化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6151-6162.DOI: 10.11949/0438-1157.20250416
收稿日期:2025-04-20
修回日期:2025-05-24
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
徐彦芹
作者简介:蔡文静(2001—),女,硕士研究生,384246686@qq.com
Received:2025-04-20
Revised:2025-05-24
Online:2025-12-31
Published:2026-01-23
Contact:
Yanqin XU
摘要:
全固态锂电作为下一代高能量密度、高安全性能储能器件,其核心固态电解质的研究备受关注。硫化物固态电解质凭借超高的离子电导率、优异的力学性能以及较好的界面兼容性,展现出显著的产业化潜力。然而,硫化物电解质仍面临空气稳定性差、界面副反应及锂枝晶生长等挑战。综述了硫化物基固态电解质的分类及其结构特性,分析了空气稳定性和电化学稳定性失效机制并总结优化策略。针对规模化制备、界面优化及薄层电解质开发等关键问题,提出了未来研究方向,为硫化物电解质的实用化提供理论参考。
中图分类号:
蔡文静, 徐彦芹. 硫化物电解质的界面调控及研究进展[J]. 化工学报, 2025, 76(12): 6151-6162.
Wenjing CAI, Yanqin XU. Interface regulation and research progress of sulfide electrolytes[J]. CIESC Journal, 2025, 76(12): 6151-6162.
| 体系类别 | 电解质 | 离子电导率/ (S·cm-1) | 文献 |
|---|---|---|---|
| Li-P-S | Li7P3S11 | 3.2×10-3 | [ |
| Li7P3S11 | 1.7×10-2 | [ | |
| β-Li3PS4 | 1.6×10-4 | [ | |
| Thio-LISICON | Li4SnS4 | 7.0×10-5 | [ |
| Li3.25Ge0.25P0.75S3.25 | 2.2×10-3 | [ | |
| LGPS | Li10SiP2S12 | 2.3×10-3 | [ |
| Li10SnP2S12 | 4×10-3 | [ | |
| Li10GeP2S12 | 1.2×10-2 | [ | |
| Li9.54Si1.74P1.44S11.7Cl0.3 | 2.5×10-2 | [ | |
| Li9.54(Si0.6Ge0.4)1.74P1.44S11.1Br0.3O0.6 | 3.2×10-2 | [ | |
| Argyrodite | Li6PS5Cl | 4.9×10-3 | [ |
| Li6PS5Br | 2.6×10-3 | [ | |
| Li6PS5I | 10-7 | [ | |
| Li5.5PS4.5Cl1.5 | 9.4×10-3 | [ | |
| Li6.6Sb0.4Si0.6S5I | 1.48×10-2 | [ |
表1 常见硫化物电解质及其离子电导率
Table 1 Common sulfide electrolytes and their ionic conductivity
| 体系类别 | 电解质 | 离子电导率/ (S·cm-1) | 文献 |
|---|---|---|---|
| Li-P-S | Li7P3S11 | 3.2×10-3 | [ |
| Li7P3S11 | 1.7×10-2 | [ | |
| β-Li3PS4 | 1.6×10-4 | [ | |
| Thio-LISICON | Li4SnS4 | 7.0×10-5 | [ |
| Li3.25Ge0.25P0.75S3.25 | 2.2×10-3 | [ | |
| LGPS | Li10SiP2S12 | 2.3×10-3 | [ |
| Li10SnP2S12 | 4×10-3 | [ | |
| Li10GeP2S12 | 1.2×10-2 | [ | |
| Li9.54Si1.74P1.44S11.7Cl0.3 | 2.5×10-2 | [ | |
| Li9.54(Si0.6Ge0.4)1.74P1.44S11.1Br0.3O0.6 | 3.2×10-2 | [ | |
| Argyrodite | Li6PS5Cl | 4.9×10-3 | [ |
| Li6PS5Br | 2.6×10-3 | [ | |
| Li6PS5I | 10-7 | [ | |
| Li5.5PS4.5Cl1.5 | 9.4×10-3 | [ | |
| Li6.6Sb0.4Si0.6S5I | 1.48×10-2 | [ |
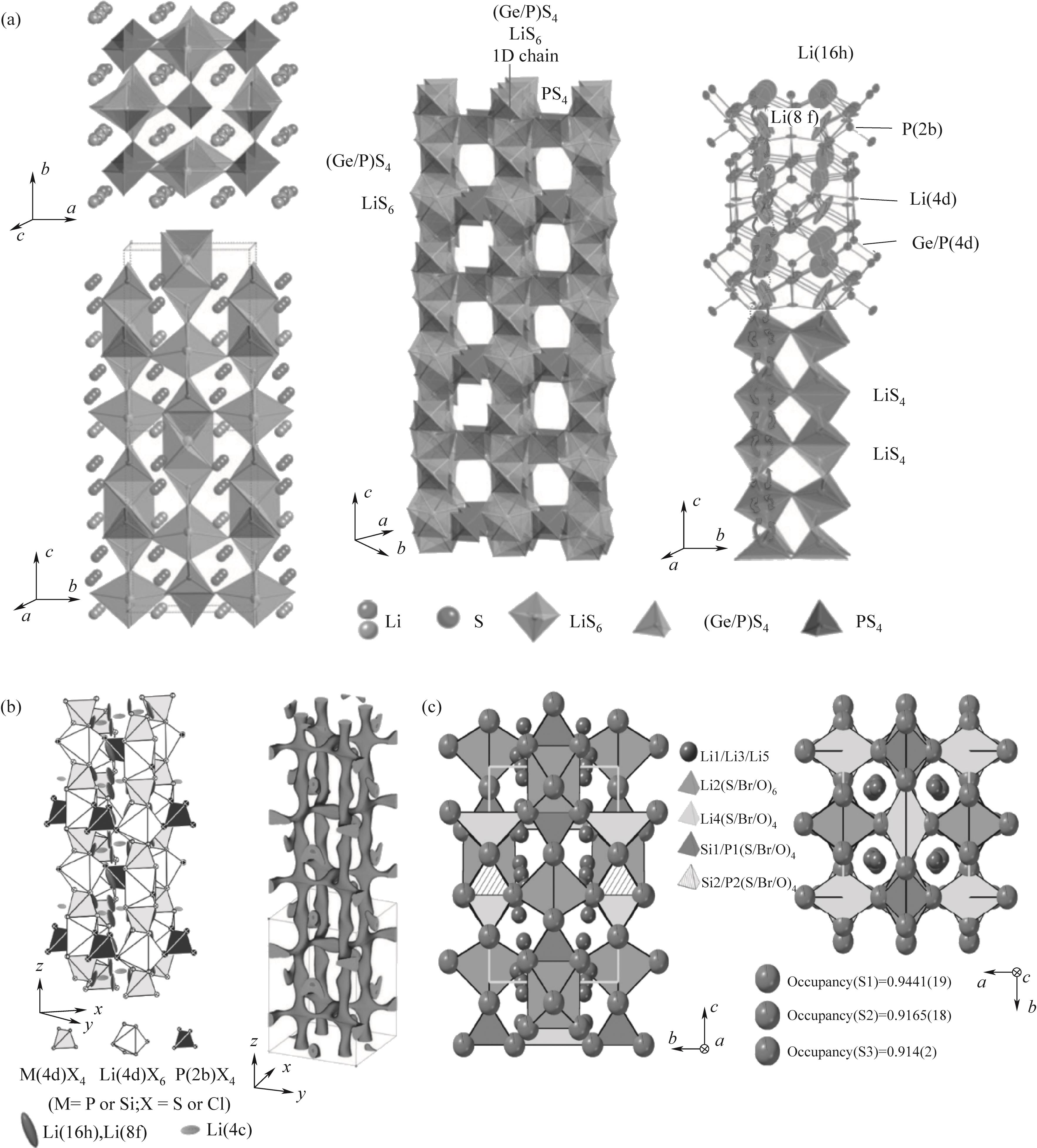
图1 (a) Li10GeP2S12结构及传导途径[26]; (b) Li9.54Si1.74P1.44S11.7Cl0.3结构[29]; (c) Li9.54(Si0.6Ge0.4)1.74P1.44S11.1Br0.3O0.6结构[5]
Fig.1 (a) The structure and conduction pathway of Li10GeP2S12[26]; (b) Li9.54Si1.74P1.44S11.7Cl0.3 structure[29]; (c) Li9.54(Si0.6Ge0.4)1.74P1.44S11.1Br0.3O0.6 structure(c)[5]
| [1] | Yan C, Xu R, Xiao Y, et al. Toward critical electrode/electrolyte interfaces in rechargeable batteries[J]. Advanced Functional Materials, 2020, 30(23): 1909887. |
