化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6196-6217.DOI: 10.11949/0438-1157.20250331
王梦涵1,2,3,4( ), 于淼1,2,3,4, 吴桐1,2,3,4(
), 于淼1,2,3,4, 吴桐1,2,3,4( )
)
收稿日期:2025-04-01
修回日期:2025-05-22
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
吴桐
作者简介:王梦涵(2001—),女,硕士研究生,1146532814@qq.com
基金资助:
Menghan WANG1,2,3,4( ), Miao YU1,2,3,4, Tong WU1,2,3,4(
), Miao YU1,2,3,4, Tong WU1,2,3,4( )
)
Received:2025-04-01
Revised:2025-05-22
Online:2025-12-31
Published:2026-01-23
Contact:
Tong WU
摘要:
锂硫(Li-S)电池作为一种具有高能量密度的储能体系,是能源领域的重点研究对象。但其在充放电过程中可溶性多硫化锂的穿梭效应及氧化还原动力学缓慢等问题,严重影响了锂硫电池的商业化发展进程。电解液作为锂硫电池体系中不可或缺的一部分,通常由锂盐、有机溶剂以及各类添加剂共同组成,在充放电过程中起到保护锂负极、传导锂离子的作用,其功能对电池的反应过程、循环寿命和安全性能等方面均产生影响。近年来,电解液添加剂备受研究者的关注,将其加入到电解液中可实现抑制多硫化物(LiPSs)穿梭、增强电极界面稳定性和提高离子电导率等功能。通过对近期相关文献的调查,介绍了含氮、氟、硫和多原子协同作用的添加剂的分子设计,及其提升电池充放电的氧化还原动力学和抑制多硫化物穿梭效应的策略及研究进展,并分析其因电负性的差异,在调控多硫化物和电极界面表现出的不同机制。简要阐述了锂盐在电解液中的重要作用,包括提供锂离子,参与电极反应过程,维持电荷平衡和保障离子传导等,为锂硫电池电解液添加剂的分子设计与实际应用拓展了思路。最后展望了锂硫电池电解液添加剂的未来发展方向。
