化工学报 ›› 2025, Vol. 76 ›› Issue (5): 2304-2312.DOI: 10.11949/0438-1157.20241213
郭彭涛1( ), 王婷1, 薛波1, 应允攀1(
), 王婷1, 薛波1, 应允攀1( ), 刘大欢1,2(
), 刘大欢1,2( )
)
收稿日期:2024-10-31
修回日期:2024-12-11
出版日期:2025-05-25
发布日期:2025-06-13
通讯作者:
应允攀,刘大欢
作者简介:郭彭涛(1998—),男,博士研究生,guo1881131265@163.com
基金资助:
Pengtao GUO1( ), Ting WANG1, Bo XUE1, Yunpan YING1(
), Ting WANG1, Bo XUE1, Yunpan YING1( ), Dahuan LIU1,2(
), Dahuan LIU1,2( )
)
Received:2024-10-31
Revised:2024-12-11
Online:2025-05-25
Published:2025-06-13
Contact:
Yunpan YING, Dahuan LIU
摘要:
开发用于纯化非常规天然气中甲烷(CH4)的吸附剂,对能源和环境的可持续发展具有重要意义。然而,CH4和N2的物理化学性质极为相似,这使得高性能吸附剂的设计成为巨大的挑战。以低极性芳香族咪唑环配体1H-苯并咪唑-5-羧酸构建超微孔金属有机框架材料(Ni-BZZA),有望实现CH4/N2混合的高效分离。该材料孔表面具有密集的氮杂环和氧原子,可作为CH4的强结合位点。Ni-BZZA的超微孔道为CH4提供了强大的约束性作用,在298 K和0.1 MPa下呈现出优异的CH4吸附量(39.1 cm3·g-1)和CH4/N2(体积比50/50)选择性(8.6)。在模拟工业条件下进行的穿透实验证实Ni-BZZA可以高效分离CH4/N2混合物。Ni-BZZA具有优异的CH4吸附量和CH4/N2选择性,说明其在非常规天然气中富集CH4方面具有极大的潜力。
中图分类号:
郭彭涛, 王婷, 薛波, 应允攀, 刘大欢. 用于CH4/N2分离的多吸附位点超微孔MOF[J]. 化工学报, 2025, 76(5): 2304-2312.
Pengtao GUO, Ting WANG, Bo XUE, Yunpan YING, Dahuan LIU. Ultramicroporous MOF with multiple adsorption sites for CH4/N2 separation[J]. CIESC Journal, 2025, 76(5): 2304-2312.
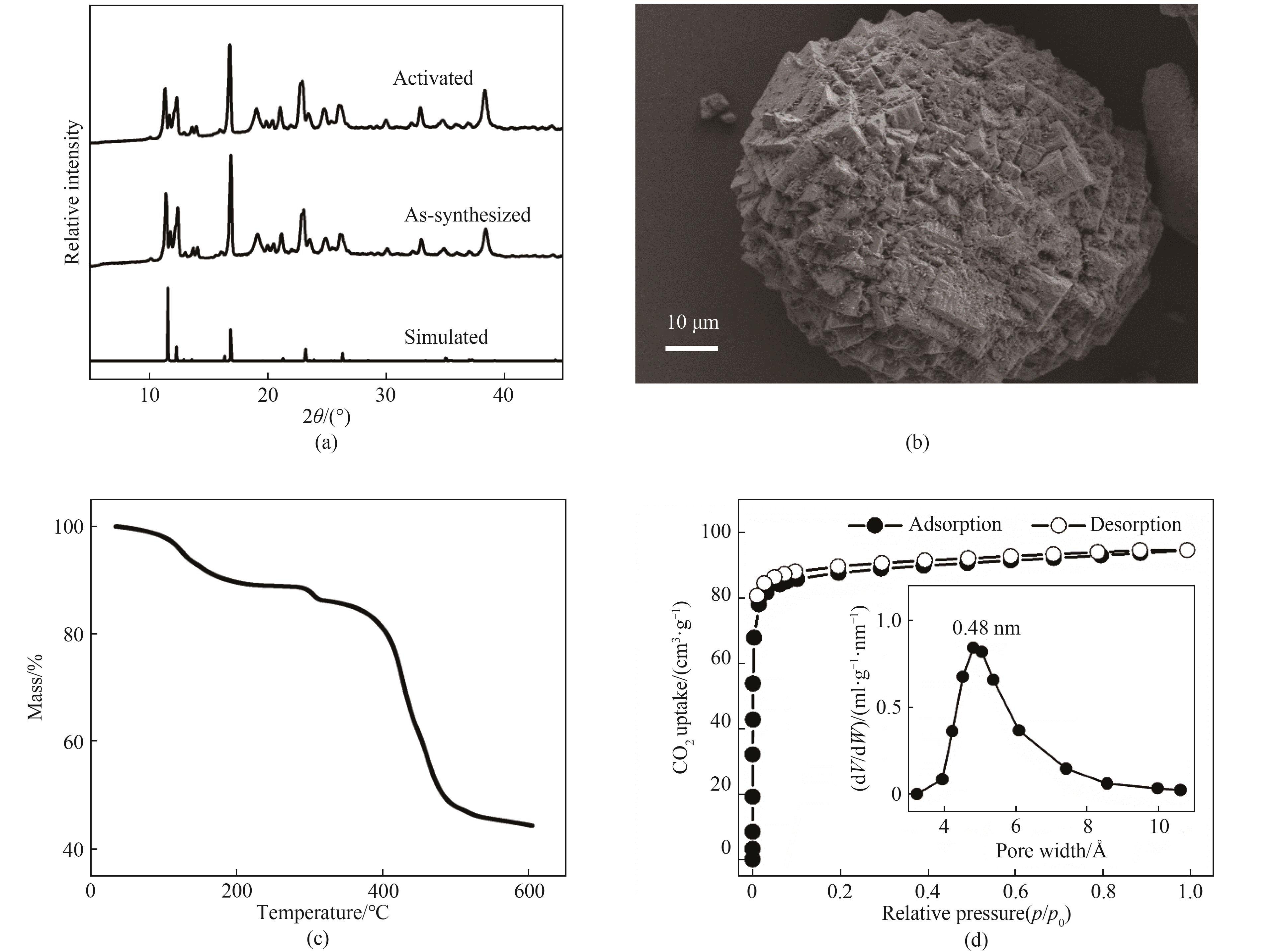
图2 (a) 刚合成和活化后Ni-BZZA的PXRD图谱;(b) Ni-BZZA的SEM图像;(c) Ni-BZZA的热重分析曲线;(d) 195 K下Ni-BZZA的CO2吸附-脱附等温线(插图:Ni-BZZA的孔径分布)(1 Å=0.1 nm)
Fig.2 (a) PXRD patterns of as-synthesized and activated Ni-BZZA; (b) SEM images of Ni-BZZA; (c) Thermogravimetric analysis curves of Ni-BZZA; and (d) CO2 adsorption-desorption isotherms of Ni-BZZA at 195 K (Inset: pore size distribution of Ni-BZZA)
| 吸附剂 | 晶体密度/(g·cm-3) | 吸附量/(cm3·g-1) | 体积吸附量/(cm3·cm-3) | 文献 |
|---|---|---|---|---|
| Ni-BZZA | 1.31 | 39.1 | 51.20 | 本研究 |
| Al-CDC | 1.27 | 32.0 | 40.64 | [ |
| Co3(C4O4)2(OH)2 | 2.18 | 9.0 | 19.81 | [ |
| STAM-1 | 1.47 | 14.2 | 20.87 | [ |
