Solena Group 旗下分公司SGH2 | 等离子增强气化 (SPEG) | 垃圾 | 11 t/d 3800 t/a | 每年将能够生产约3800 t氢气,处理42000 t垃圾 |
| 4 | 美国 | 西屋 | 等离子体气化 | 垃圾 | 48 t/d | 在运营 |
| 5 | 卢森堡 | Boson Energy | 等离子体气化 | 垃圾 | 100 kg/t | 研发阶段 |
| 6 | 美国 | startech | 等离子体 | 城市垃圾 | — | 日处理2000 t垃圾 |
| 7 | 中国 | 东方电气集团有限公司 | 等离子体气化 | 城市固废 | — | 1600 t/d |
表3 等离子体制氢国内外示范装置规模和进展
Table 3 Scale and progress of plasma hydrogen demonstration equipment at home and abroad
| 序号 | 国家和地区 | 项目方 | 技术原理 | 垃圾种类 | 产氢效率 | 进展及规模 |
|---|---|---|---|---|---|---|
| 1 | 加拿大 | PyroGenesis 公司 | 等离子体制氢 | 垃圾 | — | 技术研发 |
| 2 | 加拿大渥太华 | OMNI C | 气化和等离子体精炼系统 | 垃圾 | 产生约5000 t负碳氢/a | 200 t/d(67000 t/a) |
| 3 | 美国 | Solena Group 旗下分公司SGH2 | 等离子增强气化 (SPEG) | 垃圾 | 11 t/d 3800 t/a | 每年将能够生产约3800 t氢气,处理42000 t垃圾 |
| 4 | 美国 | 西屋 | 等离子体气化 | 垃圾 | 48 t/d | 在运营 |
| 5 | 卢森堡 | Boson Energy | 等离子体气化 | 垃圾 | 100 kg/t | 研发阶段 |
| 6 | 美国 | startech | 等离子体 | 城市垃圾 | — | 日处理2000 t垃圾 |
| 7 | 中国 | 东方电气集团有限公司 | 等离子体气化 | 城市固废 | — | 1600 t/d |

图7 (a) 等离子体气化与精炼系统; (b) 等离子体热解制氢及其储存、运输和利用示意图(LOHC:液态有机氢载体)[73]
Fig.7 (a) Plasma gasification and refining system; (b) Hydrogen production by plasma pyrolysis. Schematic representation of hydrogen production via plasma pyrolysis and renewable electricity and of its storage and transport and utilization (LOHC, liquid organic hydrogen carrier)[73]
| [1] | Zhang K, Kim W J, Park A A. Alkaline thermal treatment of seaweed for high-purity hydrogen production with carbon capture and storage potential[J]. Nature Communications, 2020, 11(1): 3783. |
| [2] | Budhraja N, Pal A, Mishra R S. Plasma reforming for hydrogen production: pathways, reactors and storage[J]. International Journal of Hydrogen Energy, 2023, 48(7): 2467-2482. |
| [3] | Kim S H, Kumar G, Chen W H, et al. Renewable hydrogen production from biomass and wastes (ReBioH2-2020)[J]. Bioresource Technology, 2021, 331: 125024. |
| [4] | Zhao W J. China's goal of achieving carbon neutrality before 2060: experts explain how[J]. National Science Review, 2022, 9(8): nwac115. |
| [5] | Kang Y Q, Cretu O, Kikkawa J, et al. Mesoporous multimetallic nanospheres with exposed highly entropic alloy sites[J]. Nature Communications, 2023, 14(1): 4182. |
| [6] | Wan C, Li G, Wang J P, et al. Modulating electronic metal-support interactions to boost visible-light-driven hydrolysis of ammonia borane: nickel-platinum nanoparticles supported on phosphorus-doped titania[J]. Angewandte Chemie International Edition, 2023, 62(40): e202305371. |
| [7] | Wang Y J, Huang L, Zhang T Y, et al. Hydrogen-rich syngas production from biomass pyrolysis and catalytic reforming using biochar-based catalysts[J]. Fuel, 2022, 313: 123006. |
| [8] | Dai H C, Dai H M. Green hydrogen production based on the co-combustion of wood biomass and porous media[J]. Applied Energy, 2022, 324: 119779. |
| [9] | Chang Y J, Chang J S, Lee D J. Gasification of biomass for syngas production: research update and stoichiometry diagram presentation[J]. Bioresource Technology, 2023, 387: 129535. |