| [2] | Lee S, Kim Y, Park C, et al. Interplay of athode-halide solid electrolyte in enhancing thermal stability of charged cathode material in all-solid-state batteries [J]. ACS Energy Letters, 2024, 9(4): 1369-1380. |
| [3] | Manthiram A, Yu X W, Wang S F. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 16103. |
| [4] | Wang Z Y, Xia J L, Ji X, et al. Lithium anode interlayer design for all-solid-state lithium-metal batteries[J]. Nature Energy, 2024, 9: 251-262. |
| [5] | Li Y X, Song S B, Kim H, et al. A lithium superionic conductor for millimeter-thick battery electrode[J]. Science, 2023, 381(6653): 50-53. |
| [6] | Bates A M, Preger Y, Torres-Castro L, et al. Are solid-state batteries safer than lithium-ion batteries?[J]. Joule, 2022, 6(4): 742-755. |
| [7] | Ma Y, Zhang R Z, Ma Y J, et al. Interface and electrode microstructure engineering for optimizing performance of the LiNiO2 cathode in all-solid-state batteries [J]. Chemistry of Materials, 2024, 36(5): 2588-2598. |
| [8] | Zhang X Y, Yang J F, Deng E L, et al. Argyrodite based all-solid-state-batteries: recent advances and perspective[J]. Energy Storage Materials, 2025, 79: 104339. |
| [9] | Famprikis T, Canepa P, Dawson J A, et al. Fundamentals of inorganic solid-state electrolytes for batteries[J]. Nature Materials, 2019, 18: 1278-1291. |
| [10] | Shah N J, Fang C, Osti N C, et al. Nanosecond solvation dynamics in a polymer electrolyte for lithium batteries[J]. Nature Materials, 2024, 23(5): 664-669. |
| [11] | Zhou S J, Liu K X, Wang Z Y, et al. An ultra-thin asymmetric solid polymer electrolyte for in situ integrated lithium-metal battery[J]. Chemical Engineering Journal, 2025, 504: 158548. |
| [12] | Kim K J, Balaish M, Wadaguchi M, et al. Solid-state Li-metal batteries: challenges and horizons of oxide and sulfide solid electrolytes and their interfaces[J]. Advanced Energy Materials, 2021, 11(1): 2002689. |
| [13] | Li J W, Li Y Y, Wang Y X, et al. Preparation, design and interfacial modification of sulfide solid electrolytes for all-solid-state lithium metal batteries[J]. Energy Storage Materials, 2025, 74: 103962. |
| [14] | Yao M X, Shi J T, Luo A H, et al. Advances in sulfide solid-state electrolytes for lithium batteries[J]. Energy Storage Materials, 2025, 75: 104018. |
| [15] | Zhang Y J, Sun J C, Li L S, et al. Advancements in the emerging rare-earth halide solid electrolytes for next-generation all-solid-state lithium batteries[J]. Coordination Chemistry Reviews, 2025, 528: 216432. |
