化工学报 ›› 2025, Vol. 76 ›› Issue (5): 2087-2100.DOI: 10.11949/0438-1157.20241307
收稿日期:2024-11-15
修回日期:2025-02-03
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
马敬,刘毅
作者简介:张越(2000—),男,博士研究生,zhangyue201941029@mail.dlut.edu.cn
基金资助:
Yue ZHANG1( ), Jiaxin LIU2,3, Jing MA2,3(
), Jiaxin LIU2,3, Jing MA2,3( ), Yi LIU1(
), Yi LIU1( )
)
Received:2024-11-15
Revised:2025-02-03
Online:2025-05-25
Published:2025-06-13
Contact:
Jing MA, Yi LIU
摘要:
海洋中约有45亿吨铀,能够满足全球核工业生产千年以上的可持续生产需求,因此海水提铀被认为是改变世界的化学分离技术之一;膜分离因效率高、能耗低、无污染等特点,被广泛用于海水提铀。金属有机骨架(MOF)由于其可变的孔道结构、丰富的活性位点、化学修饰多样性,作为膜材料在海水提铀应用领域前景广阔。综述了将膜分离技术应用于海水提铀的最新研究进展与未来发展方向,系统总结了采用MOF膜实现海水提铀的分离机理与面临的挑战。
中图分类号:
张越, 刘佳鑫, 马敬, 刘毅. 金属有机骨架膜应用于海水提铀研究进展[J]. 化工学报, 2025, 76(5): 2087-2100.
Yue ZHANG, Jiaxin LIU, Jing MA, Yi LIU. Recent progress on metal-organic framework membranes towards uranium separation from seawater[J]. CIESC Journal, 2025, 76(5): 2087-2100.
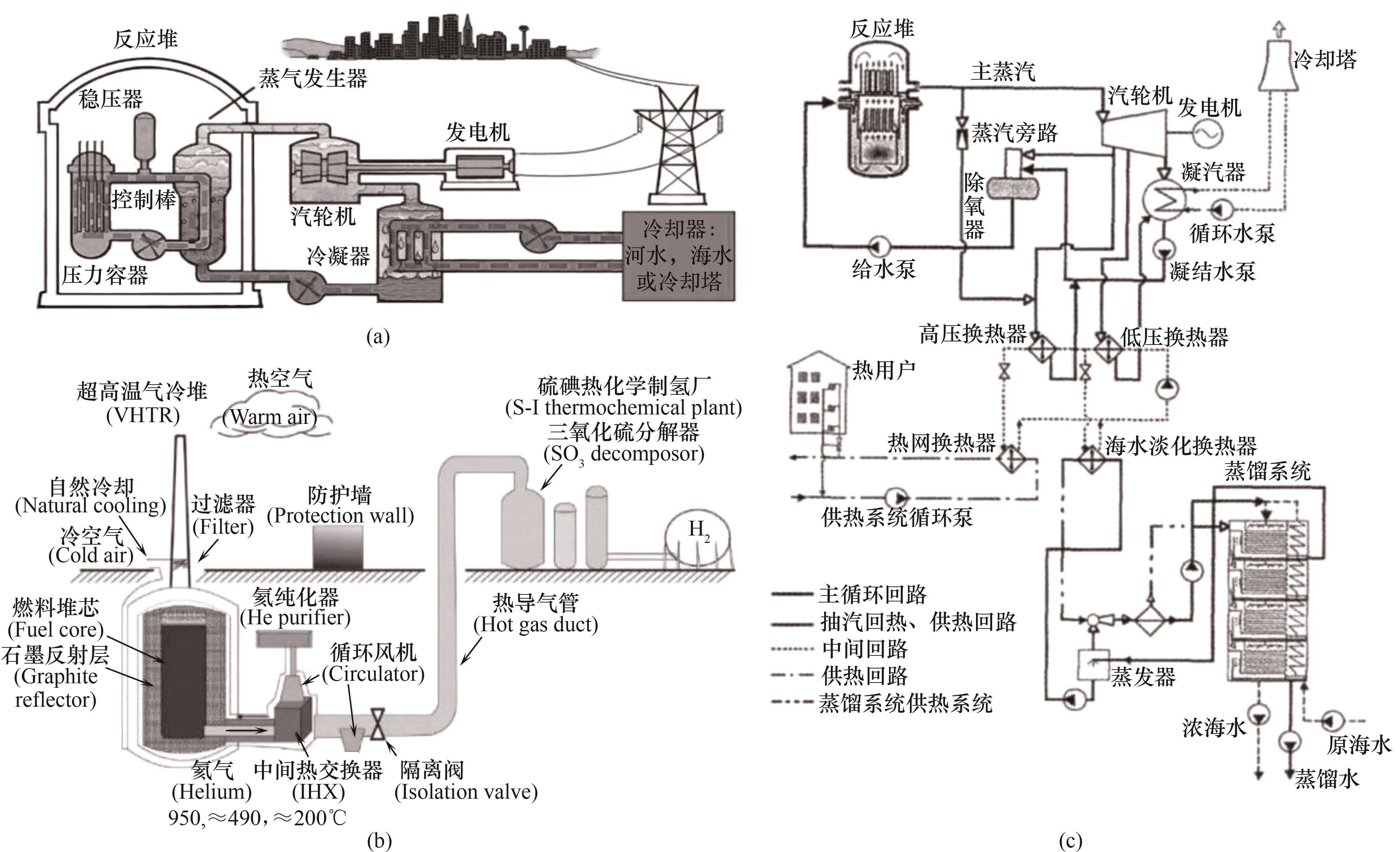
图1 (a)核能发电;(b)核能制氢;(c)核能海水淡化[3]
Fig.1 (a) Nuclear power for electricity generation; (b) Nuclear power for hydrogen production; (c) Nuclear power for seawater desalination[3]
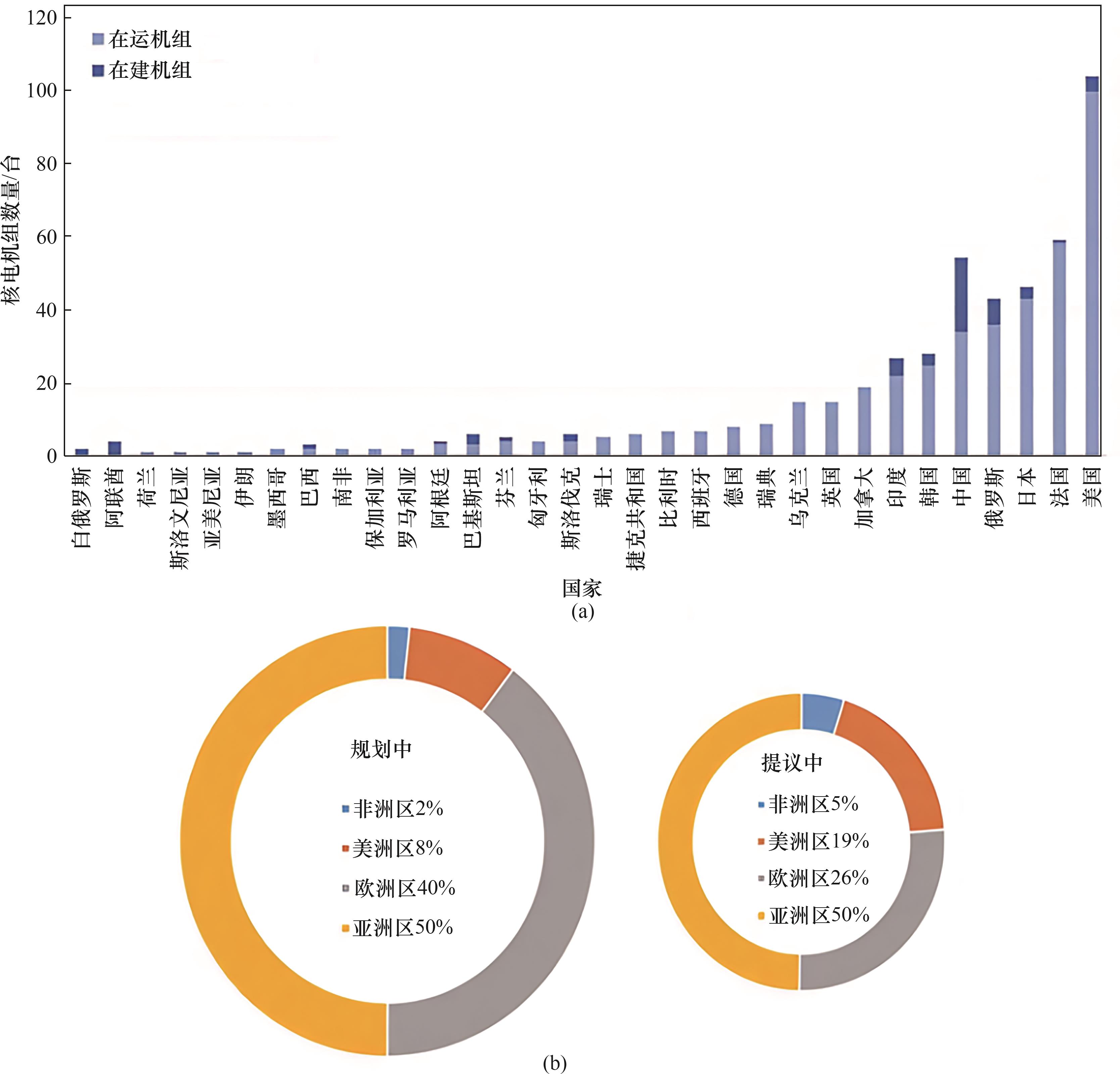
图2 (a)世界核电机组发展需求;(b)世界核电发展需求区域分布[3]
Fig.2 (a) World nuclear power unit development requirements; (b) World nuclear power development requirements in the regional distribution[3]
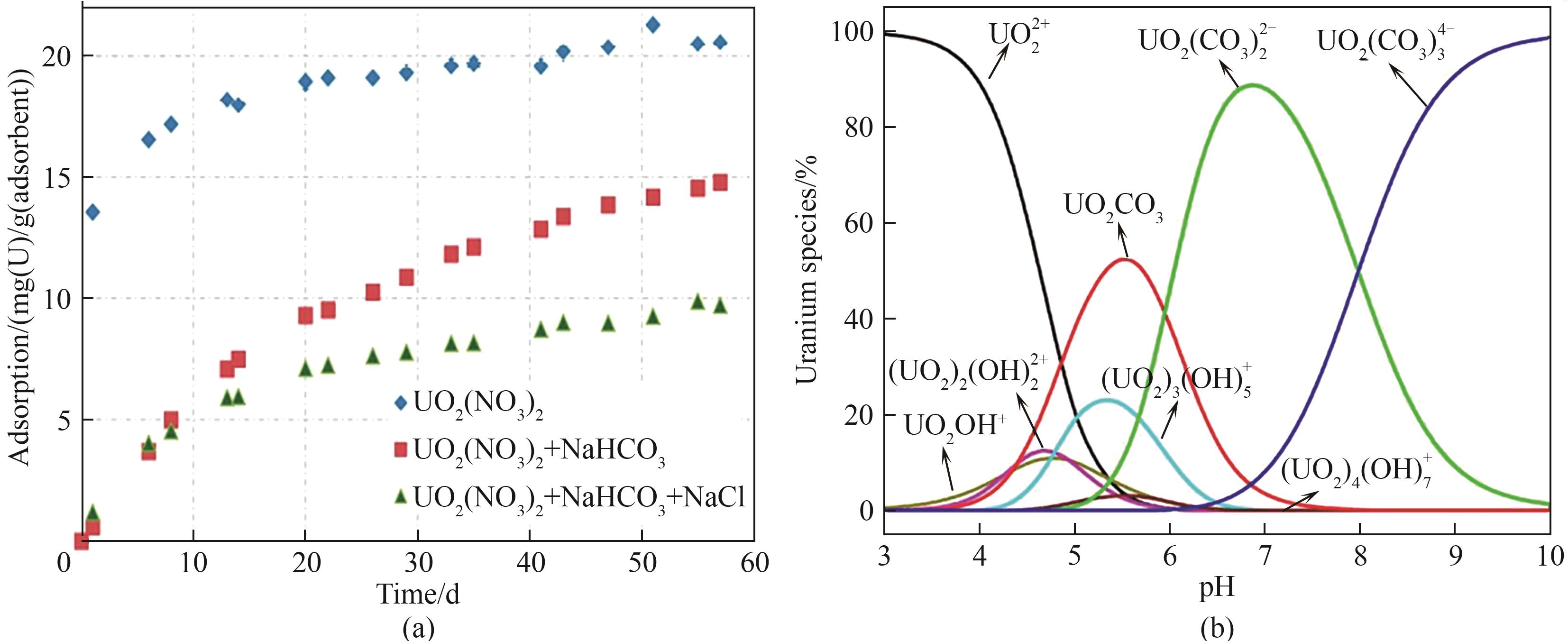
图4 (a)不同盐度对吸附剂铀吸收量的影响[42];(b) U(Ⅵ)在不同pH范围内的分布情况[42, 49]
Fig.4 (a) Effect of different salinities on the absorption of uranium by adsorbents[42]; (b) Distribution of U(Ⅵ) in different pH ranges[42, 49]
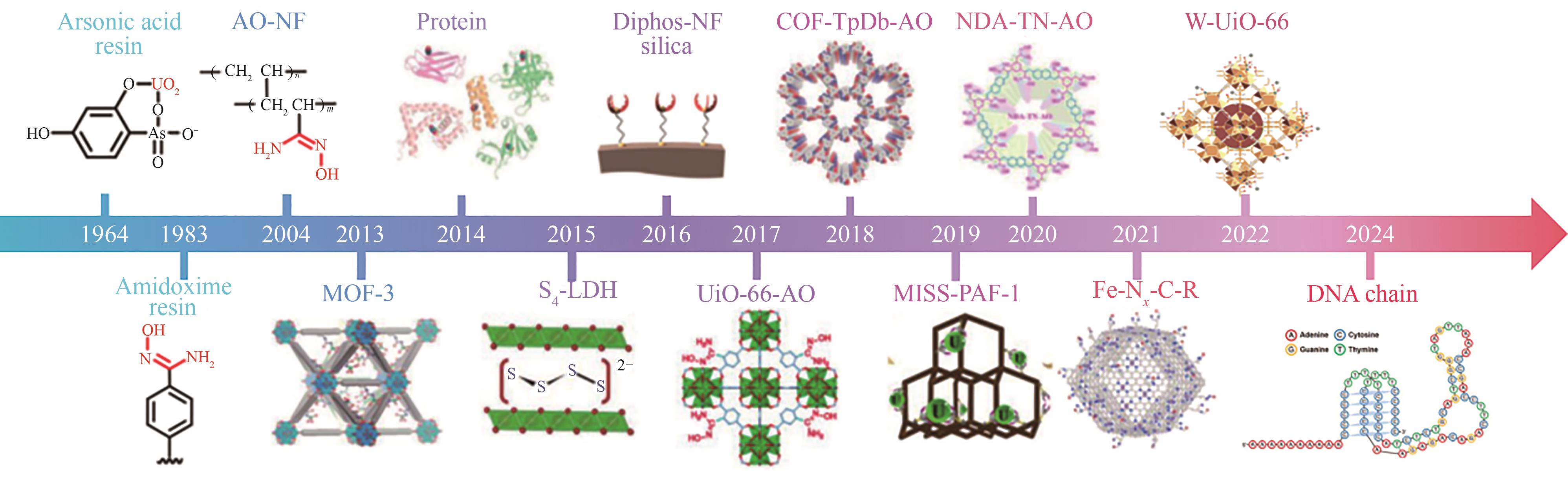
图5 1964年以来曾用于海水提铀的功能材料发展时间线[20, 39,57]
Fig.5 Timeline of the development of functional materials that have been used in seawater separation of uranium since 1964[20, 39,57]
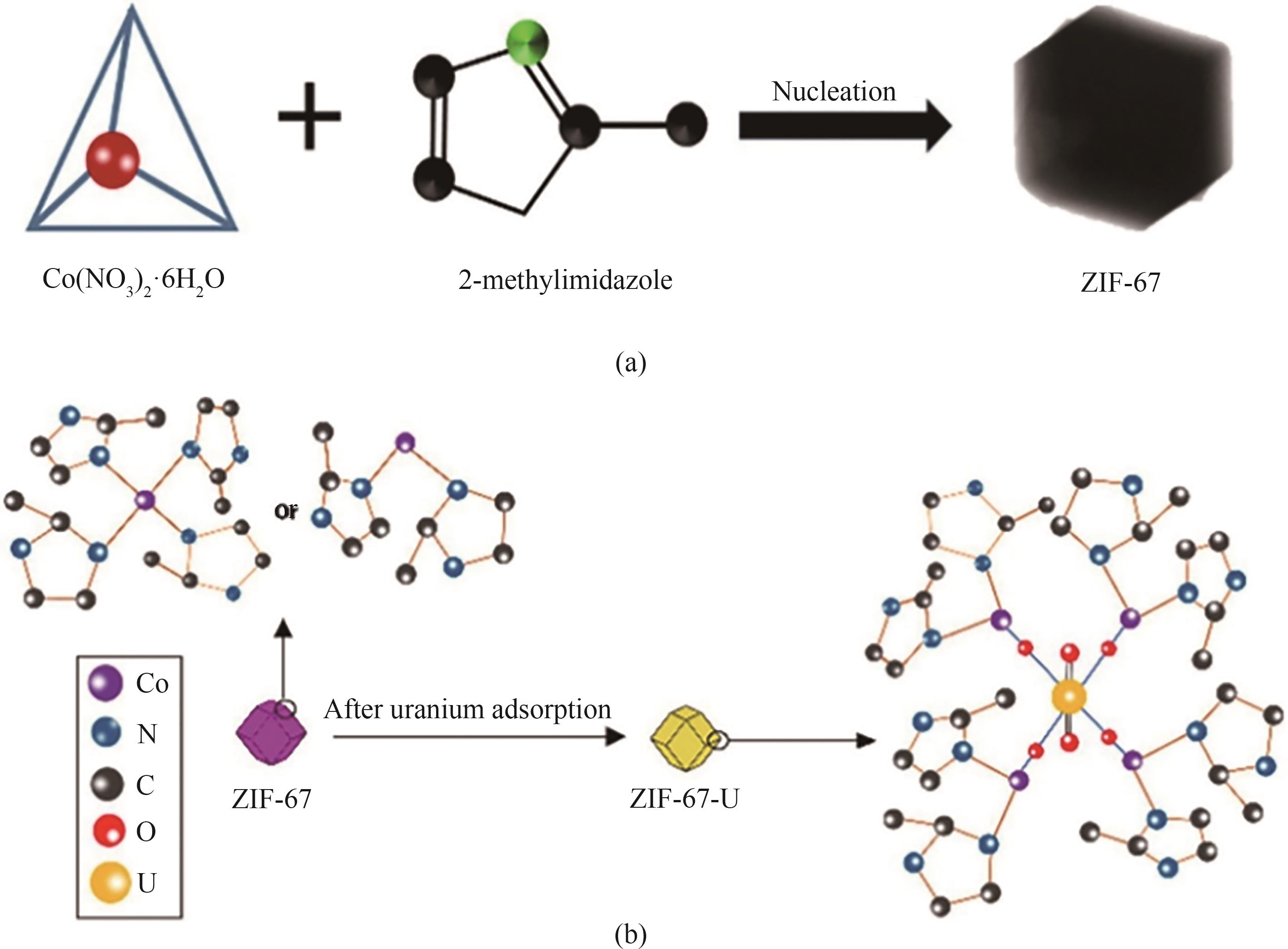
图6 (a)ZIF-67合成;(b) ZIF-67吸附分离U(Ⅵ)的机理示意图[60]
Fig.6 (a) Synthesis of ZIF-67; (b) Schematic of the mechanism for U(Ⅵ) separation by ZIF-67 adsorption[60]

图7 (a)UiO-66@PAO膜制备流程示意图;(b)UiO-66@PAO化学结构以及UiO-66@PAO MOF基膜选择性吸附铀的过程示意图[67]
Fig.7 (a) Schematic diagram of the preparation process for UiO-66@PAO membrane; (b) Schematic diagram of the chemical structure for UiO-66@PAO and the process of selective adsorption of uranium by UiO-66@PAO MOF-based membrane[67]
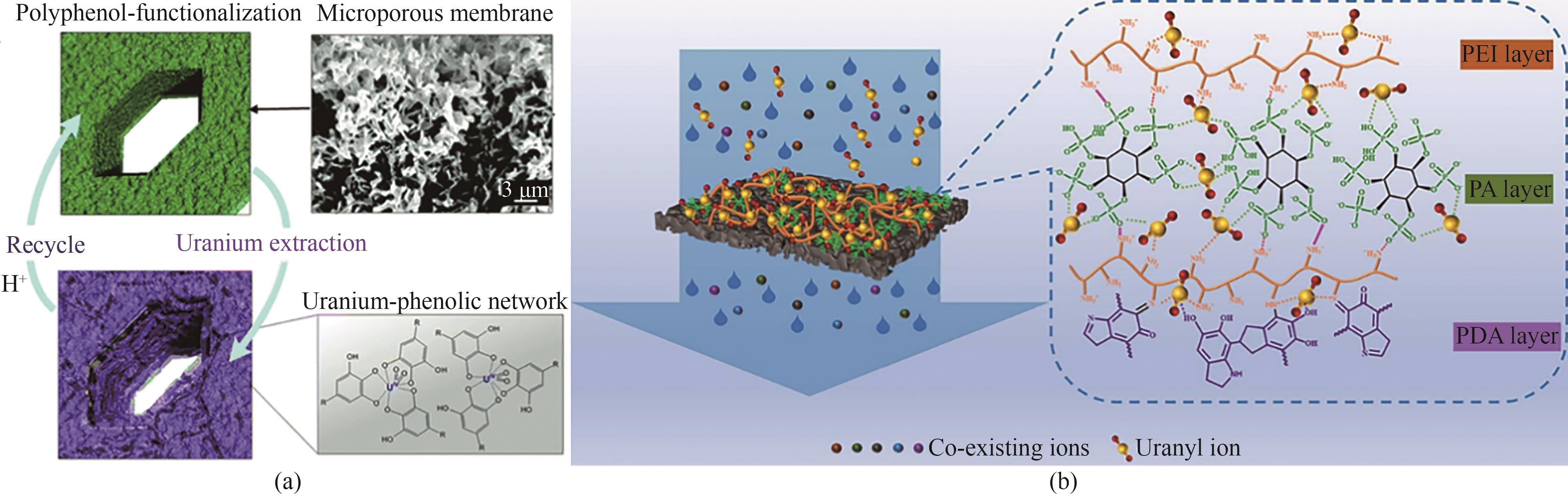
图8 (a)微孔聚酰胺膜的SEM和U(Ⅵ)捕获和释放示意图[78];(b)LAM膜在天然海水中U(Ⅵ)捕获示意图[80]
Fig.8 (a) Schematic of SEM and U(Ⅵ) capture and release by microporous polyamide membranes[78]; (b) Schematic of U(Ⅵ) capture by LAM membranes in natural seawater[80]
| 离子(分子)种类 | 水合离子(分子)直径/Å |
|---|---|
| 11.6 | |
| 9.1 | |
| 8.6 | |
| 8.2 | |
| 7.2 | |
| 6.6 | |
| 6.6 | |
| 2.8 |
表1 天然海水中UO22+、Fe3+、Ca2+、Mg2+、Na+、K+、Cl-、H2O的尺寸[88-90]
Table 1 Size of UO22+,Fe3+,Ca2+,Mg2+,Na+,K+,Cl-,H2O in the seawater[88-90]
| 离子(分子)种类 | 水合离子(分子)直径/Å |
|---|---|
| 11.6 | |
| 9.1 | |
| 8.6 | |
| 8.2 | |
| 7.2 | |
| 6.6 | |
| 6.6 | |
| 2.8 |
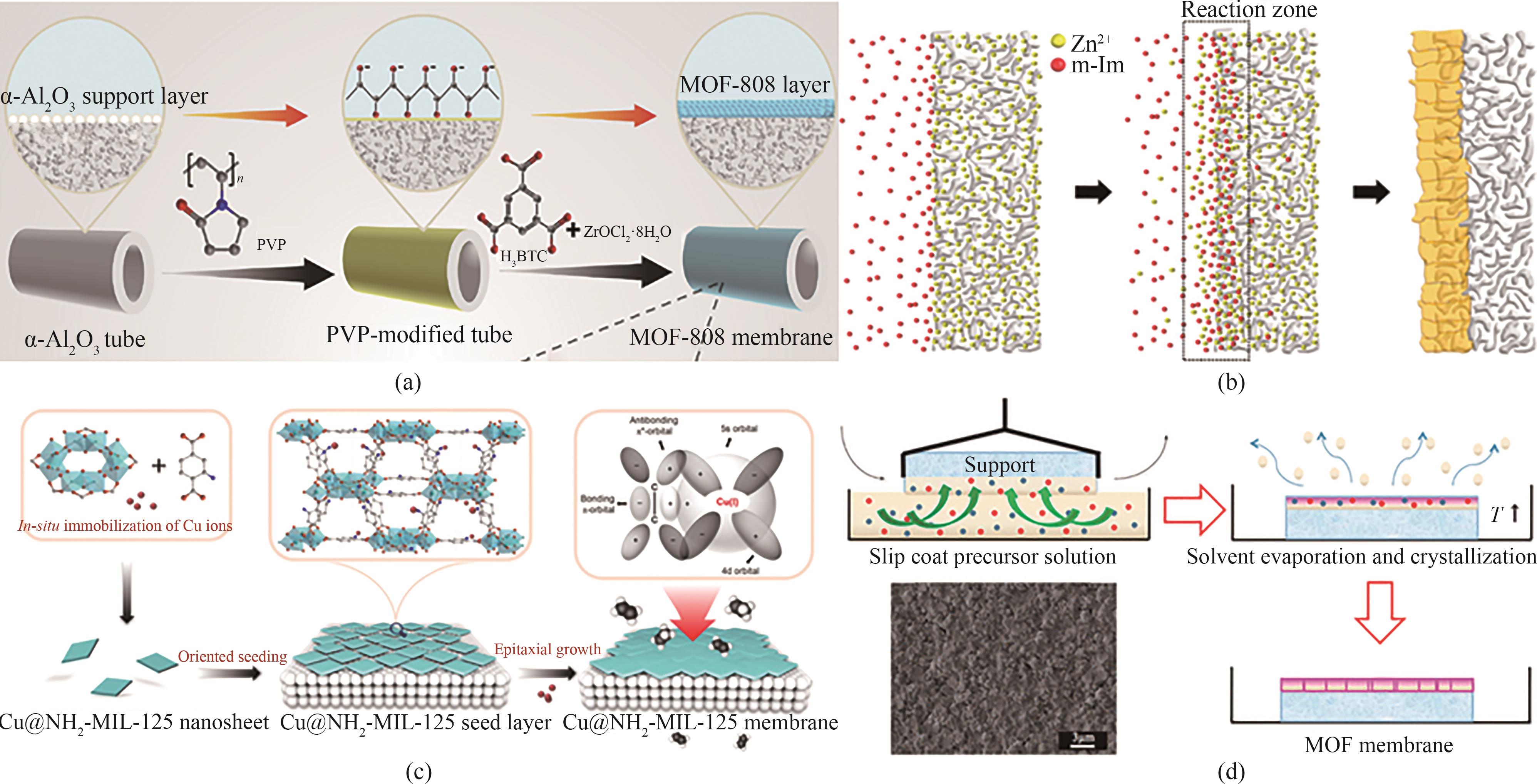
图10 (a)直接生长法制备MOF-808膜示意图[91];(b)反向扩散界面生长法制备ZIF-8膜示意图[93];(c)二次生长法制备MIL-125(Cu)膜示意图[92];(d)快速热沉积法制备ZIF-8膜示意图[94]
Fig.10 (a) Schematic diagram of MOF-808 membrane prepared by direct growth method[91]; (b) Schematic diagram of ZIF-8 membrane prepared by reverse diffusion interfacial growth method[93]; (c) Schematic diagram of MIL-125(Cu) membrane prepared by secondary growth method[92]; (d) Schematic diagram of ZIF-8 membrane prepared by rapid thermal deposition method[94]
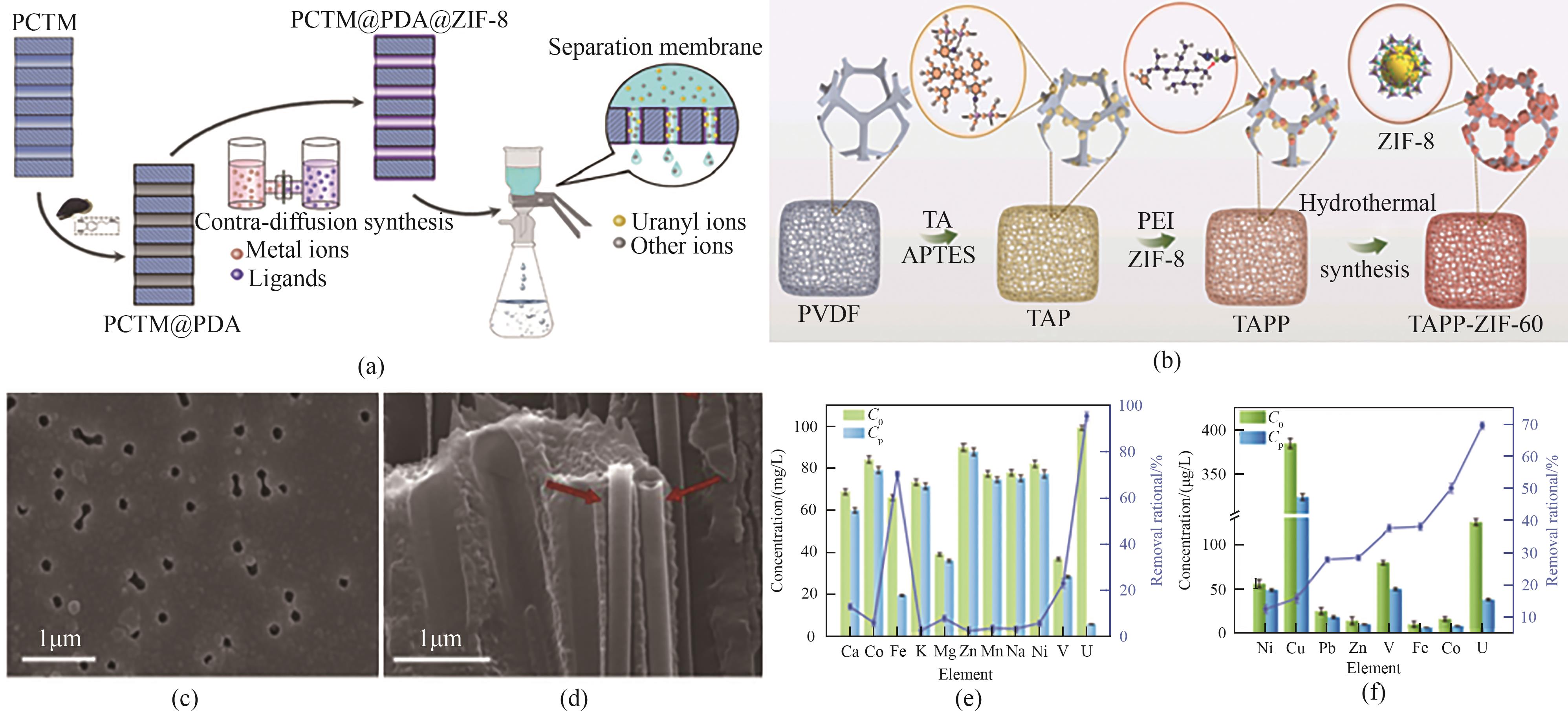
图11 (a)ZIF-8-PCTM膜制备及分离示意图;(b)TAPP-ZIF-60膜制备流程示意图;(c) ZIF-8-PCTM膜表面SEM图像;(d) ZIF-8-PCTM膜界面SEM图像[95];(e)模拟海水中高浓度共存离子对TAPP-ZIF-60铀截留率的影响;(f)天然海水中高浓度共存离子对TAPP-ZIF-60铀截留率的影响[96]