中图分类号:
王梦涵, 于淼, 吴桐. 锂硫电池电解液研究进展:分子设计与应用[J]. 化工学报, 2025, 76(12): 6196-6217.
Menghan WANG, Miao YU, Tong WU. Research progress of electrolyte for lithium-sulfur batteries: molecular design and application[J]. CIESC Journal, 2025, 76(12): 6196-6217.
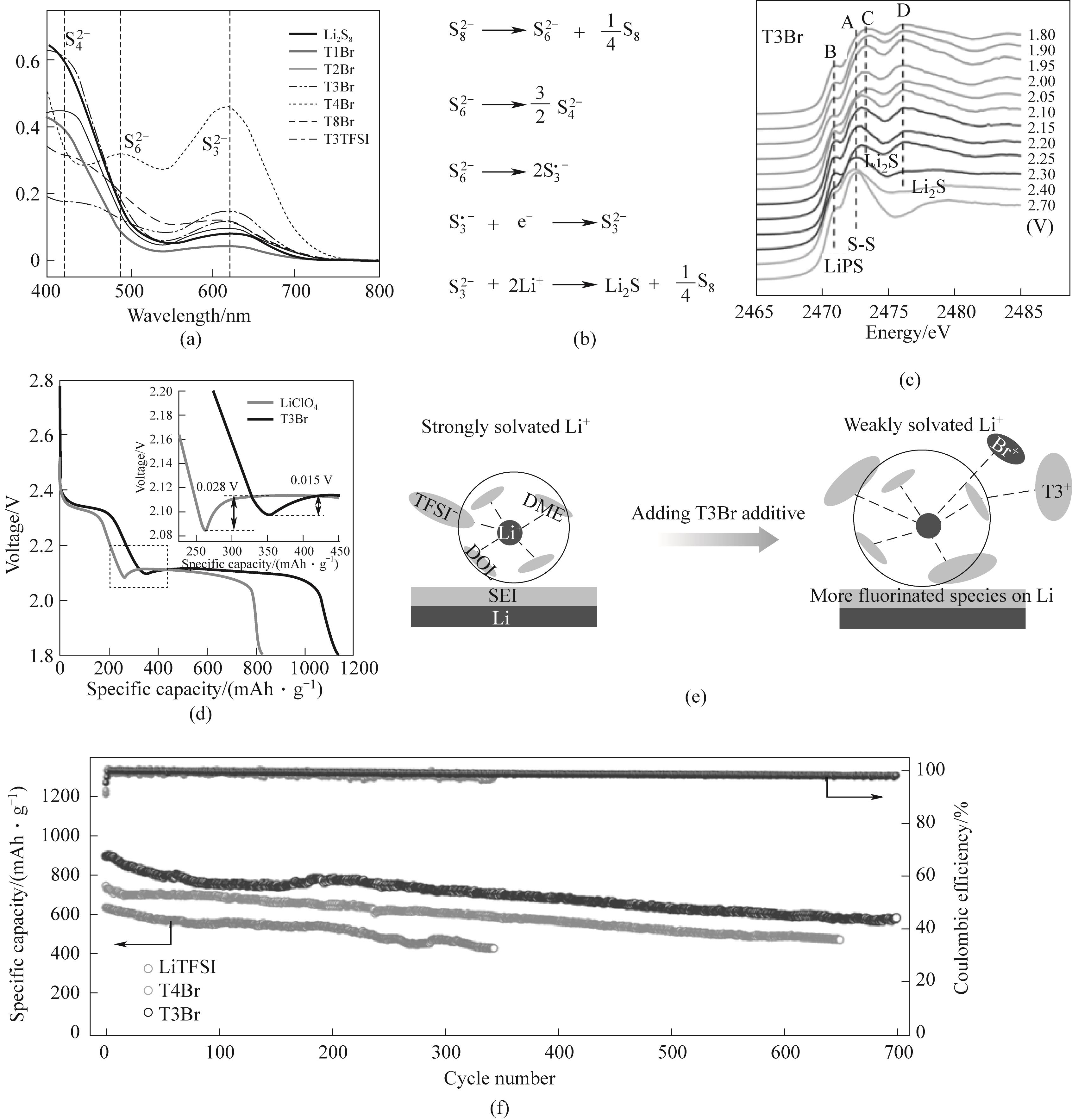
图2 (a)Li2S8溶液与QASs、T1Br、T2Br、T3Br、T4Br、T8Br和T3TFSI的紫外-可见光谱;(b)反应方程;(c)T3Br电解液中正极放电时的S K边近边结构(XANES)谱;(d)电极在T3Br和空白电解液中放电过程电压曲线;(e)空白电解液和T3Br电解液对锂表面电化学界面演变影响的示意图;(f)1C(相应电流下,一次放电持续1小时)的长循环性能[45]
Fig.2 (a) Ultraviolet-visible spectra of Li2S8 solution with QASs, T1Br, T2Br, T3Br, T4Br, T8Br and T3TFSI; (b) Reaction equation; (c) S-K-edge Near Edge Structure (XANES) Spectrum during Positive Electrode Discharge in T3Br Electrolyte; (d) Voltage curves of the electrode during the discharge