| NKMOF-8-Me | 1.37 | 39.5 | 54.11 | [ |
| MIL-160 | 1.12 | 10.5 | 11.76 | [ |
| Al-FUM-Me | 1.04 | 27.2 | 28.29 | [ |
| Ni(BTC)(PIZ) | 1.04 | 36.3 | 38.06 | [ |
| Co-MA-BPY | 1.42 | 20.6 | 29.25 | [ |
| CAU-10 | 1.16 | 16.6 | 19.26 | [ |
| Ni(OAc)2L | 0.81 | 25.7 | 20.82 | [ |
表1 298 K、1 bar下Ni-BZZA与其他吸附剂的CH4吸附量对比
Table1 Comparison of CH4 uptake of Ni-BZZA with other adsorbents at 298 K and 1 bar
| 吸附剂 | 晶体密度/(g·cm-3) | 吸附量/(cm3·g-1) | 体积吸附量/(cm3·cm-3) | 文献 |
|---|---|---|---|---|
| Ni-BZZA | 1.31 | 39.1 | 51.20 | 本研究 |
| Al-CDC | 1.27 | 32.0 | 40.64 | [ |
| Co3(C4O4)2(OH)2 | 2.18 | 9.0 | 19.81 | [ |
| STAM-1 | 1.47 | 14.2 | 20.87 | [ |
| NKMOF-8-Me | 1.37 | 39.5 | 54.11 | [ |
| MIL-160 | 1.12 | 10.5 | 11.76 | [ |
| Al-FUM-Me | 1.04 | 27.2 | 28.29 | [ |
| Ni(BTC)(PIZ) | 1.04 | 36.3 | 38.06 | [ |
| Co-MA-BPY | 1.42 | 20.6 | 29.25 | [ |
| CAU-10 | 1.16 | 16.6 | 19.26 | [ |
| Ni(OAc)2L | 0.81 | 25.7 | 20.82 | [ |
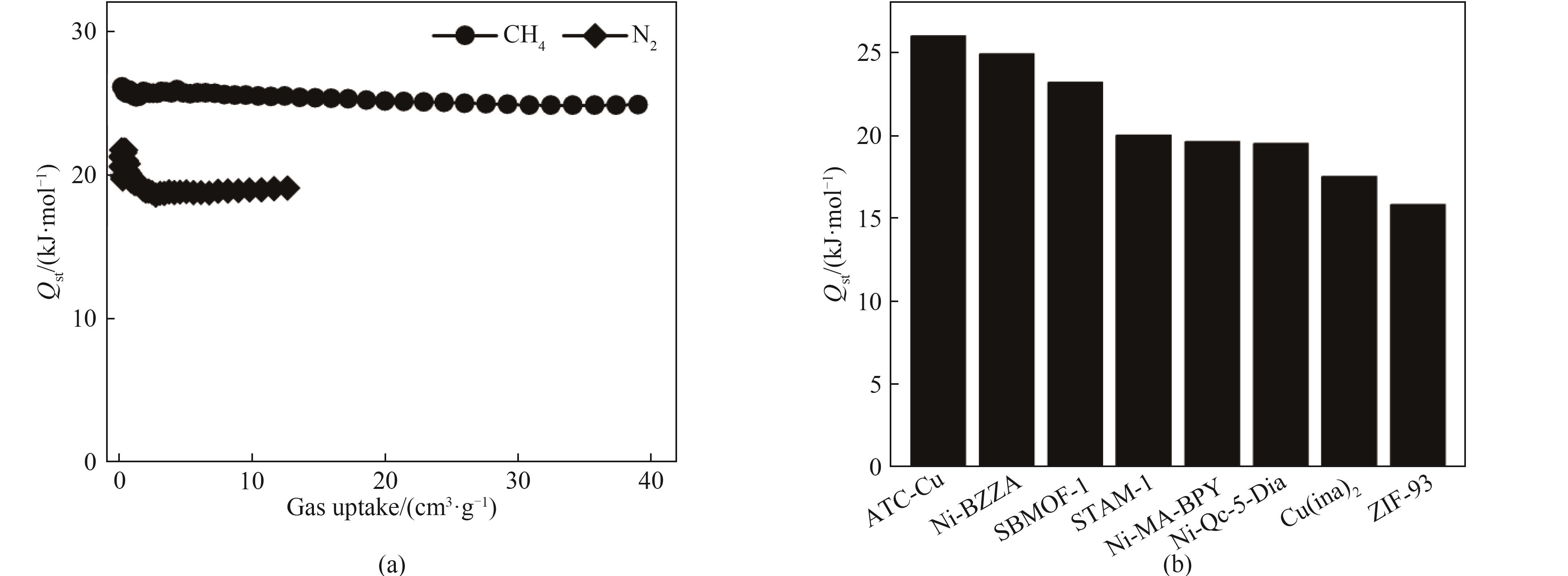
图4 (a) Ni-BZZA对CH4和N2的吸附热;(b) Ni-BZZA与其他吸附剂的吸附热对比
Fig.4 (a) Adsorption heat of Ni-BZZA for CH4 and N2; (b) Comparison of adsorption heat Qst of Ni-BZZA with other adsorbents
| Gas | bA/bar-1 | VA | bB/bar-1 | VB | R2 | ||
|---|---|---|---|---|---|---|---|
| CH4 | 30.98924 | 3.457645 | 1.025724 | 34.41176 | 0.776073 | 1.055484 | 0.9999 |
| N2 | 15.94589 | 0.691774 | 1.888699 | 8.010242 | 3.280542 | 1.146546 | 0.9999 |
表2 Dual-site Langmuir-Freundlich方程拟合298 K下Ni-BZZA的CH4和N2的吸附等温线参数
Table2 Parameters for fitting adsorption isotherms of CH4 and N2 on Ni-BZZA at 298 K using dual-site Langmuir-Freundlich equation
| Gas | bA/bar-1 | VA | bB/bar-1 | VB | R2 | ||
|---|---|---|---|---|---|---|---|
| CH4 | 30.98924 | 3.457645 | 1.025724 | 34.41176 | 0.776073 | 1.055484 | 0.9999 |
| N2 | 15.94589 | 0.691774 | 1.888699 | 8.010242 | 3.280542 | 1.146546 | 0.9999 |