Fig.11 (a) Schematic diagram of ZIF-8-PCTM membrane preparation and separation; (b) Schematic diagram of the preparation process of TAPP-ZIF-60 membrane; (c) SEM image of the surface of ZIF-8-PCTM membrane; (d) SEM image of the interface of ZIF-8-PCTM membrane[95]; (e) Effect of high concentration of coexisting ions in simulated seawater on the uranium rejection rate of TAPP-ZIF-60; (f) Effect of high concentration of co-existing ions in natural seawater on the uranium rejection rate of TAPP-ZIF-6[96]
| 序号 | 材料 | 吸附容量/(mg/g) | 时间/h | 方法 | 文献 |
|---|---|---|---|---|---|
| 1 | MOF-808-PO | 100 | 0.33 | adsorption | |
| 2 | MOF-3 | 109 | 2.50 | adsorption | |
| 3 | NU-1000-PO | 110 | 0.33 | adsorption | |
| 4 | Zn(HBTC)(L)(H2O)2 | 115 | 0.08 | adsorption | |
| 5 | Co-SLUG-35 | 118 | 4.00 | adsorption | |
| 6 | MOF-2 | 217 | 1.00 | adsorption | |
| 7 | MOF-5 | 237.0 | 0.08 | adsorption | |
| 8 | PCN-222-PA | 401.6 | 0.50 | adsorption | |
| 9 | MUU | 475 | 4.00 | adsorption | |
| 10 | GZA | 602.41 | 2.00 | adsorption | |
| 11 | HKUST-1 | 840.3 | 2.00 | adsorption | |
| 12 | ZIF-67 | 1683.8 | 2.50 | adsorption |
表2 MOF膜在模拟海水中对U(Ⅵ)的分离性能
Table 2 Separation performance of MOF membranes for U(Ⅵ) in simulated seawater
| 序号 | 材料 | 吸附容量/(mg/g) | 时间/h | 方法 | 文献 |
|---|---|---|---|---|---|
| 1 | MOF-808-PO | 100 | 0.33 | adsorption | |
| 2 | MOF-3 | 109 | 2.50 | adsorption | |
| 3 | NU-1000-PO | 110 | 0.33 | adsorption | |
| 4 | Zn(HBTC)(L)(H2O)2 | 115 | 0.08 | adsorption | |
| 5 | Co-SLUG-35 | 118 | 4.00 | adsorption | |
| 6 | MOF-2 | 217 | 1.00 | adsorption | |
| 7 | MOF-5 | 237.0 | 0.08 | adsorption | |
| 8 | PCN-222-PA | 401.6 | 0.50 | adsorption | |
| 9 | MUU | 475 | 4.00 | adsorption | |
| 10 | GZA | 602.41 | 2.00 | adsorption | |
| 11 | HKUST-1 | 840.3 | 2.00 | adsorption | |
| 12 | ZIF-67 | 1683.8 | 2.50 | adsorption |
| 序号 | 材料 | 吸附容量 | 循环次数 | 方法 | 文献 |
|---|---|---|---|---|---|
| 1 | ZIF-8 | 62.30 mg/g | 7 | adsorption | |
| 2 | SSUP | 12.33 mg/g | 6 | adsorption | |
| 3 | W-UiO-66 | 6.20 mg/g | 5 | adsorption | |
| 3 | UiO-66-NH2@CS-PDA | 5.52 mg/g | 5 | adsorption | |
| 4 | UiO-66-NH2 | 5.52 mg/g | 10 | adsorption | |
| 5 | UiO-66-AO | 5.20 mg/g | 4 | adsorption | |
| 6 | ZIF-90-ABOA | 2.80 mg/g | 5 | adsorption | |
| 7 | HF-PEI-GDAC | 3.34 μg/L | 5 | adsorption | |
| 8 | MIL-101-AO | 3.30 μg/L | 5 | adsorption |
表3 MOF膜在天然海水中对U(Ⅵ)的分离性能及循环次数
Table 3 Separation performance and cycle times of MOF membranes for U(Ⅵ) in natural seawater
| 序号 | 材料 | 吸附容量 | 循环次数 | 方法 | 文献 |
|---|---|---|---|---|---|
| 1 | ZIF-8 | 62.30 mg/g | 7 | adsorption | |
| 2 | SSUP | 12.33 mg/g | 6 | adsorption | |
| 3 | W-UiO-66 | 6.20 mg/g | 5 | adsorption | |
| 3 | UiO-66-NH2@CS-PDA | 5.52 mg/g | 5 | adsorption | |
| 4 | UiO-66-NH2 | 5.52 mg/g | 10 | adsorption | |
| 5 | UiO-66-AO | 5.20 mg/g | 4 | adsorption | |
| 6 | ZIF-90-ABOA | 2.80 mg/g | 5 | adsorption | |
| 7 | HF-PEI-GDAC | 3.34 μg/L | 5 | adsorption | |
| 8 | MIL-101-AO | 3.30 μg/L | 5 | adsorption |
| 1 | Liu Z Y, Xie Y, Wang Y F, et al. Recent advances in sorbent materials for uranium extraction from seawater[J]. Journal of Tsinghua University, 2021, 61(4): 279-301. |
| 2 | Dan W. Interpretation of the 13th Five-Year Development Plan in support of nuclear power development[J]. Chinese Nuclear Power, 2017, 10(2): 157-160. |
| 3 | 苏宏, 陈新. 核能综合利用的理论现状及发展前景探讨[J]. 东方电气评论, 2024, 38(1): 68-73. |
| Su H, Chen X. Discussion on the status and prospect of comprehensive utilization of nuclear energy[J]. Dongfang Electric Review, 2024, 38(1): 68-73. | |
| 4 | Li J, Tuo K, Fan C, et al. Hierarchical porous amidoximated metal-organic framework for highly efficient uranium extraction[J]. Small, 2024, 20(13): 2306545. |
| 5 | Ding L, Wan X Y, Zheng B W, et al. One-pot synthesis of a graphene oxide-supported Ti x Al1- x O y -based material modified with amidoxime for highly efficient uranium(Ⅵ) adsorption[J]. Journal of Materials Chemistry A, 2024, 12(14): 8381-8391. |