| [16] | Chen S J, Xie D J, Liu G Z, et al. Sulfide solid electrolytes for all-solid-state lithium batteries: structure, conductivity, stability and application[J]. Energy Storage Materials, 2018, 14: 58-74. |
| [17] | Wu J H, Shen L, Zhang Z H, et al. All-solid-state lithium batteries with sulfide electrolytes and oxide cathodes[J]. Electrochemical Energy Reviews, 2021, 4(1): 101-135. |
| [18] | Wang C H, Adair K, Sun X L. All-solid-state lithium metal batteries with sulfide electrolytes: understanding interfacial ion and electron transport[J]. Accounts of Materials Research, 2022, 3(1): 21-32. |
| [19] | Yu T, Liu Y K, Li H Y, et al. Ductile inorganic solid electrolytes for all-solid-state lithium batteries[J]. Chemical Reviews, 2025, 125(6): 3595-3662. |
| [20] | Zhang Q, Cao D X, Ma Y, et al. Sulfide-based solid-state electrolytes: synthesis, stability, and potential for all-solid-state batteries[J]. Advanced Materials, 2019, 31(44): 1901131. |
| [21] | Ribes M, Barrau B, Souquet J L. Sulfide glasses: glass forming region, structure and ionic conduction of glasses in Na2S-XS2 (X=Si; Ge), Na2S-P2S5 and Li2S-GeS2 systems[J]. Journal of Non-Crystalline Solids, 1980, 38: 271-276. |
| [22] | Yamane H, Shibata M, Shimane Y, et al. Crystal structure of a superionic conductor, Li7P3S11 [J]. Solid State Ionics, 2007, 178(15/16/17/18): 1163-1167. |
| [23] | Seino Y, Ota T, Takada K, et al. A sulphide lithium super ion conductor is superior to liquid ion conductors for use in rechargeable batteries[J]. Energy & Environmental Science, 2014, 7(2): 627-631. |
| [24] | Liu Z C, Fu W J, Andrew Payzant E, et al. Anomalous high ionic conductivity of nanoporous β-Li3PS4 [J]. Journal of the American Chemical Society, 2013, 135(3): 975-978. |
| [25] | Kaib T, Haddadpour S, Kapitein M, et al. New lithium chalcogenidotetrelates, LiChT: synthesis and characterization of the Li+-conducting tetralithium ortho-sulfidostannate Li4SnS4 [J]. Chemistry of Materials, 2012, 24(11): 2211-2219. |
| [26] | Kamaya N, Homma K, Yamakawa Y, et al. A lithium superionic conductor[J]. Nature Materials, 2011, 10: 682-686. |
| [27] | Whiteley J M, Woo J H, Hu E Y, et al. Empowering the lithium metal battery through a silicon-based superionic conductor[J]. Journal of the Electrochemical Society, 2014, 161(12): A1812-A1817. |
| [28] | Bron P, Johansson S, Zick K, et al. Li10SnP2S12: an affordable lithium superionic conductor[J]. Journal of the American Chemical Society, 2013, 135(42): 15694-15697. |