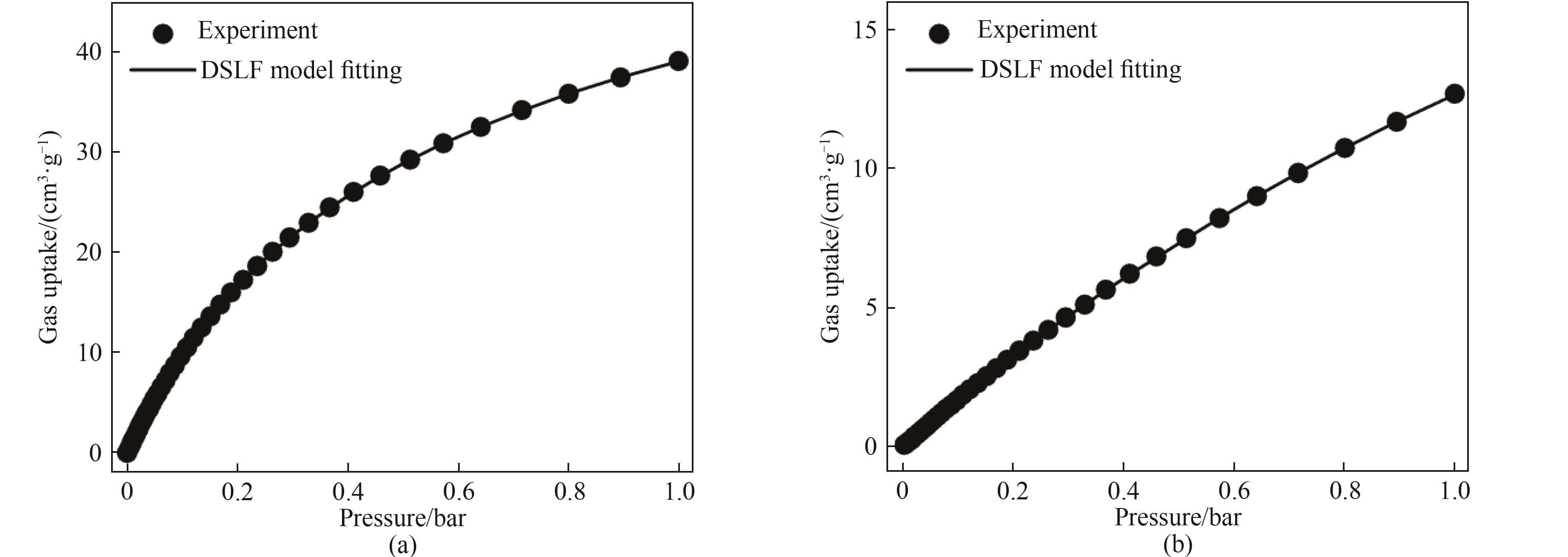
图5 Dual-site Langmuir-Frendlich方程拟合的Ni-BZZA的CH4 (a)和N2 (b)吸附等温线
Fig.5 Dual-site Langmuir-Freundlich equations fit for CH4 (a) and N2 (b) adsorption isotherms of Ni-BZZA
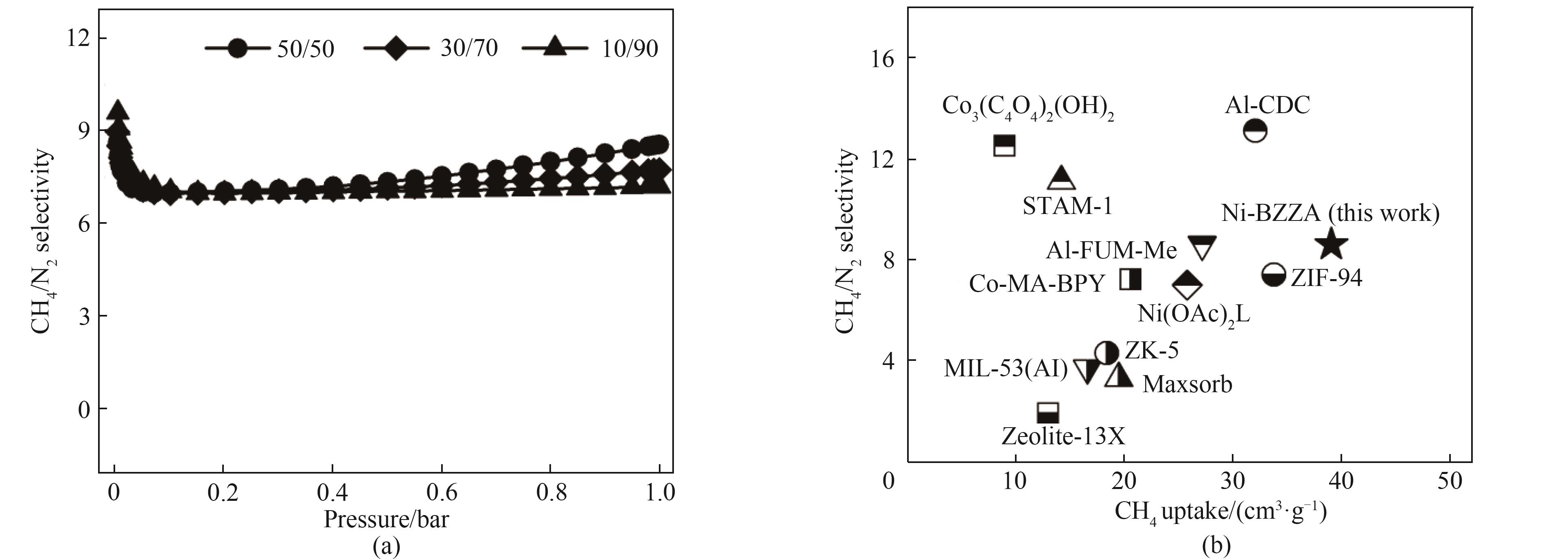
图6 (a) 298 K下Ni-BZZA的CH4/N2(体积比50/50、30/70和10/90)IAST选择性;(b) Ni-BZZA与其他吸附剂的IAST选择性和CH4吸附量对比
Fig.6 (a) CH4/N2 (50/50, 30/70 and 10/90, vol) IAST selectivity of Ni-BZZA at 298 K; (b) Comparison of IAST selectivity and CH4 uptake of Ni-BZZA with other adsorbents
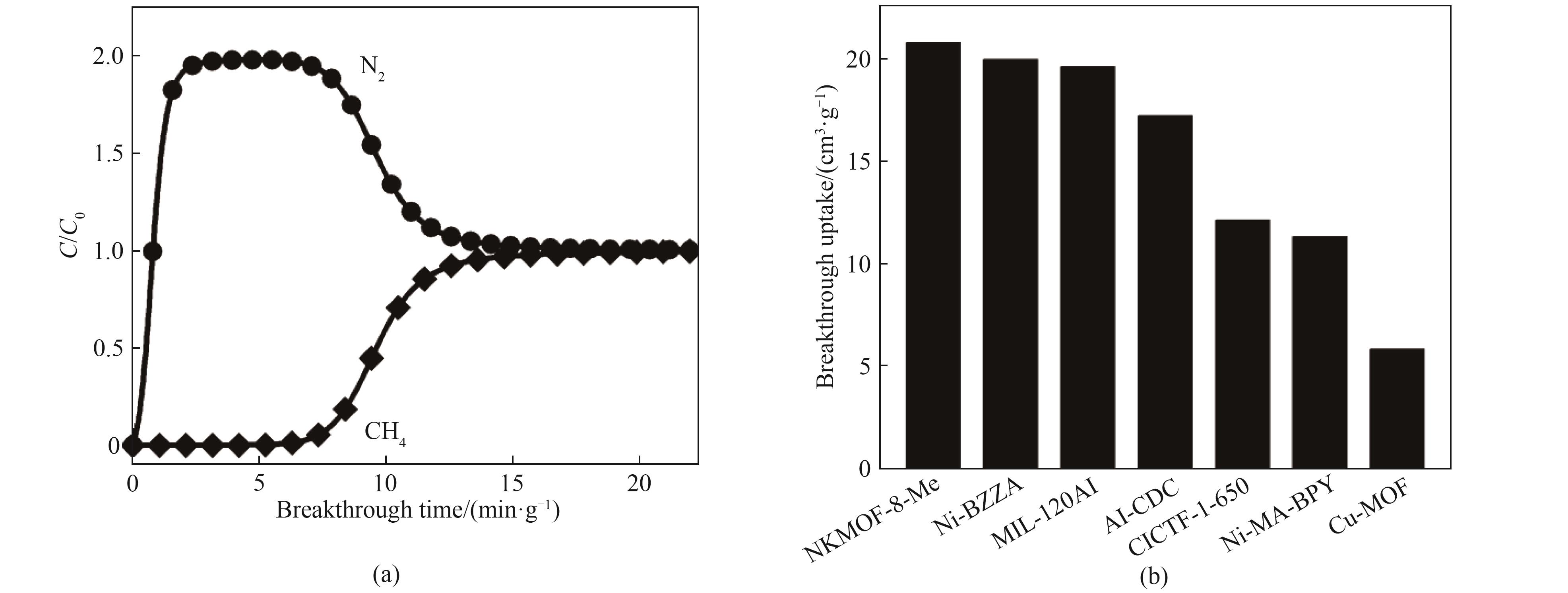
图7 (a) 在298 K下Ni-BZZA的CH4/N2(体积比50/50)混合物的穿透曲线;(b) Ni-BZZA与其他吸附剂的CH4穿透吸附量对比
Fig.7 (a) Breakthrough curves of CH4/N2 (50/50, vol) mixtures of Ni-BZZA at 298 K; (b) Comparison of CH4 breakthrough uptake of Ni-BZZA with those of other adsorbents
| 1 | Chang M, Ren J H, Wei Y, et al. Discovery of a scalable metal-organic framework with a switchable structure for efficient CH4/N2 separation[J]. Chemistry of Materials, 2023, 35(11): 4286-4296. |
| 2 | Wang S M, Shivanna M, Yang Q Y. Nickel-based metal-organic frameworks for coal-bed methane purification with record CH4/N2 selectivity[J]. Angewandte Chemie International Edition, 2022, 61(15): e202201017. |
| 3 | 马蕾, 张飞飞, 宋志强, 等. 金属有机骨架材料用于吸附分离CH4和N2的研究进展[J]. 化工进展, 2021, 40(9): 5107-5117. |
| Ma L, Zhang F F, Song Z Q, et al. Development of metal-organic frameworks in adsorptive separation of CH4-N2 [J]. Chemical Industry and Engineering Progress, 2021, 40(9): 5107-5117. | |
| 4 | Wu Y Q, Weckhuysen B M. Separation and purification of hydrocarbons with porous materials[J]. Angewandte Chemie International Edition, 2021, 60(35): 18930-18949. |
| 5 | Zhao Y L, Zhang X, Li M Z, et al. Non-CO2 greenhouse gas separation using advanced porous materials[J]. Chemical Society Reviews, 2024, 53(4): 2056-2098. |