| [10] | 严宗诚, 陈砺,王红林. 液下辉光放电等离子体重整低碳醇水溶液制氢[J]. 化工学报, 2006, 57(6): 1432-1437. |
| Yan Z C, Chen L, Wang H L. Hydrogen generation from reforming of lower alcohols aqueous solution by glow discharge plasma under liquid[J]. Journal of Chemical Industry and Engineering (China), 2006, 57(6): 1432-1437. | |
| [11] | Holladay J D, Hu J, King D L, et al. An overview of hydrogen production technologies[J]. Catalysis Today, 2009, 139(4): 244-260. |
| [12] | Song Y T, Zou X L, Gong X Z, et al. Realization of thousand-second improved confinement plasma with Super I-mode in Tokamak EAST[J]. Science Advances, 2023, 9(1): eabq5273. |
| [13] | Tanaka Y. Recent development of new inductively coupled thermal plasmas for materials processing[J]. Advances in Physics: X, 2021, 6(1): 1867637. |
| [14] | Wu Z L, Hao X D, Zhou W L, et al. N-pentane activation and products formation in a temperature-controlled dielectric barrier discharge reactor[J]. Plasma Sources Science and Technology, 2018, 27(11): 115002. |
| [15] | Wanten B, Maerivoet S, Vantomme C, et al. Dry reforming of methane in an atmospheric pressure glow discharge: confining the plasma to expand the performance[J]. Journal of CO2 Utilization, 2022, 56: 101869. |
| [16] | Phuong Pham T T, Ro K S, Chen L F, et al. Microwave-assisted dry reforming of methane for syngas production: a review[J]. Environmental Chemistry Letters, 2020, 18(6): 1987-2019. |
| [17] | Zhang X H, Wang Z W, Wu H M, et al. Propulsive effect of microwave-induced plasma jet on spark ignition of CO2-diluted CH4-air mixture[J]. Combustion and Flame, 2021, 229: 111400. |
| [18] | Gautam R, Kumar S, Upadhyayula S. A comprehensive review on recent breakthroughs in hydrogen production from hydrogen sulfide decomposition: harnessing the power of plasma[J]. Renewable and Sustainable Energy Reviews, 2024, 202: 114735. |
| [19] | 丁天英, 刘景林, 赵天亮, 等. 非热等离子体烃类燃料氧化重整反应器的研究进展[J]. 化工学报, 2015, 66(3):872-879. |
| Ding T Y, Liu J L, Zhao T L, et al. Progress of non-thermal plasma reactors for oxidative reforming of hydrocarbon fuel[J]. CIESC Journal, 2015, 66(3): 872-879. | |
| [20] | 王军锋, 张俊杰, 张伟, 等. 液相放电等离子体分解甲醇制氢:电极配置的优化[J]. 化工学报, 2024, 75(9): 3277-3286. |
| Wang J F, Zhang J J, Zhang W, et al. Liquid-phase discharge plasma decomposition of methanol for hydrogen production: optimization of electrode configuration[J]. CIESC Journal, 2024, 75(9): 3277-3286. | |
| [21] | Vadikkeettil Y, Subramaniam Y, Murugan R, et al. Plasma assisted decomposition and reforming of greenhouse gases: a review of current status and emerging trends[J]. Renewable and Sustainable Energy Reviews, 2022, 161: 112343. |
| [22] | Shao S S, Ye Z A, Sun J Y, et al. A review on the application of non-thermal plasma (NTP) in the conversion of biomass: catalyst preparation, thermal utilization and catalyst regeneration[J]. Fuel, 2022, 330: 125420. |