| [29] | Kato Y, Hori S, Saito T, et al. High-power all-solid-state batteries using sulfide superionic conductors[J]. Nature Energy, 2016, 1(4): 16030. |
| [30] | Yu C, Ganapathy S, Hageman J, et al. Facile synthesis toward the optimal structure-conductivity characteristics of the argyrodite Li6PS5Cl solid-state electrolyte[J]. ACS Applied Materials & Interfaces, 2018, 10(39): 33296-33306. |
| [31] | Yu C, Hageman J, Ganapathy S, et al. Tailoring Li6PS5Br ionic conductivity and understanding of its role in cathode mixtures for high performance all-solid-state Li-S batteries[J]. Journal of Materials Chemistry A, 2019, 7(17): 10412-10421. |
| [32] | Zhang J, Li L J, Zheng C, et al. Silicon-doped argyrodite solid electrolyte Li6PS5I with improved ionic conductivity and interfacial compatibility for high-performance all-solid-state lithium batteries[J]. ACS Applied Materials & Interfaces, 2020, 12(37): 41538-41545. |
| [33] | Adeli P, Bazak J D, Park K H, et al. Boosting solid-state diffusivity and conductivity in lithium superionic argyrodites by halide substitution[J]. Angewandte Chemie International Edition, 2019, 58(26): 8681-8686. |
| [34] | Zhou L D, Assoud A, Zhang Q, et al. New family of argyrodite thioantimonate lithium superionic conductors[J]. Journal of the American Chemical Society, 2019, 141(48): 19002-19013. |
| [35] | Dietrich C, Weber D A, Culver S, et al. Synthesis, structural characterization, and lithium ion conductivity of the lithium thiophosphate Li2P2S6 [J]. Inorganic Chemistry, 2017, 56(11): 6681-6687. |
| [36] | Hayashi A, Hama S, Mizuno F, et al. Characterization of Li2S-P2S5 glass-ceramics as a solid electrolyte for lithium secondary batteries[J]. Solid State Ionics, 2004, 175(1/2/3/4): 683-686. |
| [37] | Murayama M, Kanno R, Kawamoto Y, et al. Structure of the thio-LISICON, Li4GeS4 [J]. Solid State Ionics, 2002, 154: 789-794. |
| [38] | Zhou P F, Wang J B, Cheng F Y, et al. A solid lithium superionic conductor Li11AlP2S12 with a thio-LISICON analogous structure[J]. Chemical Communications, 2016, 52(36): 6091-6094. |
| [39] | Sahu G, Lin Z, Li J C, et al. Air-stable, high-conduction solid electrolytes of arsenic-substituted Li4SnS4 [J]. Energy & Environmental Science, 2014, 7(3): 1053-1058. |
| [40] | Zhang P, Li L, Du P, et al. Li8.2SiP1.4S9.6: a novel sulfide solid electrolyte for lithium-ion battery[J]. Journal of Energy Storage, 2025, 123: 116789. |
| [41] | Deiseroth H J, Kong S T, Eckert H, et al. Li6PS5X: a class of crystalline Li-rich solids with an unusually high Li+ mobility[J]. Angewandte Chemie International Edition, 2008, 47(4): 755-758. |