process in T3Br and blank electrolyte; (e) Schematic diagram of the influence of blank electrolytes and T3Br electrolytes on the evolution of the electrochemical interface on the lithium surface; (f) Long cycle performance of 1C (Under the corresponding current, one discharge lasts for 1 hour)[45]
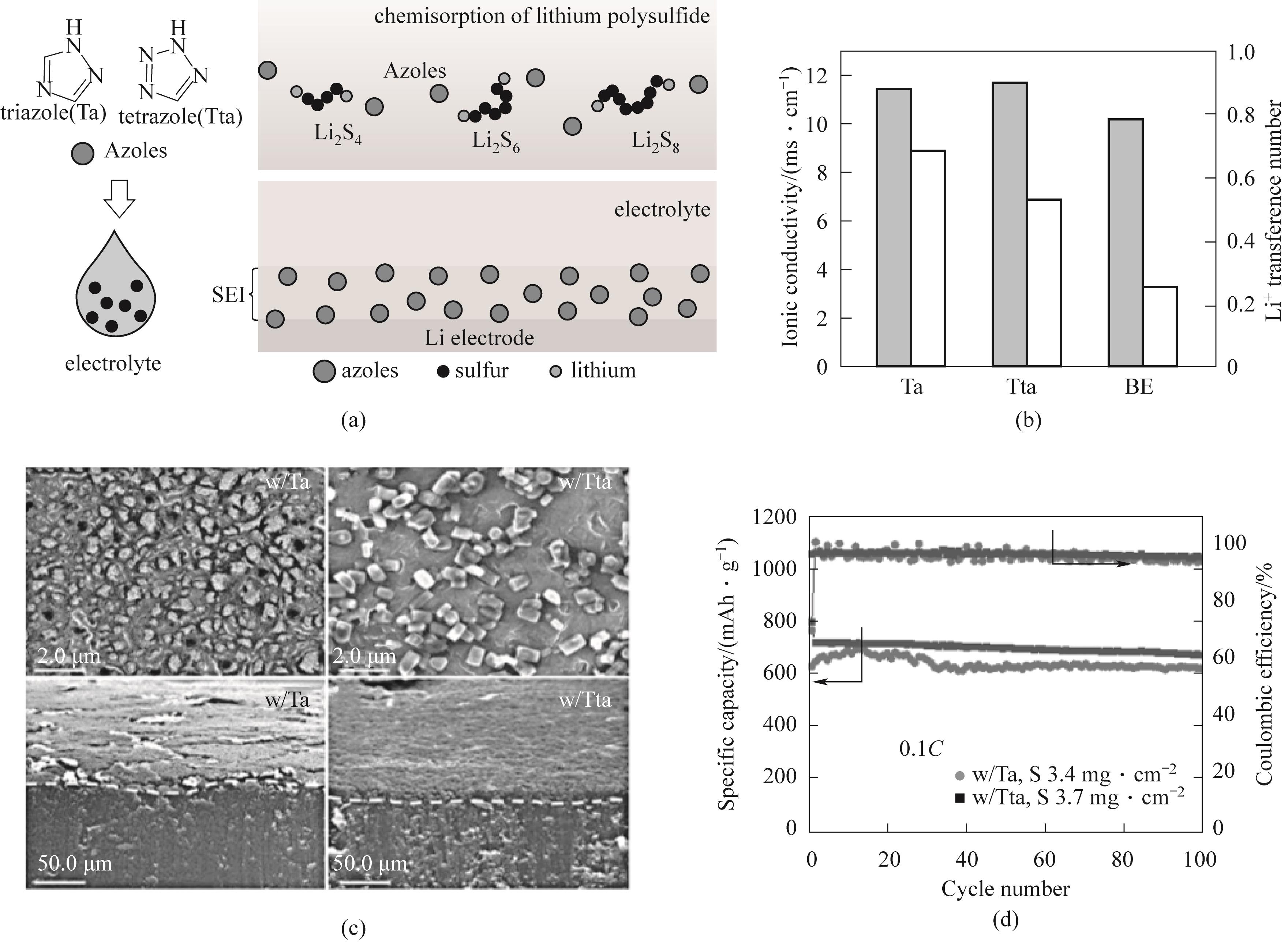
图3 (a)唑-Li2S n (n=4,6,8)配合物的结构及唑类化合物参与固体电解质界面膜的形成并抑制锂枝晶形成示意图;(b)离子电导率和 Li+转移数;(c)循环50次后Li|Li对称电池锂负极扫描电子显微镜(SEM)图相应横截面SEM图像;(d)0.1C,硫负载为3.4 mg·cm-2、3.7 mg·cm-2,使用Ta、Tta基电解液的高负载Li-S电池的循环性能[46]
Fig.3 (a) The structure of the oxazole Li2S n (n=4,6,8) complex and the schematic diagram of the oxazole compounds participating in the formation of solid electrolyte interface facial mask and inhibiting the formation of lithium dendrites; (b) Ionic conductivity and Li+transfer number; (c) S After 50 cycles, the scanning electron