| [23] | Wei R R, Yin K X, Zhang R Q, et al. Techno-economic and thermodynamic analysis of hydrogen production process via plasma co-gasification of coal and biomass[J]. Energy, 2025, 314: 134241. |
| [24] | Wang W T, Ma Y, Chen G X, et al. Enhanced hydrogen production using a tandem biomass pyrolysis and plasma reforming process[J]. Fuel Processing Technology, 2022, 234: 107333. |
| [25] | Elhambakhsh A, Van Duc Long N, Lamichhane P, et al. Recent progress and future directions in plasma-assisted biomass conversion to hydrogen[J]. Renewable Energy, 2023, 218: 119307. |
| [26] | Zheng Y Y, Marek E J, Scott S A. H2 production from a plasma-assisted chemical looping system from the partial oxidation of CH4 at mild temperatures[J]. Chemical Engineering Journal, 2020, 379: 122197. |
| [27] | Liu L N, Dai J, Das S, et al. Plasma-catalytic CO2 reforming of toluene over hydrotalcite-derived NiFe/(Mg, Al)O x Catalysts[J]. JACS Au, 2023, 3(3): 785-800. |
| [28] | 王群, 臧鑫芝, 孙慧慧, 等. Mn基金属有机骨架(MOFs)催化剂制备及介质阻挡放电(DBD)等离子体协同催化降解甲苯[J]. 环境化学, 2023, 42(11): 3767-3778. |
| Wang Q, Zang X Z, Sun H H, et al. Preparation of Mn-based metal-organic frameworks (MOFs) catalysts and its synergistic catalysis on toluene degradation with dielectric barrier discharge (DBD) plasma[J]. Environmental Chemistry, 2023, 42(11): 3767-3778. | |
| [29] | Wang N, Otor H O, Rivera-Castro G, et al. Plasma catalysis for hydrogen production: a bright future for decarbonization[J]. ACS Catalysis, 2024, 14(9): 6749-6798. |
| [30] | Yang G, Hu Q, Hu J H, et al. Hydrogen-rich syngas production from biomass gasification using biochar-based nanocatalysts[J]. Bioresource Technology, 2023, 379: 129005. |
| [31] | Mishra K, Singh Siwal S, Kumar Saini A, et al. Recent update on gasification and pyrolysis processes of lignocellulosic and algal biomass for hydrogen production[J]. Fuel, 2023, 332: 126169. |
| [32] | 刘昊霖. 生物质气化制氢过程中的焦油脱除研究[D]. 杭州: 浙江科技学院, 2021. |
| Liu H L. Study on tar removal in hydrogen production from biomass gasification[D]. Hangzhou: Zhejiang University of Science & Technology, 2021. | |
| [33] | Bridgwater A V. Renewable fuels and chemicals by thermal processing of biomass[J]. Chemical Engineering Journal, 2003, 91(2/3): 87-102. |
| [34] | Zhang L H, Xu C B, Champagne P. Overview of recent advances in thermo-chemical conversion of biomass[J]. Energy Conversion and Management, 2010, 51(5): 969-982. |
| [35] | Ren J, Cao J P, Zhao X Y, et al. Recent advances in syngas production from biomass catalytic gasification: a critical review on reactors, catalysts, catalytic mechanisms and mathematical models[J]. Renewable and Sustainable Energy Reviews, 2019, 116: 109426. |
| [36] | Ashok J, Dewangan N, Das S, et al. Recent progress in the development of catalysts for steam reforming of biomass tar model reaction[J]. Fuel Processing Technology, 2020, 199: 106252. |
| [37] | Gao N B, Salisu J, Quan C, et al. Modified nickel-based catalysts for improved steam reforming of biomass tar: a critical review[J]. Renewable and Sustainable Energy Reviews, 2021, 145: 111023. |