| [42] | Kong S, Gün Ö, Koch B, et al. Structural characterisation of the Li argyrodites Li7PS6 and Li7PSe6 and their solid solutions: quantification of site preferences by MAS-NMR spectroscopy[J]. Chemistry – A European Journal, 2010, 16(17): 5138-5147. |
| [43] | Hanghofer I, Brinek M, Eisbacher S L, et al. Substitutional disorder: structure and ion dynamics of the argyrodites Li6PS5Cl, Li6PS5Br and Li6PS5I[J]. Physical Chemistry Chemical Physics, 2019, 21(16): 8489-8507. |
| [44] | Adeli P, Bazak J D, Huq A, et al. Influence of aliovalent cation substitution and mechanical compression on Li-ion conductivity and diffusivity in argyrodite solid electrolytes[J]. Chemistry of Materials, 2021, 33(1): 146-157. |
| [45] | Zhang Z R, Zhang J X, Jia H H, et al. Enhancing ionic conductivity of solid electrolyte by lithium substitution in halogenated Li-Argyrodite[J]. Journal of Power Sources, 2020, 450: 227601. |
| [46] | Bai X T, Duan Y, Zhuang W D, et al. Research progress in Li-argyrodite-based solid-state electrolytes[J]. Journal of Materials Chemistry A, 2020, 8(48): 25663-25686. |
| [47] | Pearson R G. Hard and soft acids and bases[J]. Journal of the American Chemical Society, 1963, 85(22): 3533-3539. |
| [48] | Lu P S, Wu D X, Chen L Q, et al. Air stability of solid-state sulfide batteries and electrolytes[J]. Electrochemical Energy Reviews, 2022, 5(3): 3. |
| [49] | Xu H J, Cao G Q, Shen Y L, et al. Enabling argyrodite sulfides as superb solid-state electrolyte with remarkable interfacial stability against electrodes[J]. Energy & Environmental Materials, 2022, 5(3): 852-864. |
| [50] | Liu H, Zhu Q S, Liang Y H, et al. Versatility of Sb-doping enabling argyrodite electrolyte with superior moisture stability and Li metal compatibility towards practical all-solid-state Li metal batteries[J]. Chemical Engineering Journal, 2023, 462: 142183. |
| [51] | Li G Y, Wu S P, Zheng H P, et al. Sn-O dual-substituted chlorine-rich argyrodite electrolyte with enhanced moisture and electrochemical stability[J]. Advanced Functional Materials, 2023, 33(11): 2211805. |
| [52] | Zhang C J. Tuning the composition[J]. Nature Energy, 2023, 8(8): 772. |
| [53] | Tan D H S, Banerjee A, Chen Z, et al. Author correction: from nanoscale interface characterization to sustainable energy storage using all-solid-state batteries[J]. Nature Nanotechnology, 2021, 16: 479. |
| [54] | Liang Y H, Liu H, Wang G X, et al. Challenges, interface engineering, and processing strategies toward practical sulfide-based all-solid-state lithium batteries[J]. InfoMat, 2022, 4(5): e12292. |