| 6 | Lelieveld J, Klingmüller K, Pozzer A, et al. Effects of fossil fuel and total anthropogenic emission removal on public health and climate[J]. Proceedings of the National Academy of Sciences of the United States of America, 2019, 116(15): 7192-7197. |
| 7 | Yu Y X, Shang M Y, Kong L T, et al. Influence of ligands within Al-based metal-organic frameworks for selective separation of methane from unconventional natural gas[J]. Chemosphere, 2023, 321: 138160. |
| 8 | Xing G Y, Cong S Z, Wang B, et al. A high-performance N2-selective MXene membrane with double selectivity mechanism for N2/CH4 separation[J]. Small, 2024, 20(14): 2309360. |
| 9 | Kim J, Maiti A, Lin L C, et al. New materials for methane capture from dilute and medium-concentration sources[J]. Nature Communications, 2013, 4: 1694. |
| 10 | Zhang Z, Poulter B, Knox S, et al. Anthropogenic emission is the main contributor to the rise of atmospheric methane during 1993—2017[J]. National Science Review, 2021, 9(5): nwab200. |
| 11 | Qadir S, Li D F, Gu Y M, et al. Experimental and numerical investigations on the separation performance of [Cu(INA)2] adsorbent for CH4 recovery by VPSA from oxygen-bearing coal mine methane[J]. Chemical Engineering Journal, 2021, 408: 127238. |
| 12 | 郑卫, 马文君. 变压吸附气体分离技术的应用进展[J]. 当代化工研究, 2024(15): 11-13. |
| Zheng W, Ma W J. Overview of the application progress of pressure swing adsorption gas separation technology[J]. Modern Chemical Research, 2024(15): 11-13. | |
| 13 | Qian Z L, Zhou Y W, Yang Y, et al. Methane recovery from low-grade unconventional natural gas by the integrated mode of the conventional/improved vacuum pressure swing adsorption processes[J]. Fuel, 2023, 331: 125717. |