| [38] | Qin T, Yuan S F. Research progress of catalysts for catalytic steam reforming of high temperature tar: a review[J]. Fuel, 2023, 331: 125790. |
| [39] | Plis P, Wilk R K. Theoretical and experimental investigation of biomass gasification process in a fixed bed gasifier[J]. Energy, 2011, 36(6): 3838-3845. |
| [40] | Meng X M, de Jong W, Fu N J, et al. Biomass gasification in a 100 kWth steam-oxygen blown circulating fluidized bed gasifier: effects of operational conditions on product gas distribution and tar formation[J]. Biomass and Bioenergy, 2011, 35(7): 2910-2924. |
| [41] | Rapagnà S, Jand N, Kiennemann A, et al. Steam-gasification of biomass in a fluidised-bed of olivine particles[J]. Biomass and Bioenergy, 2000, 19(3): 187-197. |
| [42] | Kong G, Zhang X, Wang K J, et al. Coupling biomass gasification and inline co-steam reforming: synergistic effect on promotion of hydrogen production and tar removal[J]. Fuel Processing Technology, 2023, 243: 107689. |
| [43] | Liu H B, Chen T H, Zhang X L, et al. Effect of additives on catalytic cracking of biomass gasification tar over a nickel-based catalyst[J]. Chinese Journal of Catalysis, 2010, 31(4): 409-414. |
| [44] | Wu C F, Dupont V, Nahil M A, et al. Investigation of Ni/SiO2 catalysts prepared at different conditions for hydrogen production from ethanol steam reforming[J]. Journal of the Energy Institute, 2017, 90(2): 276-284. |
| [45] | Zhang R Q, Wang Y C, Brown R C. Steam reforming of tar compounds over Ni/olivine catalysts doped with CeO2 [J]. Energy Conversion and Management, 2007, 48(1): 68-77. |
| [46] | Mukai D, Murai Y, Higo T, et al. Effect of Pt addition to Ni/La0.7Sr0.3AlO3- δ catalyst on steam reforming of toluene for hydrogen production[J]. Applied Catalysis A: General, 2014, 471: 157-164. |
| [68] | Wnukowski M, Jamróz P. Microwave plasma treatment of simulated biomass syngas: interactions between the permanent syngas compounds and their influence on the model tar compound conversion[J]. Fuel Processing Technology, 2018, 173: 229-242. |
| [69] | Favas J, Monteiro E, Rouboa A. Hydrogen production using plasma gasification with steam injection[J]. International Journal of Hydrogen Energy, 2017, 42(16): 10997-11005. |
| [70] | Xu J Q, Xia P, Zhang Q, et al. Coke resistance of Ni-based catalysts enhanced by cold plasma treatment for CH4-CO2 reforming: review[J]. International Journal of Hydrogen Energy, 2021, 46(45): 23174-23189. |
| [71] | Pan W, Meng J G, Gu T T, et al. Plasma-catalytic steam reforming of benzene as a tar model compound over Ni-HAP and Ni-γAl2O3 catalysts: insights into the importance of steam and catalyst support[J]. Fuel, 2023, 339: 127327. |
| [72] | Liang W J, Ma L, Liu H, et al. Toluene degradation by non-thermal plasma combined with a ferroelectric catalyst[J]. Chemosphere, 2013, 92(10): 1390-1395. |
| [73] | Chen G X, Tu X, Homm G, et al. Plasma pyrolysis for a sustainable hydrogen economy[J]. Nature Reviews Materials, 2022, 7: 333-334. |