| [55] | Wagner C. The electrical conductivity of semi-conductors involving inclusions of another phase[J]. Journal of Physics and Chemistry of Solids, 1972, 33(5): 1051-1059. |
| [56] | Maier J. Ionic conduction in space charge regions[J]. Progress in Solid State Chemistry, 1995, 23(3): 171-263. |
| [57] | Liang C C. Conduction characteristics of the lithium iodide-aluminum oxide solid electrolytes[J]. Journal of the Electrochemical Society, 1973, 120(10): 1289. |
| [58] | Haruyama J, Sodeyama K, Han L Y, et al. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery[J]. Chemistry of Materials, 2014, 26(14): 4248-4255. |
| [59] | Xiao Y H, Miara L J, Wang Y, et al. Computational screening of cathode coatings for solid-state batteries[J]. Joule, 2019, 3(5): 1252-1275. |
| [60] | Strauss F, Teo J H, Maibach J, et al. Li2ZrO3-coated NCM622 for application in inorganic solid-state batteries: role of surface carbonates in the cycling performance[J]. ACS Applied Materials & Interfaces, 2020, 12(51): 57146-57154. |
| [61] | Luo Q Y, Yu C, Wei C C, et al. Enabling superior electrochemical performances of Li10SnP2S12-based all-solid-state batteries using lithium halide electrolytes[J]. Ceramics International, 2023, 49(7): 11485-11493. |
| [62] | Sakuda A, Hayashi A, Tatsumisago M. Interfacial observation between LiCoO2 electrode and Li2S-P2S5 solid electrolytes of all-solid-state lithium secondary batteries using transmission electron microscopy[J]. Chemistry of Materials, 2010, 22(3): 949-956. |
| [63] | Kim K H, Iriyama Y, Yamamoto K, et al. Characterization of the interface between LiCoO2 and Li7La3Zr2O12 in an all-solid-state rechargeable lithium battery[J]. Journal of Power Sources, 2011, 196(2): 764-767. |
| [64] | Zhu Y Z, He X F, Mo Y F. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations[J]. ACS Applied Materials & Interfaces, 2015, 7(42): 23685-23693. |
| [65] | Han F D, Zhu Y Z, He X F, et al. Electrochemical stability of Li10GeP2S12 and Li7La3Zr2O12 solid electrolytes[J]. Advanced Energy Materials, 2016, 6(8): 1501590. |
| [66] | Dong Z L, Gan Y, Martins V, et al. Novel sulfide-chloride solid-state electrolytes with tunable anion ratio for highly stable solid-state sodium-ion batteries[J]. Advanced Materials, 2025, n/a(n/a): 2503107. |
| [67] | Auvergniot J, Cassel A, Ledeuil J B, et al. Interface stability of argyrodite Li6PS5Cl toward LiCoO2, LiNi1/3Co1/3Mn1/3O2, and LiMn2O4 in bulk all-solid-state batteries[J]. Chemistry of Materials, 2017, 29(9): 3883-3890. |