microscope (SEM) image of the Li | Li symmetric battery’s lithium negative electrode corresponds to the cross-sectional SEM image; (d) 0.1C, Cycle performance of high load Li-S batteries with sulfur loadings of 3.4 mg·cm-2 and 3.7 mg·cm-2 using Ta and Tta based electrolytes[46]
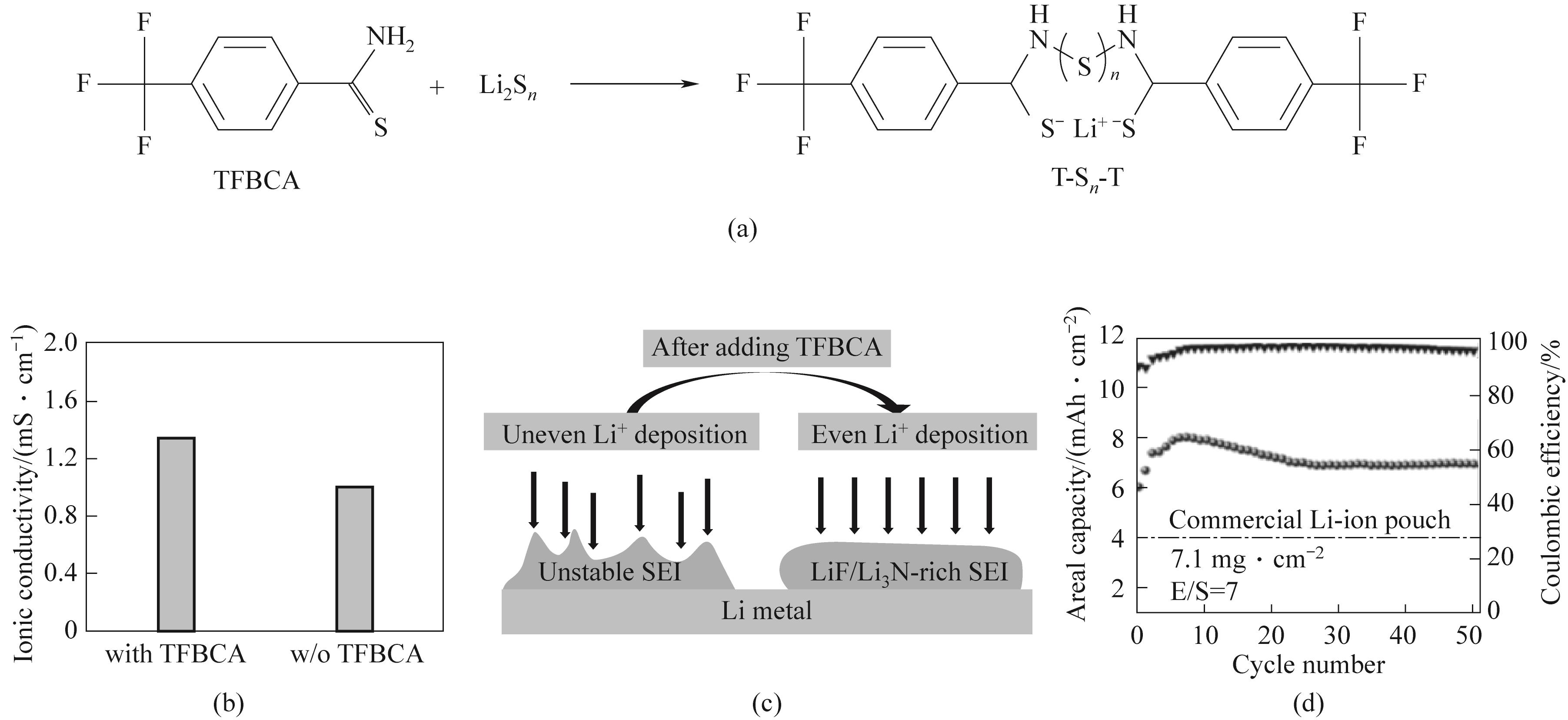
图4 (a)TFBCA和多硫化物之间反应的示意图;(b)含和不含TFBCA的电解液的离子电导率;(c)含和不含TFBCA的SEI示意图;(d)0.1C,硫负载为7.1 mg·cm-2条件下使用TFBCA电池循环性能[47]
Fig.4 (a) Schematic diagram of the reaction between TFBCA and polysulfides; (b) Ionic conductivity of electrolytes with and without TFBCA; (c) SEI schematic diagram with and without TFBCA; (d) Cycle performance of TFBCA battery under 0.1C, sulfur load of 7.1 mg·cm-2[47]
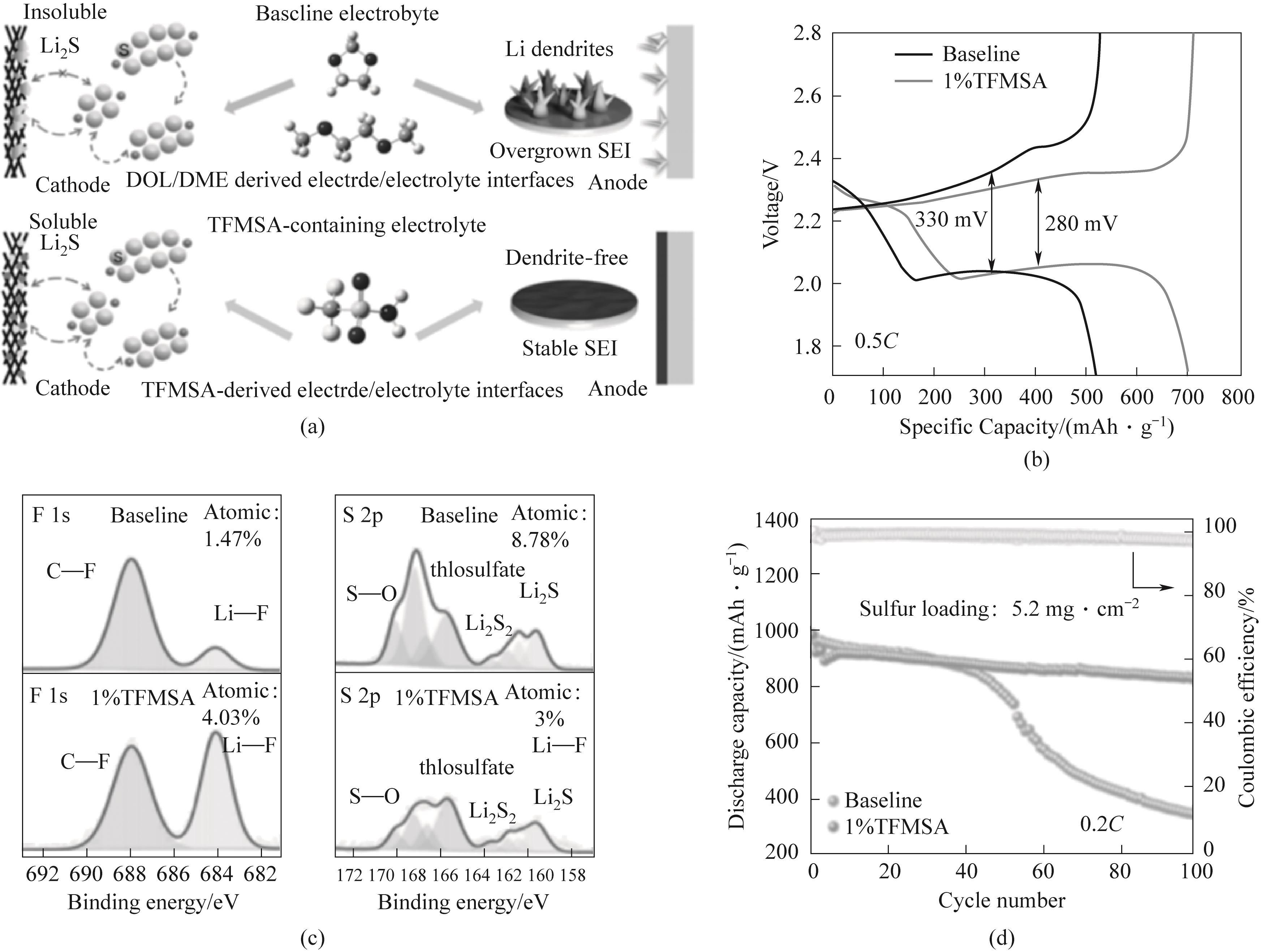
图5 (a)TFMSA添加剂对锂硫电池电极/电解液界面的影响示意图;(b)含空白电解液和含TFMSA电解液电池在0.5C倍率下的充放电曲线;(c)使用空白电解液和TFMSA电解液循环后的表面F 1s和S 2p XPS光谱;(d)0.2C,硫负载为5.2 mg·cm-2锂硫电池循环性能[50]