| 14 | Guo Y, Hu J L, Liu X W, et al. Scalable solvent-free preparation of [Ni3(HCOO)6] frameworks for highly efficient separation of CH4 from N2 [J]. Chemical Engineering Journal, 2017, 327: 564-572. |
| 15 | Li J R, Kuppler R J, Zhou H C. Selective gas adsorption and separation in metal-organic frameworks[J]. Chemical Society Reviews, 2009, 38(5): 1477-1504. |
| 16 | Nandanwar S U, Corbin D R, Shiflett M B. A review of porous adsorbents for the separation of nitrogen from natural gas[J]. Industrial & Engineering Chemistry Research, 2020, 59(30): 13355-13369. |
| 17 | 刘伟, 田林宇, 王宪飞, 等. 金属有机框架材料分离碳四烃的研究进展[J]. 现代化工, 2020, 40(3): 16-21. |
| Liu W, Tian L Y, Wang X F, et al. Study progress in separation of C4 hydrocarbons by metal-organic frameworks[J]. Modern Chemical Industry, 2020, 40(3): 16-21. | |
| 18 | Jiang Y, Jia S J, Liu X Q, et al. Selective adsorption of ethane over ethylene through a metal-organic framework bearing dense alkyl groups[J]. Separation and Purification Technology, 2022, 295: 121330. |
| 19 | Wang J, Zhang Y, Zhang P X, et al. Optimizing pore space for flexible-robust metal-organic framework to boost trace acetylene removal[J]. Journal of the American Chemical Society, 2020, 142(21): 9744-9751. |
| 20 | Cadiau A, Adil K, Bhatt P M, et al. A metal-organic framework-based splitter for separating propylene from propane[J]. Science, 2016, 353(6295): 137-140. |
| 21 | Li T, Jia X X, Chen H, et al. Tuning the pore environment of MOFs toward efficient CH4/N2 separation under humid conditions[J]. ACS Applied Materials & Interfaces, 2022, 14(13): 15830-15839. |
| 22 | Kivi C E, Gelfand B S, Dureckova H, et al. 3D porous metal-organic framework for selective adsorption of methane over dinitrogen under ambient pressure[J]. Chemical Communications, 2018, 54(100): 14104-14107. |
| 23 | 肖思杰, 廖小诺, 郭志奋, 等. 多孔材料吸附分离甲烷氮气研究进展[J]. 云南化工, 2023, 50(11): 1-4. |