| [47] | Oemar U, Ang M L, Hee W F, et al. Perovskite La x M1- x Ni0.8Fe0.2O3 catalyst for steam reforming of toluene: crucial role of alkaline earth metal at low steam condition[J]. Applied Catalysis B: Environmental, 2014, 148: 231-242. |
| [48] | 孙成伟, 沈洁, 任雪梅, 等. 等离子气化技术用于固体废物处理的研究进展[J]. 物理学报, 2021, 70(9): 72-85. |
| Sun C W, Shen J, Ren X M, et al. Research progress of plasma gasification technology for solid waste treatment[J]. Acta Physica Sinica, 2021, 70(9): 72-85. | |
| [49] | Du C M, Mo J M, Li H X. Renewable hydrogen production by alcohols reforming using plasma and plasma-catalytic technologies: challenges and opportunities[J]. Chemical Reviews, 2015, 115(3): 1503-1542. |
| [50] | Aleknaviciute I, Karayiannis T G, Collins M W, et al. Methane decomposition under a corona discharge to generate CO x -free hydrogen[J]. Energy, 2013, 59: 432-439. |
| [51] | Zhu X B, Gao X, Qin R, et al. Plasma-catalytic removal of formaldehyde over Cu-Ce catalysts in a dielectric barrier discharge reactor[J]. Applied Catalysis B: Environmental, 2015, 170: 293-300. |
| [52] | Guo Y F, Ye D Q, Chen K F, et al. Toluene removal by a DBD-type plasma combined with metal oxides catalysts supported by nickel foam[J]. Catalysis Today, 2007, 126(3/4): 328-337. |
| [53] | Wang T, Chen S, Wang H Q, et al. In-plasma catalytic degradation of toluene over different MnO2 polymorphs and study of reaction mechanism[J]. Chinese Journal of Catalysis, 2017, 38(5): 793-803. |
| [54] | Liu L N, Wang Q, Ahmad S, et al. Steam reforming of toluene as model biomass tar to H2-rich syngas in a DBD plasma-catalytic system[J]. Journal of the Energy Institute, 2018, 91(6): 927-939. |
| [55] | Zeng Y X, Zhu X B, Mei D H, et al. Plasma-catalytic dry reforming of methane over γ-Al2O3 supported metal catalysts[J]. Catalysis Today, 2015, 256: 80-87. |
| [56] | Huang X Y, Cheng D G, Chen F Q, et al. Reaction pathways of hemicellulose and mechanism of biomass pyrolysis in hydrogen plasma: a density functional theory study[J]. Renewable Energy, 2016, 96: 490-497. |
| [57] | Qi H Q, Xu H W, Zhang J F, et al. Thermodynamic and techno-economic analyses of hydrogen production from different algae biomass by plasma gasification[J]. International Journal of Hydrogen Energy, 2023, 48(92): 35895-35906. |
| [58] | 石秀娟, 梁文俊, 尹国彬, 等. 低温等离子体协同Mn基催化剂降解氯苯研究[J]. 化工学报, 2022, 73(10): 4472-4483. |
| Shi X J, Liang W J, Yin G B, et al. Degradation of chlorobenzene by non-thermal plasma with Mn based catalyst[J]. CIESC Journal, 2022, 73(10): 4472-4483. | |
| [59] | Zhou Y, Chu R Z, Fan L L, et al. Conversion mechanism of thermal plasma-enhanced CH4-CO2 reforming system to syngas under the non-catalytic conditions[J]. Science of the Total Environment, 2023, 866: 161453. |
| [60] | Neyts E C, Ostrikov K K, Sunkara M K, et al. Plasma catalysis: synergistic effects at the nanoscale[J]. Chemical Reviews, 2015, 115(24): 13408-13446. |