| 6 | Yu F T, Li C Y, Li W R, et al. π-skeleton tailoring of olefin-linked covalent organic frameworks achieving low exciton binding energy for photo-enhanced uranium extraction from seawater[J]. Advanced Functional Materials, 2024, 34(1): 2307230. |
| 7 | Chu J, Huang Q G, Dong Y H, et al. Enrichment of uranium in seawater by glycine cross-linked graphene oxide membrane[J]. Chemical Engineering Journal, 2022, 444: 136602. |
| 8 | The nuclear fuel report: expanded summary—Global scenarios for demand and supply availability 2021—2040[R]. World Nuclear Association, 2022. |
| 9 | Abney C W, Mayes R T, Saito T, et al. Materials for the recovery of uranium from seawater[J]. Chemical Reviews, 2017, 117(23): 13935-14013. |
| 10 | Zhao C X, Liu J N, Li B Q, et al. Multiscale construction of bifunctional electrocatalysts for long-lifespan rechargeable zinc-air batteries[J]. Advanced Functional Materials, 2020, 30(36): 2003619. |
| 11 | Ye X X, Chi R Y, Wu Z H, et al. A biomass fiber adsorbent grafted with phosphate/amidoxime for efficient extraction of uranium from seawater by synergistic effect[J]. Journal of Environmental Management, 2023, 337: 117658. |
| 12 | Song Y C, Deng B L, Wang K, et al. Highly-efficient adsorbent materials for uranium extraction from seawater[J]. Journal of Environmental Chemical Engineering, 2024, 12(5): 113967. |
| 13 | Kuo L J, Pan H B, Wai C M, et al. Investigations into the reusability of amidoxime-based polymeric adsorbents for seawater uranium extraction[J]. Industrial & Engineering Chemistry Research, 2017, 56(40): 11603-11611. |
| 14 | Li Y, Zheng Y J, Ahamd Z, et al. Strategies for designing highly efficient adsorbents to capture uranium from seawater[J]. Coordination Chemistry Reviews, 2023, 491: 215234. |
| 15 | Chang X, Hu P Z, Liu H L, et al. ZIF-8 modified graphene oxide/sodium alginate 3D elastic spheres for uranium trapping in seawater[J]. Desalination, 2023, 549: 116371. |
| 16 | Zhao S L, Feng T T, Cao M, et al. Ferrocene-based 2D metal-organic framework nanosheet for highly efficient photocatalytic seawater uranium recovery[J]. Chemical Engineering Journal, 2024, 498: 155228. |
| 17 | Liu T, Gu A P, Wei T, et al. Ligand-assistant iced photocatalytic reduction to synthesize atomically dispersed Cu implanted metal-organic frameworks for photo-enhanced uranium extraction from seawater[J]. Small, 2023, 19(26): 2208002. |
| 18 | Chen C, Fei L Y, Wang B Y, et al. MOF-based photocatalytic membrane for water purification: a review[J]. Small, 2024, 20(1): 2305066. |
| 19 | Ma X J, Meihaus K R, Yang Y J, et al. Photocatalytic extraction of uranium from seawater using covalent organic framework nanowires[J]. Journal of the American Chemical Society, 2024, 146(33): 23566-23573. |
| 20 | Wang W, Luo Q, Li J Y, et al. Single-atom tungsten engineering of MOFs with biomimetic antibiofilm activity toward robust uranium extraction from seawater[J]. Chemical Engineering Journal, 2022, 431: 133483. |
| 21 | Zhang B T, Shan X H, Yu J Q, et al. Facile synthesis of TiO2-PAN photocatalytic membrane with excellent photocatalytic performance for uranium extraction from seawater[J]. Separation and Purification Technology, 2024, 328: 125026. |
| 22 | Yu F T, Zhu Z Q, Wang S P, et al. Tunable perylene-based donor-acceptor conjugated microporous polymer to significantly enhance photocatalytic uranium extraction from seawater[J]. Chemical Engineering Journal, 2021, 412: 127558. |
| 23 | Park Y S, Lee J, Jang M J, et al. High-performance anion exchange membrane alkaline seawater electrolysis[J]. Journal of Materials Chemistry A, 2021, 9(15): 9586-9592. |
| 24 | Li H, Chen S S, Song Y, et al. Preparation of antibioadhering materials containing quaternary phosphonium salt for uranium extraction from seawater[J]. ACS Applied Polymer Materials, 2024, 6(7): 3796-3804. |
| 25 | Foster R I, Amphlett J T M, Kim K W, et al. SOHIO process legacy waste treatment: uranium recovery using ion exchange[J]. Journal of Industrial and Engineering Chemistry, 2020, 81: 144-152. |