| Xiao S J, Liao X N, Guo Z F, et al. Research progress of porous material for adsorption separation of methane and nitrogen[J]. Yunnan Chemical Technology, 2023, 50(11): 1-4. | |
| 24 | Zhang F F, Shang H, Zhai B L, et al. Thermodynamic-kinetic synergistic separation of CH4/N2 on a robust aluminum-based metal-organic framework[J]. AIChE Journal, 2023, 69(6): e18079. |
| 25 | Qadir S, Gu Y M, Ali S, et al. A thermally stable isoquinoline based ultra-microporous metal-organic framework for CH4 separation from coal mine methane[J]. Chemical Engineering Journal, 2022, 428: 131136. |
| 26 | Hu J L, Sun T J, Liu X W, et al. Separation of CH4/N2 mixtures in metal-organic frameworks with 1D micro-channels[J]. RSC Advances, 2016, 6(68): 64039-64046. |
| 27 | Tan Y X, He Y P, Zhang J. Temperature-/pressure-dependent selective separation of CO2 or benzene in a chiral metal-organic framework material[J]. ChemSusChem, 2012, 5(8): 1597-1601. |
| 28 | Myers A L, Prausnitz J M. Thermodynamics of mixed-gas adsorption[J]. AIChE Journal, 1965, 11(1): 121-127. |
| 29 | Li L Y, Yang L F, Wang J W, et al. Highly efficient separation of methane from nitrogen on a squarate-based metal-organic framework[J]. AIChE Journal, 2018, 64(10): 3681-3689. |
| 30 | Nguyen P T K, Nguyen H T D, Pham H Q, et al. Synthesis and selective CO2 capture properties of a series of hexatopic linker-based metal-organic frameworks[J]. Inorganic Chemistry, 2015, 54(20): 10065-10072. |
| 31 | Guo P T, Ying Y P, Liu D H. One scalable and stable metal-organic framework for efficient separation of CH4/N2 mixture[J]. ACS Applied Materials & Interfaces, 2024, 16(6): 7338-7344. |
| 32 | Shi Q, Wang J, Shang H, et al. Effective CH4 enrichment from N2 by SIM-1 via a strong adsorption potential SOD cage[J]. Separation and Purification Technology, 2020, 230: 115850. |