Fig.5 (a) Schematic diagram of the effect of TFMSA additive on the electrode/electrolyte interface of lithium sulfur batteries; (b) Charge discharge curves of batteries containing blank electrolyte and TFMSA electrolyte at 0.5C rate; (c) Surface F 1s and S 2p XPS spectra after cycling with blank electrolyte and TFMSA electrolyte; (d) 0.2C, Cycle performance of lithium sulfur battery with sulfur load of 5.2 mg·cm-2[50]
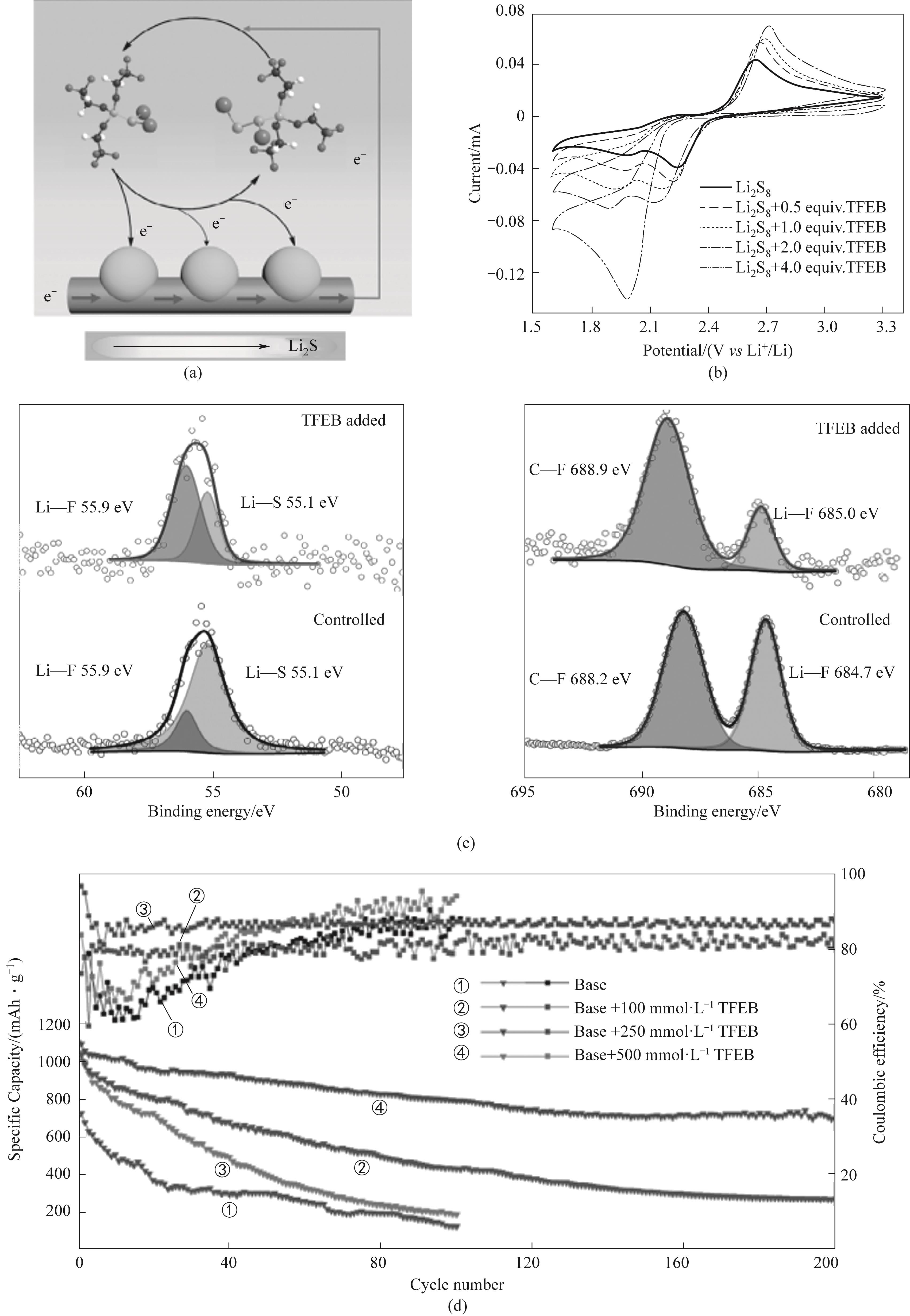
图6 (a)TFEB-Li2S x 介质氧化还原催化硫转化;(b)2 mmol·L-1 Li2S8正极电解液与不同浓度TFEB在200 mV·S-1下的循环伏安图;(c)添加TFEB和不添加TFEB电解液中循环锂负极的XPS光谱;(d)0.1C,硫负载为5 mg·cm-2不同TFEB含量的锂硫电池循环稳定性[51]
Fig.6 (a) TFEB-Li2S x medium redox catalytic sulfur conversion; (b) Cyclic voltammetry of 2 mmol·L-1 Li2S8 positive electrode electrolyte with different concentrations of TFEB at 200 mV·S-1; (c) XPS spectra of circulating