| 26 | Jegan G, Sreenivasulu B, Suresh A, et al. Experimental and theoretical studies on solvent extraction of uranium(Ⅵ) with hexapropyl and hexabutyl phosphoramide extractants[J]. Solvent Extraction and Ion Exchange, 2022, 40(3): 312-332. |
| 27 | Abdeshahi A, Sadeghi M H, Ghoddocy Nejad D, et al. Recovery of uranium from phosphate ore of Iran mine(part Ⅱ): Solvent extraction of uranium from wet-process phosphoric acid[J]. Nuclear Engineering and Design, 2024, 421: 113093. |
| 28 | Tsutsui N, Ban Y, Sagawa H, et al. Solvent extraction of uranium with N,N-di(2-ethylhexyl) octanamide from nitric acid medium[J]. Solvent Extraction and Ion Exchange, 2017, 35(6): 439-449. |
| 29 | Liu C, Hsu P C, Xie J, et al. A half-wave rectified alternating current electrochemical method for uranium extraction from seawater[J]. Nature Energy, 2017, 2(4): 17007. |
| 30 | Chi F T, Zhang S, Wen J, et al. Highly efficient recovery of uranium from seawater using an electrochemical approach[J]. Industrial & Engineering Chemistry Research, 2018, 57(23): 8078-8084. |
| 31 | Feng H C, Dong H H, He P, et al. Nickel single atom mediated phosphate functionalization of moss derived biochar effectively enhances electrochemical uranium extraction from seawater[J]. Journal of Materials Chemistry A, 2024, 12(13): 7896-7905. |
| 32 | Zhu L E, Zhang C H, Ma F Q, et al. Hierarchical self-assembled polyimide microspheres functionalized with amidoxime groups for uranium-containing wastewater remediation[J]. ACS Applied Materials & Interfaces, 2023, 15(4): 5577-5589. |
| 33 | Cai W Q, Wang Y Q, Chen L, et al. High-efficiency adsorptive removal of U(Ⅵ) on magnetic mesoporous carbon/Sr-doped hydroxyapatite composites[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2024, 683: 132975. |
| 34 | Xing C, Bernicot B, Arrachart G, et al. Application of ultra/nano filtration membrane in uranium rejection from fresh and salt waters[J]. Separation and Purification Technology, 2023, 314: 123543. |
| 35 | 莫滨宇, 张雅馨, 刘国振, 等.面向一/二价离子分离的金属有机骨架膜研究进展[J]. 化工学报, 2024, 75(4): 1183-1197. |
| Mo B Y, Zhang Y X, Liu G Z, et al. Recent progress of metal-organic framework membranes for mono/divalent ions separation[J]. CIESC Journal, 2024, 75(4): 1183-1197. | |
| 36 | Koros W J, Zhang C. Materials for next-generation molecularly selective synthetic membranes[J]. Nature Materials, 2017, 16(3): 289-297. |
| 37 | Park H B, Kamcev J, Robeson L M, et al. Maximizing the right stuff: the trade-off between membrane permeability and selectivity[J]. Science, 2017, 356(6343): eaab0530. |
| 38 | Albolkany M K, Liu C Y, Wang D Y, et al. Molecular surgery at microporous MOF for mesopore generation and renovation[J]. Angewandte Chemie International Edition, 2021, 60(26): 14601-14608. |
| 39 | Wu Y, Xie Y H, Liu X L, et al. Functional nanomaterials for selective uranium recovery from seawater: material design, extraction properties and mechanisms[J]. Coordination Chemistry Reviews, 2023, 483: 215097. |
| 40 | Singh B K, Asim M, Salkenova Z, et al. Engineered sorbents for selective uranium sequestration from seawater[J]. ACS ES&T Water, 2024, 4(2): 325-345. |
| 41 | Krestou A, Panias D. Uranium (Ⅵ) speciation diagrams in the U O 2 2 + /CO 3 2 - /H2O system at 25℃[J]. Eur. J. Miner. Process. Environ. Prot., 2004, 4(2): 113-129. |
| 42 | Zhang D, Fang L, Liu L J, et al. Uranium extraction from seawater by novel materials: a review[J]. Separation and Purification Technology, 2023, 320: 124204. |
| 43 | Wang W, Yang K, Zhu Q H, et al. MOFs-based materials with confined space: opportunities and challenges for energy and catalytic conversion[J]. Small, 2024, 20(37): 2311449. |