| 33 | Chang M, Zhao Y J, Liu D H, et al. Methane-trapping metal-organic frameworks with an aliphatic ligand for efficient CH4/N2 separation[J]. Sustainable Energy & Fuels, 2020, 4(1): 138-142. |
| 34 | Chang M, Zhao Y J, Yang Q Y, et al. Microporous metal-organic frameworks with hydrophilic and hydrophobic pores for efficient separation of CH4/N2 mixture[J]. ACS Omega, 2019, 4(11): 14511-14516. |
| 35 | Chang M, Wang F, Wei Y, et al. Separation of CH4/N2 by an ultra-stable metal-organic framework with the highest breakthrough selectivity[J]. AIChE Journal, 2022, 68(9): e17794. |
| 36 | Huang Z H, Hu P, Liu J, et al. Enhancing CH4/N2 separation performance within aluminum-based metal-organic frameworks: influence of the pore structure and linker polarity[J]. Separation and Purification Technology, 2022, 286: 120446. |
| 37 | Chang M, Yan T A, Wei Y, et al. Enhancing CH4 capture from coalbed methane through tuning van der Waals affinity within isoreticular Al-based metal-organic frameworks[J]. ACS Applied Materials & Interfaces, 2022, 14(22): 25374-25384. |
| 38 | Chen Y, Wang Y, Wang Y, et al. Improving CH4 uptake and CH4/N2 separation in pillar-layered metal-organic frameworks using a regulating strategy of interlayer channels[J]. AIChE Journal, 2022, 68(11): e17819. |
| 39 | Liu X W, Gu Y M, Sun T J, et al. Water resistant and flexible MOF materials for highly efficient separation of methane from nitrogen[J]. Industrial & Engineering Chemistry Research, 2019, 58(44): 20392-20400. |
| 40 | Chang M, Ren J H, Yang Q Y, et al. A robust calcium-based microporous metal-organic framework for efficient CH4/N2 separation[J]. Chemical Engineering Journal, 2021, 408: 127294. |
| 41 | Wang Q M, Shen D M, Bülow M, et al. Metallo-organic molecular sieve for gas separation and purification[J]. Microporous and Mesoporous Materials, 2002, 55(2): 217-230. |