lithium negative electrode with and without TFEB electrolyte; (d) 0.1C, Cycle stability of lithium sulfur batteries with sulfur loading of 5 mg·cm-2 and different TFEB contents[51]
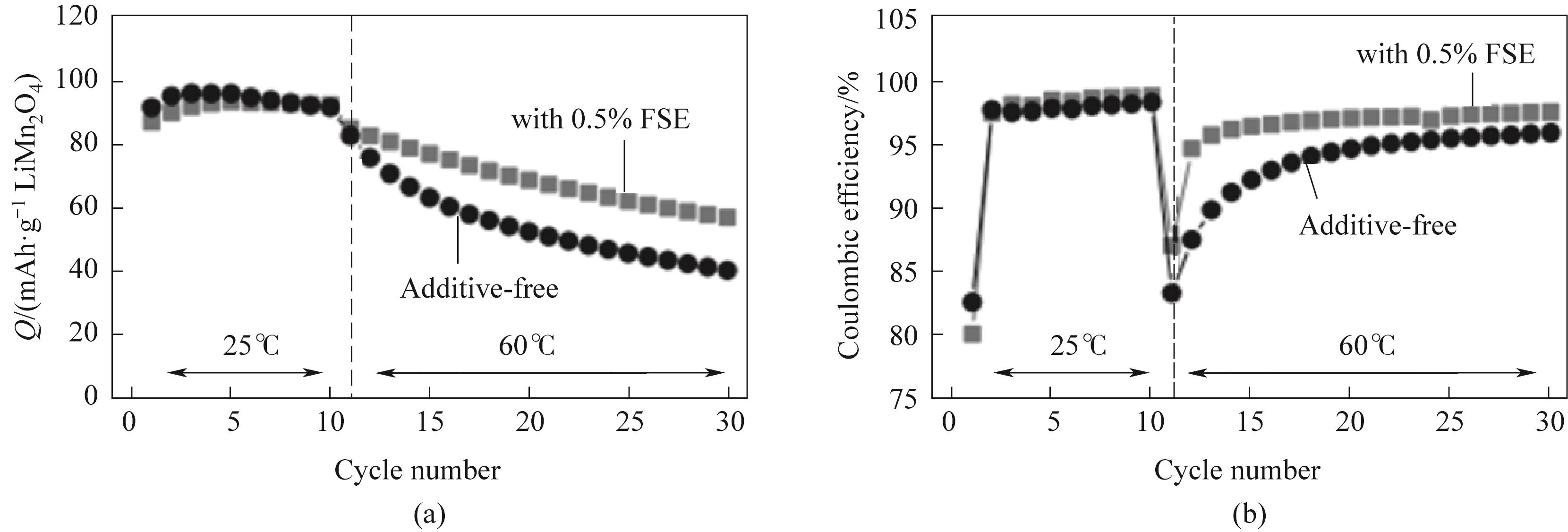
图7 (a)石墨/LiMn2O4电池的放电容量和(b)库仑效率(10 mA·g-1 LiMn2O4)在无FSE和0.5%(质量分数)FSE添加条件下,在室温下进行10次初始循环后,再在60℃下进行第11至30次循环[53]
Fig.7 (a) Discharge capacity and (b) Coulombic efficiency (10 mA·g-1 LiMn2O4) of graphite/LiMn2O4 battery under the conditions of no FSE and 0.5% (mass) FSE addition, after 10 initial cycles at room temperature, the 11th to 30th cycles were carried out at 60℃[53]
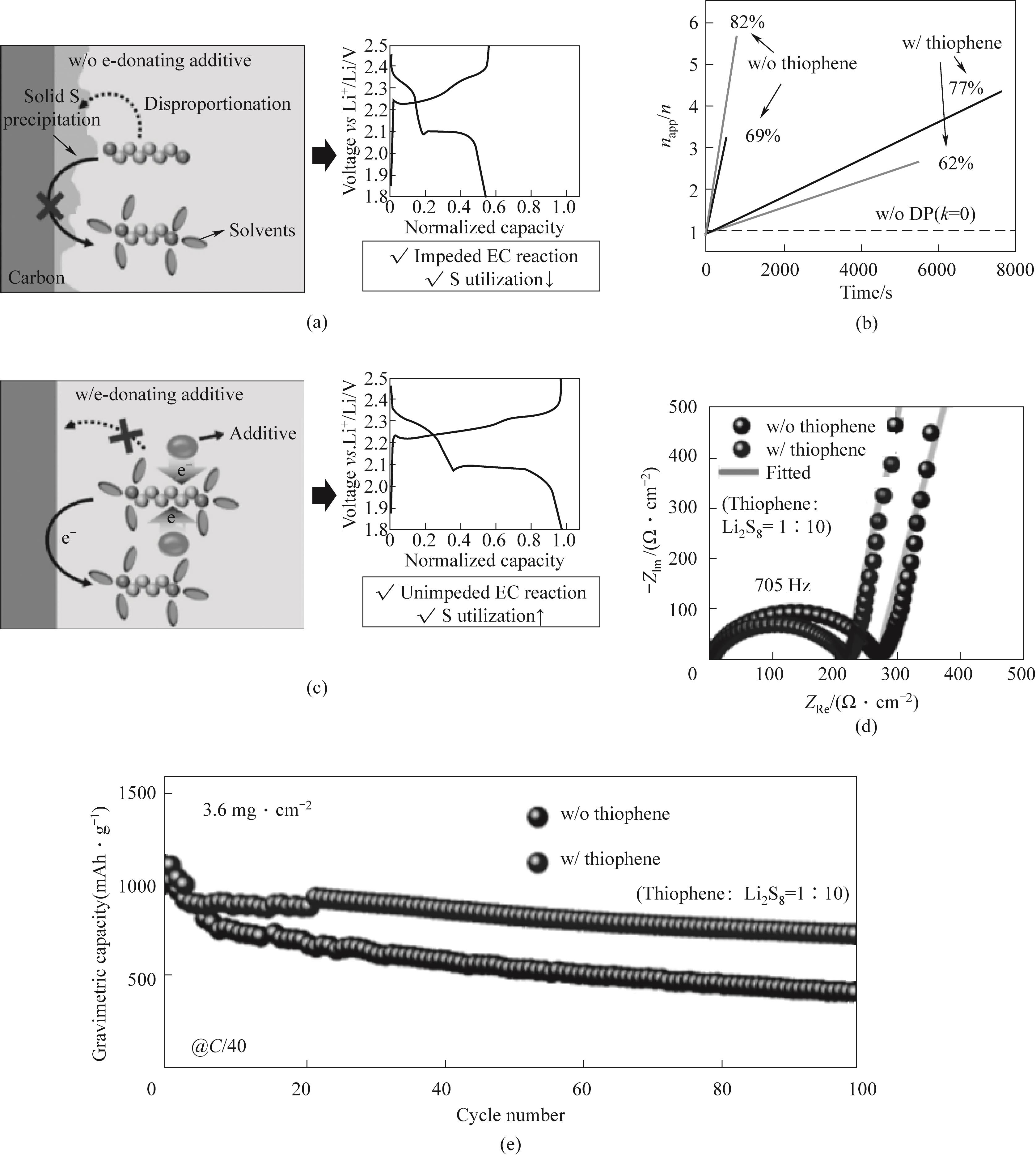
图8 正极上多硫化锂的反应路径和Li-S电池的充放电曲线无添加剂(a)和有添加剂(c);napp/n图(b);(d)有和没有噻吩电池的对称硫/碳电池在105至10-2 Hz的频率范围内具有10 mV幅度扰动的电化学阻抗谱(EIS)图;(e)C/40,硫负载为3.6 mg·cm-2锂硫电池电化学性能[62]