| 44 | Endrizzi F, Leggett C J, Rao L F. Scientific basis for efficient extraction of uranium from seawater(Ⅰ): Understanding the chemical speciation of uranium under seawater conditions[J]. Industrial & Engineering Chemistry Research, 2016, 55(15): 4249-4256. |
| 45 | Ladshaw A P, Das S, Liao W P, et al. Experiments and modeling of uranium uptake by amidoxime-based adsorbent in the presence of other ions in simulated seawater[J]. Industrial & Engineering Chemistry Research, 2016, 55(15): 4241-4248. |
| 46 | He N N, Li H, Li L Y, et al. Polyguanidine-modified adsorbent to enhance marine applicability for uranium recovery from seawater[J]. Journal of Hazardous Materials, 2021, 416: 126192. |
| 47 | Lin Z M, Chen L F, Ye Z X, et al. Film-like chitin/polyethylenimine biosorbent for highly efficient removal of uranyl-carbonate compounds from water[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105340. |
| 48 | Zhang A Y, Uchiyama G, Asakura T. pH effect on the uranium adsorption from seawater by a macroporous fibrous polymeric material containing amidoxime chelating functional group[J]. Reactive and Functional Polymers, 2005, 63(2): 143-153. |
| 49 | Shahid M U, Najam T, Islam M, et al. Engineering of metal organic framework (MOF) membrane for waste water treatment: synthesis, applications and future challenges[J]. Journal of Water Process Engineering, 2024, 57: 104676. |
| 50 | Prasad T L, Saxena A K, Tewari P K, et al. An engineering scale study on radiation grafting of polymeric adsorbents for recovery of heavy metal ions from seawater[J]. Nuclear Engineering and Technology, 2009, 41(8): 1101-1108. |
| 51 | Sekiguchi K, Saito K, Konishi S, et al. Effect of seawater temperature on uranium recovery from seawater using amidoxime adsorbents[J]. Industrial & Engineering Chemistry Research, 1994, 33(3): 662-666. |
| 52 | Wang J L, Zhuang S T. Extraction and adsorption of U(Ⅵ) from aqueous solution using affinity ligand-based technologies: an overview[J]. Reviews in Environmental Science and Bio/Technology, 2019, 18(3): 437-452. |
| 53 | Li B Y, Sun Q, Zhang Y M, et al. Functionalized porous aromatic framework for efficient uranium adsorption from aqueous solutions[J]. ACS Applied Materials & Interfaces, 2017, 9(14): 12511-12517. |
| 54 | Huang W, Yin Z, Hu X T, et al. Pore functionalization, single-crystal transformation and selective CO2 adsorption in chemical stable pillared-layer Co(Ⅱ) based metal-organic framework[J]. Inorganic Chemistry Communications, 2021, 131: 108758. |
| 55 | Salih Z I, Guo Y J, Zheng J J, et al. Effect of modified linkers of MOF-5 on enhancing interaction energies: a theoretical study[J]. Computational and Theoretical Chemistry, 2015, 1058: 28-33. |
| 56 | Mo G L, Wang L X, Luo J H. Controlled thermal treatment of NH2-MIL-125(Ti) for drastically enhanced photocatalytic reduction of Cr (Ⅵ)[J]. Separation and Purification Technology, 2021, 277: 119643. |
| 57 | Liang W B, Zhang X, Wang L Q, et al. Designing biomimetic two-dimensional channels for uranium separation from seawater[J]. Chemical Science, 2024, 15(27): 10455-10463. |
| 58 | Wang W, Luo Q, Li J Y, et al. Single-atom tungsten engineering of MOFs with biomimetic antibiofilm activity toward robust uranium extraction from seawater[J]. Chemical Engineering Journal, 2022, 431: 133483. |
| 59 | Qin X, Qin X, Xu X, et al. The membrane-based desalination: focus on MOFs and COFs[J]. Desalination, 2023, 557: 116598. |
| 60 | Su S Z, Che R, Liu Q, et al. Zeolitic imidazolate framework-67: a promising candidate for recovery of uranium(Ⅵ) from seawater[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2018, 547: 73-80. |
| 61 | Chen L, Bai Z L, Zhu L, et al. Ultrafast and efficient extraction of uranium from seawater using an amidoxime appended metal-organic framework[J]. ACS Applied Materials & Interfaces, 2017, 9(38): 32446-32451. |