| [68] | Li Y X, Daikuhara S, Hori S, et al. Oxygen substitution for Li-Si-P-S-Cl solid electrolytes toward purified Li10GeP2S12-type phase with enhanced electrochemical stabilities for all-solid-state batteries[J]. Chemistry of Materials, 2020, 32(20): 8860-8867. |
| [69] | Oh P P, Yun D J, Choi D J H, et al. Development of high-energy anodes for all-solid-state lithium batteries based on sulfide electrolytes[J]. Angewandte Chemie International Edition, 2022, 61(25): e202201249. |
| [70] | Jing S H, Wang K, Li S J, et al. An all-in-one approach for sulfide solid electrolyte with bidirectional stabilization shells enabling 4.6 V all-solid-state lithium batteries[J]. Energy Storage Materials, 2025, 76: 104131. |
| [71] | Hu X, Zhang Z J, Zhang X, et al. External-pressure–electrochemistry coupling in solid-state lithium metal batteries[J]. Nature Reviews Materials, 2024, 9(5): 305-320. |
| [72] | Lu Y, Zhao C Z, Yuan H, et al. Critical current density in solid-state lithium metal batteries: mechanism, influences, and strategies[J]. Advanced Functional Materials, 2021, 31(18): 2009925. |
| [73] | Liu J, Yuan H, Liu H, et al. Unlocking the failure mechanism of solid state lithium metal batteries[J]. Advanced Energy Materials, 2022, 12(4): 2100748. |
| [74] | Porz L, Swamy T, Sheldon B W, et al. Mechanism of lithium metal penetration through inorganic solid electrolytes[J]. Advanced Energy Materials, 2017, 7(20): 1701003. |
| [75] | Kasemchainan J, Zekoll S, Spencer Jolly D, et al. Critical stripping current leads to dendrite formation on plating in lithium anode solid electrolyte cells[J]. Nature Materials, 2019, 18(10): 1105-1111. |
| [76] | Yang D X, Gao D X, Jiang D M, et al. Grain boundary electronic insulation for high-performance all-solid-state lithium batteries[J]. Angewandte Chemie International Edition, 2023, 62(5): e202215680. |
| [77] | Pang M C, Yang K, Brugge R, et al. Interactions are important: Linking multi-physics mechanisms to the performance and degradation of solid-state batteries[J]. Materials Today, 2021, 49: 145-183. |
| [78] | Mangani L R, Villevieille C. Mechanical vs. chemical stability of sulphide-based solid-state batteries. Which one is the biggest challenge to tackle? Overview of solid-state batteries and hybrid solid state batteries[J]. Journal of Materials Chemistry A, 2020, 8(20): 10150-10167. |
| [79] | Kato A, Yamamoto M, Sakuda A, et al. Mechanical properties of Li2S-P2S5 glasses with lithium halides and application in all-solid-state batteries[J]. ACS Applied Energy Materials, 2018, 1(3): 1002-1007. |