| [61] | Indrawan N, Mohammad S, Kumar A, et al. Modeling low temperature plasma gasification of municipal solid waste[J]. Environmental Technology & Innovation, 2019, 15: 100412. |
| [62] | Tamošiūnas A, Valatkevičius P, Valinčius V, et al. Biomass conversion to hydrogen-rich synthesis fuels using water steam plasma[J]. Comptes Rendus Chimie, 2016, 19(4): 433-440. |
| [63] | Wang C, Liu T, Xiao R, et al. High-purity hydrogen obtained via a plasma-assisted chemical looping process using perovskite-supported iron oxides as oxygen carriers[J]. Energy & Fuels, 2023, 37(18): 14141-14149. |
| [64] | Tamošiūnas A, Gimžauskaitė D, Aikas M, et al. Biomass gasification to syngas in thermal water vapor arc discharge plasma[J]. Biomass Conversion and Biorefinery, 2023, 13(18): 16373-16384. |
| [65] | 余淼霏, 杜胜男, 米俊锋, 等. 低温等离子体协同催化处理VOCs的研究进展[J]. 环境工程, 2022, 40(8): 213-219, 212. |
| Yu M F, Du S N, Mi J F, et al. Research progress of low-temperature plasma synergistic catalytic treatment of VOCs [J]. Environmental Engineering, 2022, 40(8): 213-219, 212. | |
| [66] | 叶凯, 刘香华, 姜月, 等. 低温等离子体协同CeO2/13X催化降解甲苯[J]. 化工学报, 2021, 72(7): 3706-3715. |
| Ye K, Liu X H, Jiang Y, et al. Combing low-temperature plasma with CeO2/13X for toluene degradation[J]. CIESC Journal, 2021, 72(7): 3706-3715. | |
| [67] | Saleem F, Zhang K, Harvey A P. Decomposition of benzene as a tar analogue in CO2 and H2 carrier gases, using a non-thermal plasma[J]. Chemical Engineering Journal, 2019, 360: 714-720. |
| [1] | 张涵川, 尚超, 吕文祥, 黄德先, 张亚宁. 基于无监督时序聚类的催化裂化装置工况划分识别与产率预测方法[J]. 化工学报, 2025, 76(6): 2781-2790. |
| [2] | 何军, 李勇, 赵楠, 何孝军. 碳负载硒掺杂硫化钴在锂硫电池中的性能研究[J]. 化工学报, 2025, 76(6): 2995-3008. |
| [3] | 宋粉红, 王文光, 郭亮, 范晶. C元素修饰g-C3N4对TiO2的调控及复合材料光催化产氢性能研究[J]. 化工学报, 2025, 76(6): 2983-2994. |
| [4] | 王智超, 刘冬妹, 熊敏, 周利, 吉旭, 党亚固. 可再生能源发电制氢与炼油企业氢气网络耦合系统的多周期调度优化[J]. 化工学报, 2025, 76(6): 2802-2812. |
| [5] | 张畅, 解强, 沙雨桐, 王炳杰, 梁鼎成, 刘金昌. 低灰低硅竹炭的制备及衍生硬炭的电化学性能[J]. 化工学报, 2025, 76(6): 3073-3083. |
| [6] | 李愽龙, 蒋雨希, 任傲天, 秦雯琪, 傅杰, 吕秀阳. TS-1/In-TS-1催化果糖一步法醇解制备乳酸甲酯连续化试验[J]. 化工学报, 2025, 76(6): 2678-2686. |
| [7] | 杨盛华, 孙阳杰, 薛晓君, 米杰, 王建成, 冯宇. 缺陷型金属氧化物脱除气体污染物研究进展[J]. 化工学报, 2025, 76(6): 2469-2482. |
| [8] | 卢丽丽, 李晨, 陈柳云, 谢新玲, 罗轩, 苏通明, 秦祖赠, 纪红兵. BiOBr的形貌调控及其光催化CO2还原性能的研究[J]. 化工学报, 2025, 76(6): 2687-2700. |
| [9] | 龚丽芳, 任美慧, 蒋吉春, 郭光召, 胡红云, 黄永达, 姚洪. 垃圾焚烧烟气中芳香烃化合物在线监测和选择性催化还原脱除研究[J]. 化工学报, 2025, 76(6): 3018-3028. |
| [10] | 王一非, 任婧杰, 毕明树, 叶昊天. 基于本质安全与经济性的环己烷氧化工艺参数多目标优化研究[J]. 化工学报, 2025, 76(6): 2722-2732. |
| [11] | 茅雨洁, 路晓飞, 锁显, 杨立峰, 崔希利, 邢华斌. 工业气体中微量氧深度脱除催化剂研究进展[J]. 化工学报, 2025, 76(5): 1997-2010. |
| [12] | 刘璐, 万开, 王文玥, 王太, 汤建成, 王少恒. 基于氦膨胀制冷的正仲氢转化耦合流动换热研究[J]. 化工学报, 2025, 76(4): 1513-1522. |
| [13] | 张静, 元跃, 刘艳梅, 王智文, 陈涛. 生物法制备衣康酸研究进展[J]. 化工学报, 2025, 76(3): 909-921. |
| [14] | 戴文智, 沈雄健, 宋晓博, 杨新乐. 生物质双级蒸发双回热有机朗肯循环系统环境分析[J]. 化工学报, 2025, 76(3): 1230-1242. |
| [15] | 万俊, 宋佳芮, 范春煌, 魏乐乐, 聂依娜, 刘琳. 高效空穴转移助力光催化碱性甲醇-水溶液制氢[J]. 化工学报, 2025, 76(3): 1064-1075. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号