| 62 | Chen X, Wan C X, Yu R, et al. Fabrication of amidoximated polyacrylonitrile nanofibrous membrane by simultaneously biaxial stretching for uranium extraction from seawater[J]. Desalination, 2020, 486: 114447. |
| 63 | Wang Y, Zhang Y P, Li Q, et al. Amidoximated cellulose fiber membrane for uranium extraction from simulated seawater[J]. Carbohydrate Polymers, 2020, 245: 116627. |
| 64 | Wang Y, Lin Z W, Liu Q, et al. Simple one-step synthesis of woven amidoximated natural material bamboo strips for uranium extraction from seawater[J]. Chemical Engineering Journal, 2021, 425: 131538. |
| 65 | Yu R, Lu Y R, Zhang X S, et al. Amidoxime-modified ultrathin polyethylene fibrous membrane for uranium extraction from seawater[J]. Desalination, 2022, 539: 115965. |
| 66 | Shi S, Qian Y X, Mei P P, et al. Robust flexible poly(amidoxime) porous network membranes for highly efficient uranium extraction from seawater[J]. Nano Energy, 2020, 71: 104629. |
| 67 | Wang J W, Sun Y, Zhao X M, et al. A poly(amidoxime)-modified MOF macroporous membrane for high-efficient uranium extraction from seawater[J]. e-Polymers, 2022, 22(1): 399-410. |
| 68 | Yu Y, Liu J Y, Liu Q, et al. High-performance polyamidoxime porous membrane prepared by the in situ modification/nonsolvent-induced phase separation strategy for uranium extraction from seawater[J]. ACS Applied Materials & Interfaces, 2024, 16(37): 49778-49789. |
| 69 | Chen J Q, Gao J Z, Lv H T, et al. Preparation of polydopamine-functionalized polyamidoxime membrane for uranium recovery from seawater[J]. Applied Surface Science, 2023, 634: 157604. |
| 70 | Xiao F, Cheng Y X, Zhou P C, et al. Fabrication of novel carboxyl and amidoxime groups modified luffa fiber for highly efficient removal of uranium(Ⅵ) from uranium mine water[J]. Journal of Environmental Chemical Engineering, 2021, 9(4): 105681. |
| 71 | Yu Q H, Yuan Y H, Wen J, et al. A universally applicable strategy for construction of anti-biofouling adsorbents for enhanced uranium recovery from seawater[J]. Advanced Science, 2019, 6(13): 1900002. |
| 72 | Ahmad Z, Li Y, Yang J J, et al. A membrane-supported bifunctional poly(amidoxime-ethyleneimine) network for enhanced uranium extraction from seawater and wastewater[J]. Journal of Hazardous Materials, 2022, 425: 127995. |
| 73 | Ma L, Gao J, Huang C, et al. UiO-66-NH-(AO) MOFs with a new ligand BDC-NH-(CN) for efficient extraction of uranium from seawater[J]. ACS Applied Materials & Interfaces, 2021, 13(48): 57831-57840. |
| 74 | Yao Y Y, Liao J, Xu X, et al. Hydrazide and amidoxime dual functional membranes for uranium extraction from seawater[J]. Journal of Materials Chemistry A, 2024, 12(17): 10528-10538. |
| 75 | Fu M T, Huang C, Ma L, et al. Solar enhanced uranium extraction from seawater with the efficient strategy of MXene loaded nano-porous polyamidoxime membrane[J]. Separation and Purification Technology, 2024, 332: 125803. |
| 76 | Liu T, Zhang R Q, Chen M W, et al. Vertically aligned polyamidoxime/graphene oxide hybrid sheets’membrane for ultrafast and selective extraction of uranium from seawater[J]. Advanced Functional Materials, 2022, 32(14): 2111049. |
| 77 | Suresh P, Duval C E. Poly(acid)-functionalized membranes to sequester uranium from seawater[J]. Industrial & Engineering Chemistry Research, 2020, 59(26): 12212-12222. |
| 78 | Luo W, Xiao G, Tian F, et al. Engineering robust metal-phenolic network membranes for uranium extraction from seawater[J]. Energy & Environmental Science, 2019, 12(2): 607-614. |
| 79 | Peng Y G, Zhang Y X, Tan Q, et al. Bioinspired construction of uranium ion trap with abundant phosphate functional groups[J]. ACS Applied Materials & Interfaces, 2021, 13(23): 27049-27056. |
| 80 | Yu Y, Liu J Y, Chen S S, et al. Bioinspired electrostatic layer-by-layer assembly membranes constructed based on mild strategy for uranium extraction from seawater[J]. Chemical Engineering Journal, 2024, 486: 149783. |