| 42 | Yang J F, Tang X, Liu J Q, et al. Down-sizing the crystal size of ZK-5 zeolite for its enhanced CH4 adsorption and CH4/N2 separation performances[J]. Chemical Engineering Journal, 2021, 406: 126599. |
| [1] | 张耀辉, 班宇杰, 杨维慎. 以蒸气加工法制备和修饰金属-有机框架膜[J]. 化工学报, 2025, 76(5): 2070-2086. |
| [2] | 杨雅南, 常胜然, 薛松林, 潘建明, 邢卫红. 基于光、电驱动促进海水中铀和锂提取的研究进展[J]. 化工学报, 2025, 76(5): 1927-1942. |
| [3] | 杨紫博, 王有发, 岳寒松, 远双杰, 耿付江, 李晴晴, 奥德, 李斌, 叶茂, 顾振杰, 乔志华. MOF玻璃基气体分离膜的研究进展[J]. 化工学报, 2025, 76(5): 2158-2168. |
| [4] | 牛宏斌, 邱丽, 杨景轩, 张忠林, 郝晓刚, 赵忠凯, 阿布里提, 官国清. 筒体直径对旋风分离器性能的影响及其流场机制[J]. 化工学报, 2025, 76(5): 2367-2376. |
| [5] | 朱迪, 高守建, 方望熹, 靳健. 水蒸气诱导相分离构筑海绵孔结构超亲水聚醚砜膜及其油/水乳液分离性能研究[J]. 化工学报, 2025, 76(5): 2397-2409. |
| [6] | 茅雨洁, 路晓飞, 锁显, 杨立峰, 崔希利, 邢华斌. 工业气体中微量氧深度脱除催化剂研究进展[J]. 化工学报, 2025, 76(5): 1997-2010. |
| [7] | 时任泽, 丁秋燕, 袁振军, 那健, 刘见华, 郭树虎, 赵雄, 李洪, 高鑫. 4N电子级二乙氧基甲基硅烷的纯化技术研究[J]. 化工学报, 2025, 76(5): 2186-2197. |
| [8] | 郭明钢, 杨晓航, 代岩, 米盼盼, 马世鑫, 贺高红, 肖武, 崔福军. 贫氦管输天然气提氦多元化产品耦合工艺优化设计[J]. 化工学报, 2025, 76(5): 2251-2261. |
| [9] | 唐磊, 王振菲, 李聪利, 杨佳辉, 郑浩, 石琪, 董晋湘. Co-MOF-74和Mg-MOF-74的CO工作吸附容量及操作条件[J]. 化工学报, 2025, 76(5): 2279-2293. |
| [10] | 李紫鹃, 谭晓艳, 吴永盛, 杨陈怡, 陈红, 毕小刚, 刘捷, 喻发全. 分子模拟研究三维扭曲催化芳烃-降冰片烯环化聚合物膜的CO2/N2分离机理[J]. 化工学报, 2025, 76(5): 2348-2357. |
| [11] | 花敬贤, 罗宇荣, 顾亚伟, 吴婷婷, 潘宜昌, 邢卫红. 超薄取向ZIF-8膜的制备及乙烯/乙烷高效分离[J]. 化工学报, 2025, 76(5): 2209-2218. |
| [12] | 李艳, 雷美丽, 李鑫钢. 基于分离性能的顺序式模拟移动床结构调控策略[J]. 化工学报, 2025, 76(5): 2219-2229. |
| [13] | 巴雅琪, 吴涛, 邸安頔, 陆安慧. 多孔炭材料用于低碳烃分离的研究进展[J]. 化工学报, 2025, 76(5): 2136-2157. |
| [14] | 谈朋, 李雪梅, 刘晓勤, 孙林兵. 基于柔性MOFs的磁响应复合材料及其丙烯吸附性能研究[J]. 化工学报, 2025, 76(5): 2230-2240. |
| [15] | 向昕辰, 鲁丹, 赵影, 姚之侃, 寇瑞强, 郑丹军, 周志军, 张林. 聚酰胺纳滤膜表面季铵化提高荷正电性及其锂镁分离性能[J]. 化工学报, 2025, 76(5): 2377-2386. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号