| [80] | Masias A, Felten N, Garcia-Mendez R, et al. Elastic, plastic, and creep mechanical properties of lithium metal[J]. Journal of Materials Science, 2019, 54(3): 2585-2600. |
| [81] | Yu S, Siegel D J. Grain boundary softening: a potential mechanism for lithium metal penetration through stiff solid electrolytes[J]. ACS Applied Materials & Interfaces, 2018, 10(44): 38151-38158. |
| [82] | Doux J M, Nguyen H, Tan D H S, et al. Stack pressure considerations for room-temperature all-solid-state lithium metal batteries[J]. Advanced Energy Materials, 2020, 10(1): 1903253. |
| [83] | Wenzel S, Randau S, Leichtweiß T, et al. Direct observation of the interfacial instability of the fast ionic conductor Li10GeP2S12 at the lithium metal anode[J]. Chemistry of Materials, 2016, 28(7): 2400-2407. |
| [84] | Krauskopf T, Richter F H, Zeier W G, et al. Physicochemical concepts of the lithium metal anode in solid-state batteries[J]. Chemical Reviews, 2020, 120(15): 7745-7794. |
| [85] | Fan X L, Ji X, Han F D, et al. Fluorinated solid electrolyte interphase enables highly reversible solid-state Li metal battery[J]. Science Advances, 2018, 4(12): eaau9245. |
| [1] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [2] | 罗佳欣, 袁艳. 压电材料在固态金属二次电池中的研究进展[J]. 化工学报, 2025, 76(8): 3822-3833. |
| [3] | 王珺仪, 夏章讯, 景粉宁, 王素力. 基于重整气的高温聚合物电解质膜燃料电池电化学阻抗谱弛豫时间分布研究[J]. 化工学报, 2025, 76(7): 3509-3520. |
| [4] | 高凤凤, 程慧峰, 杨博, 郝晓刚. 电驱动NiFeMn LDH/CNTs/PVDF膜电极选择性提取钨酸根离子[J]. 化工学报, 2025, 76(7): 3350-3360. |
| [5] | 王子恒, 李文怀, 周嵬. 图形电极在固体氧化物燃料电池中的应用[J]. 化工学报, 2025, 76(7): 3153-3171. |
| [6] | 李长宇, 曾强, 肖杰, 张阳杰, 张政, 林元华. PVDF对LATP基固态电解质膜界面修饰研究[J]. 化工学报, 2025, 76(6): 2974-2982. |
| [7] | 盛全康, 陈奥, 陈龙, 张禹, 陈韶云, 胡成龙. 铅笔芯上原位生长有序聚苯胺阵列及其电化学储能[J]. 化工学报, 2025, 76(4): 1875-1884. |
| [8] | 徐桂培, 孙倩, 赖洁文, 卢毅锋, 邸会芳, 黄辉, 王振兵. 电化学双电层电容器失效机理的研究进展[J]. 化工学报, 2025, 76(3): 951-962. |
| [9] | 钟晓航, 许卫, 张文, 许莉, 王宇新. 碱性水电解制氢中铁杂质的影响研究进展[J]. 化工学报, 2025, 76(2): 519-531. |
| [10] | 王梦涵, 于淼, 吴桐. 锂硫电池电解液研究进展:分子设计与应用[J]. 化工学报, 2025, 76(12): 6196-6217. |
| [11] | 双舒炎, 张伟, 王家乐, 王军锋. 基于多孔镍电极的液相放电等离子体分解甲醇制氢实验研究[J]. 化工学报, 2025, 76(11): 5753-5763. |
| [12] | 赵海霞, 刘洋, 王雷, 高凤凤, 郭志远, 张盼盼, 汪婧, 纪志永. 电化学法油气田采出水同步提锂溴及制溴化锂[J]. 化工学报, 2025, 76(10): 5213-5224. |
| [13] | 王天闻, 闫肃, 赵梦园, 杨天让, 刘建国. 固体氧化物电池空气电极铬中毒机理及抗铬性能研究进展[J]. 化工学报, 2024, 75(6): 2091-2108. |
| [14] | 孙铭泽, 黄鹤来, 牛志强. 铂基氧还原催化剂:从单晶电极到拓展表面纳米材料[J]. 化工学报, 2024, 75(4): 1256-1269. |
| [15] | 郭邦军, 贾理男, 张希. 全固态硫化物锂电池中NCM正极及其界面研究[J]. 化工学报, 2024, 75(3): 743-759. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