| 81 | Tyagi A, Sharma H, Yadav A K, et al. Pristine and postsynthetically modified UiO-66-NH2 (Ce) MOF for efficient capture of uranium from aqueous solutions[J]. Industrial & Engineering Chemistry Research, 2024, 63(25): 10892-10902. |
| 82 | Liu T, Zhang X B, Wang H, et al. Photothermal enhancement of uranium capture from seawater by monolithic MOF-bonded carbon sponge[J]. Chemical Engineering Journal, 2021, 412: 128700. |
| 83 | Bai Z Q, Yuan L Y, Zhu L, et al. Introduction of amino groups into acid-resistant MOFs for enhanced U(Ⅵ) sorption[J]. Journal of Materials Chemistry A, 2015, 3(2): 525-534. |
| 84 | Yu Y, Liu J Y, Liu Q, et al. Quaternized polyethyleneimine-polyacrylonitrile crosslinked membrane with excellent performance synthetized by homogeneous strategy for efficient uranium extraction from seawater[J]. Desalination, 2023, 565: 116828. |
| 85 | Cao M, Peng Q, Wang Y, et al. High-efficiency uranium extraction from seawater by low-cost natural protein hydrogel[J]. International Journal of Biological Macromolecules, 2023, 242: 124792. |
| 86 | Schneider E, Sachde D. The cost of recovering uranium from seawater by a braided polymer adsorbent system[J]. Science & Global Security, 2013, 21(2): 134-163. |
| 87 | Kim J, Tsouris C, Oyola Y, et al. Uptake of uranium from seawater by amidoxime-based polymeric adsorbent: field experiments, modeling, and updated economic assessment[J]. Industrial & Engineering Chemistry Research, 2014, 53(14): 6076-6083. |
| 88 | Tansel B, Sager J, Rector T, et al. Significance of hydrated radius and hydration shells on ionic permeability during nanofiltration in dead end and cross flow modes[J]. Separation and Purification Technology, 2006, 51(1): 40-47. |
| 89 | Tansel B. Significance of thermodynamic and physical characteristics on permeation of ions during membrane separation: hydrated radius, hydration free energy and viscous effects[J]. Separation and Purification Technology, 2012, 86: 119-126. |
| 90 | Wu M M, Yan J H, Ji T T, et al. Synthesis of (222)-oriented defect-rich MOF-808 membranes towards high-efficiency uranium rejection[J]. Journal of Membrane Science, 2025, 717: 123570. |
| 91 | Wu M M, Sun Y W, Ji T T, et al. Fabrication of water-stable MOF-808 membrane for efficient salt/dye separation[J]. Journal of Membrane Science, 2023, 686: 122023. |
| 92 | Sun Y, Hu S, Yan J, et al. Oriented ultrathin π-complexation MOF membrane for ethylene/ethane and flue gas separations[J]. Angewandte Chemie International Edition, 2023, 62(43): 202311336. |
| 93 | Kwon H T, Jeong H K. In situ synthesis of thin zeolitic-imidazolate framework ZIF-8 membranes exhibiting exceptionally high propylene/propane separation[J]. Journal of the American Chemical Society, 2013, 135(29): 10763-10768. |
| 94 | Shah M N, Gonzalez M A, McCarthy M C, et al. An unconventional rapid synthesis of high performance metal-organic framework membranes[J]. Langmuir, 2013, 29(25): 7896-7902. |
| 95 | Yu B X, Ye G, Chen J, et al. Membrane-supported 1D MOF hollow superstructure array prepared by polydopamine-regulated contra-diffusion synthesis for uranium entrapment[J]. EnvironmentalPollution, 2019, 253: 39-48. |
| 96 | Tan H H, Tang Y, Hou Z W, et al. Antimicrobial polymer-based zeolite imidazolate framework composite membranes for uranium extraction from wastewater and seawater[J]. Journal of Colloid and Interface Science, 2025, 677: 435-445. |
| 97 | Carboni M, Abney C W, Liu S B, et al. Highly porous and stable metal-organic frameworks for uranium extraction[J]. Chemical Science, 2013, 4(6): 2396-2402. |
| 98 | Wu Y H, Pang H W, Yao W, et al. Synthesis of rod-like metal-organic framework (MOF-5) nanomaterial for efficient removal of U(Ⅵ): batch experiments and spectroscopy study[J]. Science Bulletin, 2018, 63(13): 831-839. |