Fig.8 Reaction pathway of lithium polysulfide on the positive electrode and charge discharge curve of Li-S battery (a) without additives and (c) additives; (b) napp/n diagram; (d) Electrochemical impedance spectroscopy (EIS) plots of symmetric sulfur/carbon batteries with and without thiophene batteries exhibiting a 10 mV amplitude perturbation in the frequency range of 105 to 10-2 Hz; (e) C/40, Electrochemical performance of lithium sulfur battery with sulfur loading of 3.6 mg·cm-2[62]
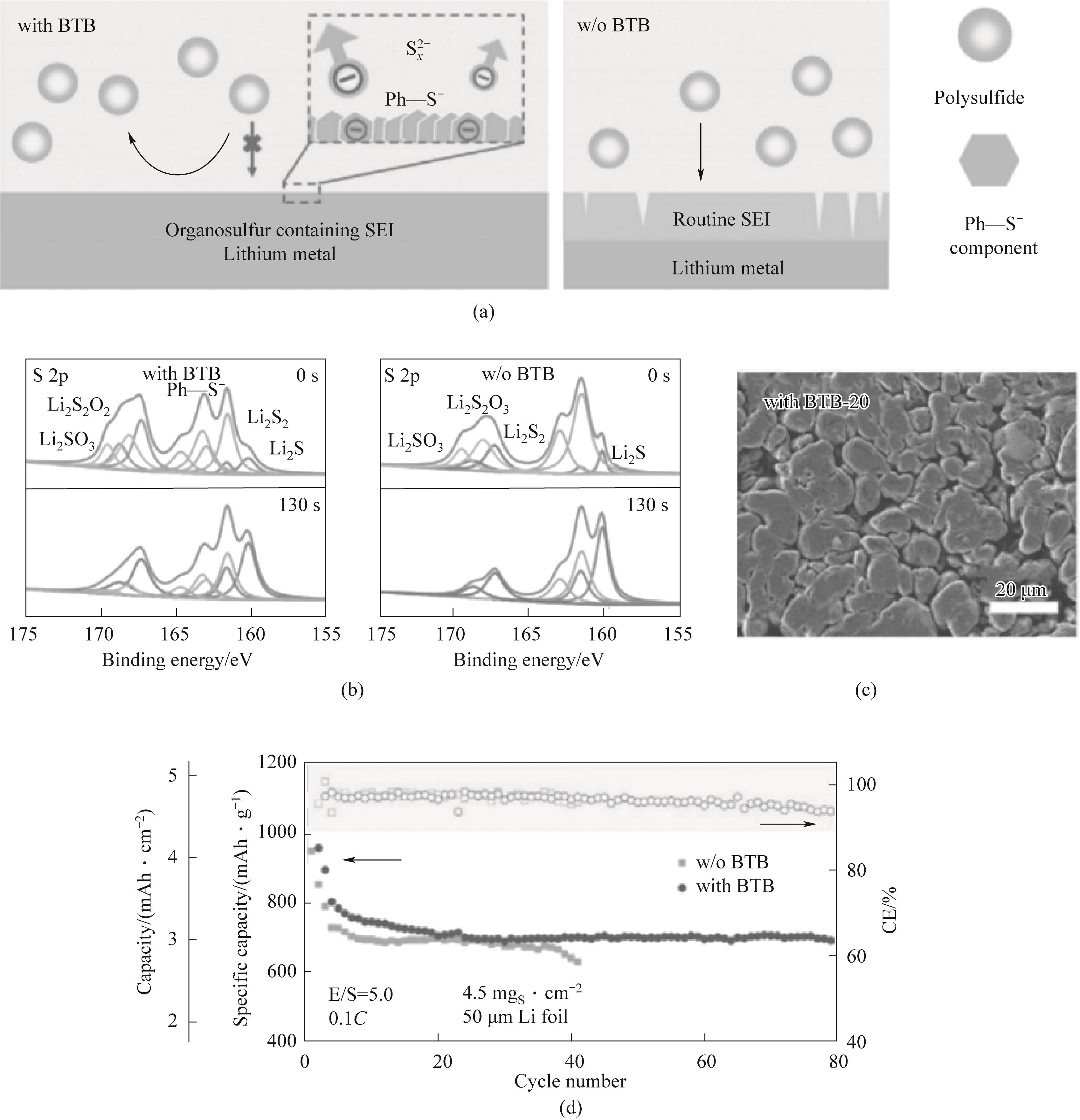
图9 (a)固体电解质界面膜的形成示意图;(b)5次循环后锂负极的含和不含BTB添加剂的SEI的XPS深度剖面;(c)20次循环后Li-S电池中含和不含BTB添加剂的循环锂负极的SEM图;(d)0.1C,高负载S正极、低E/S比及超薄锂负极时的循环性能[63]
Fig. 9 (a) Schematic diagram of the formation of solid electrolyte interface facial mask; (b) XPS depth profiles of SEI with and without BTB additive for lithium negative electrode after 5 cycles; (c) SEM of cycling Li negative electrode with and without BTB additive in Li-S