| 99 | Zhang W, Dong X T, Mu Y X, et al. Constructing adjacent phosphine oxide ligands confined in mesoporous Zr-MOFs for uranium capture from acidic medium[J]. Journal of Materials Chemistry A, 2021, 9(31): 16685-16691. |
| 100 | Guo X J, Chen R R, Liu Q, et al. Graphene oxide and silver ions coassisted zeolitic imidazolate framework for antifouling and uranium enrichment from seawater[J]. ACS Sustainable Chemistry & Engineering, 2019, 7(6): 6185-6195. |
| 101 | Feng Y F, Jiang H, Li S N, et al. Metal-organic frameworks HKUST-1 for liquid-phase adsorption of uranium[J]. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2013, 431: 87-92. |
| 102 | Wang L L, Luo F, Dang L L, et al. Ultrafast high-performance extraction of uranium from seawater without pretreatment using an acylamide-and carboxyl-functionalized metal-organic framework[J]. Journal of Materials Chemistry A, 2015, 3(26): 13724-13730. |
| 103 | Li J Q, Gong L L, Feng X F, et al. Direct extraction of U(Ⅵ) from alkaline solution and seawater via anion exchange by metal-organic framework[J]. Chemical Engineering Journal, 2017, 316: 154-159. |
| 104 | Feng L J, Wang D H, Feng T T, et al. In situ synthesis of uranyl-imprinted nanocage for selective uranium recovery from seawater[J]. Angewandte Chemie International Edition, 2022, 61(13): e202101015. |
| 105 | Yuan D Y, Yu Q H, Wen D J, et al. Ultrafast and highly selective uranium extraction from seawater by hydrogel-like spidroin-based protein fiber[J]. Angewandte Chemie International Edition, 2019, 58(34): 11785-11790. |
| 106 | Qin X D, Yang W T, Yang W K, et al. Covalent modification of ZIF-90 for uranium adsorption from seawater[J]. Microporous and Mesoporous Materials, 2021, 323: 111231. |
| 107 | Bai Z H, Liu Q, Zhang H S, et al. A novel 3D reticular anti-fouling bio-adsorbent for uranium extraction from seawater: polyethylenimine and guanidyl functionalized hemp fibers[J]. Chemical Engineering Journal, 2020, 382: 122555. |
| 108 | Liu L J, Fang Y G, Meng Y J, et al. Efficient adsorbent for recovering uranium from seawater prepared by grafting amidoxime groups on chloromethylated MIL-101(Cr) via diaminomaleonitrile intermediate[J]. Desalination, 2020, 478: 114300. |
| 109 | Yan J H, Sun Y W, Ji T T, et al. Facile synthesis of oriented Zr-MOF membrane under complete room-temperature condition with superb selectivity for carbon capture[J]. Industrial & Engineering Chemistry Research, 2023, 62(14): 5973-5983. |
| [1] | 胡嘉朗, 姜明源, 金律铭, 张永刚, 胡鹏, 纪红兵. 机器学习辅助MOFs高通量计算筛选及气体分离研究进展[J]. 化工学报, 2025, 76(5): 1973-1996. |
| [2] | 王金月, 谢恩泽, 马翰泽, 袁晟, 何光伟, 姜忠义. 单原子层分离膜:进展与展望[J]. 化工学报, 2025, 76(5): 1943-1959. |
| [3] | 陆艳秋, 狄扬, 石文博, 殷聪聪, 汪勇. 基于新型有机多孔聚合物的智能响应膜研究进展[J]. 化工学报, 2025, 76(5): 2101-2118. |
| [4] | 齐昊, 王玉杰, 李申辉, 邹琦, 刘轶群, 赵之平. 双金属Co/Zn-ZIFs中C3H6和C3H8吸附和扩散行为分子模拟研究[J]. 化工学报, 2025, 76(5): 2313-2326. |
| [5] | 陶春珲, 李印辉, 傅钰, 段然, 赵泽一, 唐羽丰, 张罡, 马和平. 不同吸附剂对低浓度Kr气的选择性吸附与纯化[J]. 化工学报, 2025, 76(5): 2358-2366. |
| [6] | 赵浩帆, 任豪杰, 刘宗凯, 董冠英, 张亚涛. MOFs玻璃膜在气体分离领域的研究进展[J]. 化工学报, 2025, 76(5): 2042-2054. |
| [7] | 郭明钢, 杨晓航, 代岩, 米盼盼, 马世鑫, 贺高红, 肖武, 崔福军. 贫氦管输天然气提氦多元化产品耦合工艺优化设计[J]. 化工学报, 2025, 76(5): 2251-2261. |
| [8] | 程刘惠美, 闫军营, 刘慧情, 王治澎, 王报英, 徐铜文, 汪耀明. 双极膜电渗析在醇水体系的应用研究进展[J]. 化工学报, 2025, 76(5): 1960-1972. |
| [9] | 张耀辉, 班宇杰, 杨维慎. 以蒸气加工法制备和修饰金属-有机框架膜[J]. 化工学报, 2025, 76(5): 2070-2086. |
| [10] | 杨紫博, 王有发, 岳寒松, 远双杰, 耿付江, 李晴晴, 奥德, 李斌, 叶茂, 顾振杰, 乔志华. MOF玻璃基气体分离膜的研究进展[J]. 化工学报, 2025, 76(5): 2158-2168. |
| [11] | 朱迪, 高守建, 方望熹, 靳健. 水蒸气诱导相分离构筑海绵孔结构超亲水聚醚砜膜及其油/水乳液分离性能研究[J]. 化工学报, 2025, 76(5): 2397-2409. |
| [12] | 顾栋, 皮行健, 张叠, 张瑛. 不同粒径CAU-1/PI混合基质膜的构建与H2/CO2分离性能研究[J]. 化工学报, 2025, 76(5): 2410-2418. |
| [13] | 马瑞洁, 黄子轩, 关雪倩, 陈光进, 刘蓓. ZIF-8/DMPU浆液分离C2H6/ CH4混合气研究[J]. 化工学报, 2025, 76(5): 2262-2269. |
| [14] | 高冰冰, 许诺, 白云翔, 张春芳, 杨永强, 董亮亮. 氦气分离聚合物膜[J]. 化工学报, 2025, 76(5): 2119-2135. |
| [15] | 郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号