battery after 20 cycles; (d) 0.1C, Cycle performance of high load S positive electrode, low E/S ratio, and ultra-thin Li negative electrode[63]
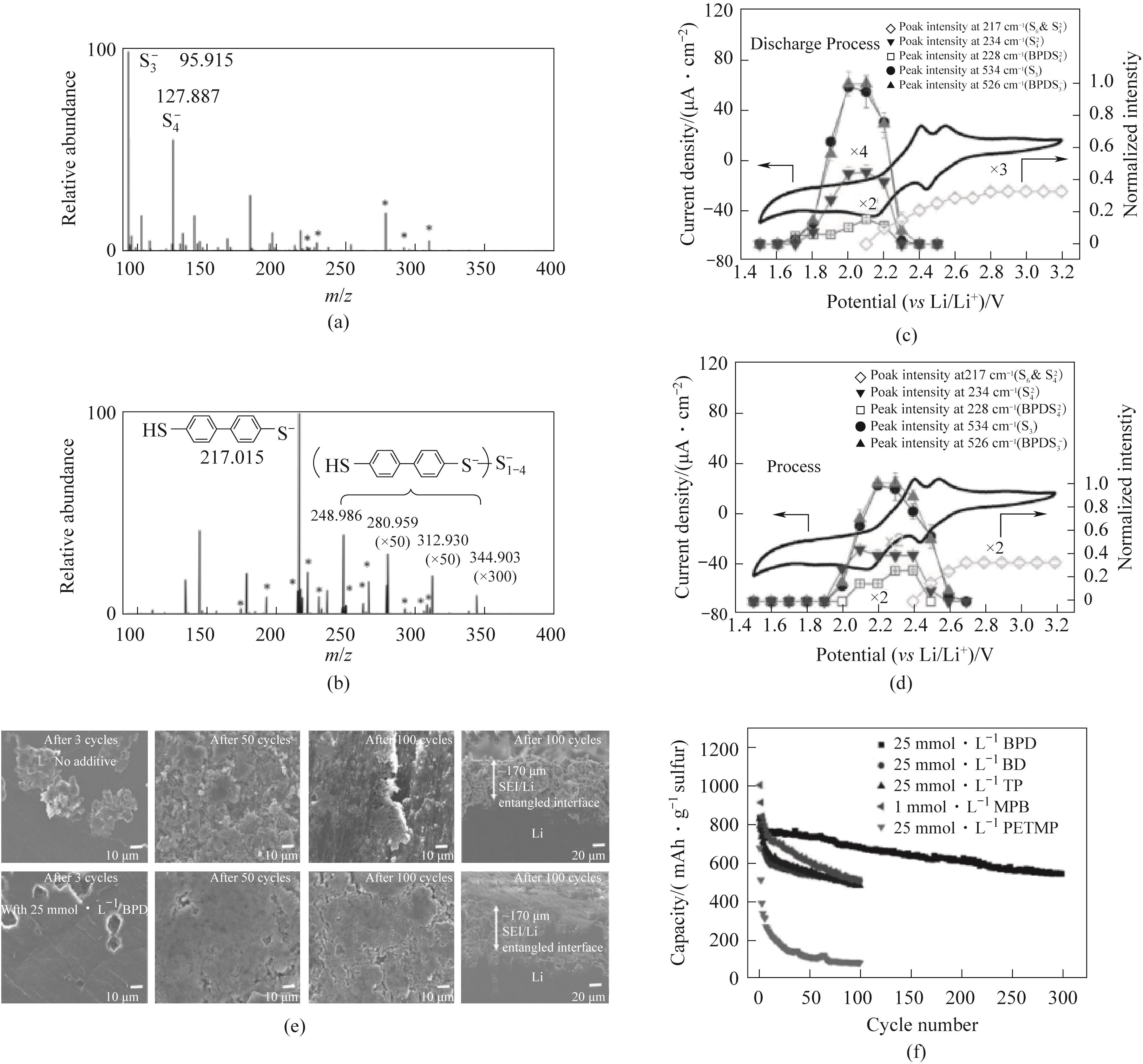
图10 (a)未加入和(b)加入BPD的Li2S4溶液ESI-MS图;在(c)放电和(d)充电过程中,添加5 mmol·L-1 BPD的碱性电解液中获得的硫-碳正极的CV和在217、228、234、526和534 cm-1处的拉曼峰;(e)0.1C循环后,在有和没有添加25 mmol·L-1的BPD的锂负极SEM图;(f)0.1C不同硫醇基添加剂对硫碳正极的循环性能[64]
Fig.10 (a) not included; (b) Electrospray ionization mass spectrum of Li2S4 solution added with BPD; The CV and Raman peaks at 217, 228, 234, 526, and 534 cm-1 of the sulfur carbon cathode obtained in alkaline electrolyte with 5 mmol·L-1 BPD during (c) discharge and (d) charge processes; (e) SEM of lithium negative electrode with and without the addition of 25 mmol·L-1 BPD after 0.1C cycling; (f) The cyclic performance of sulfur carbon cathode with different thiol based additives at 0.1C[64]
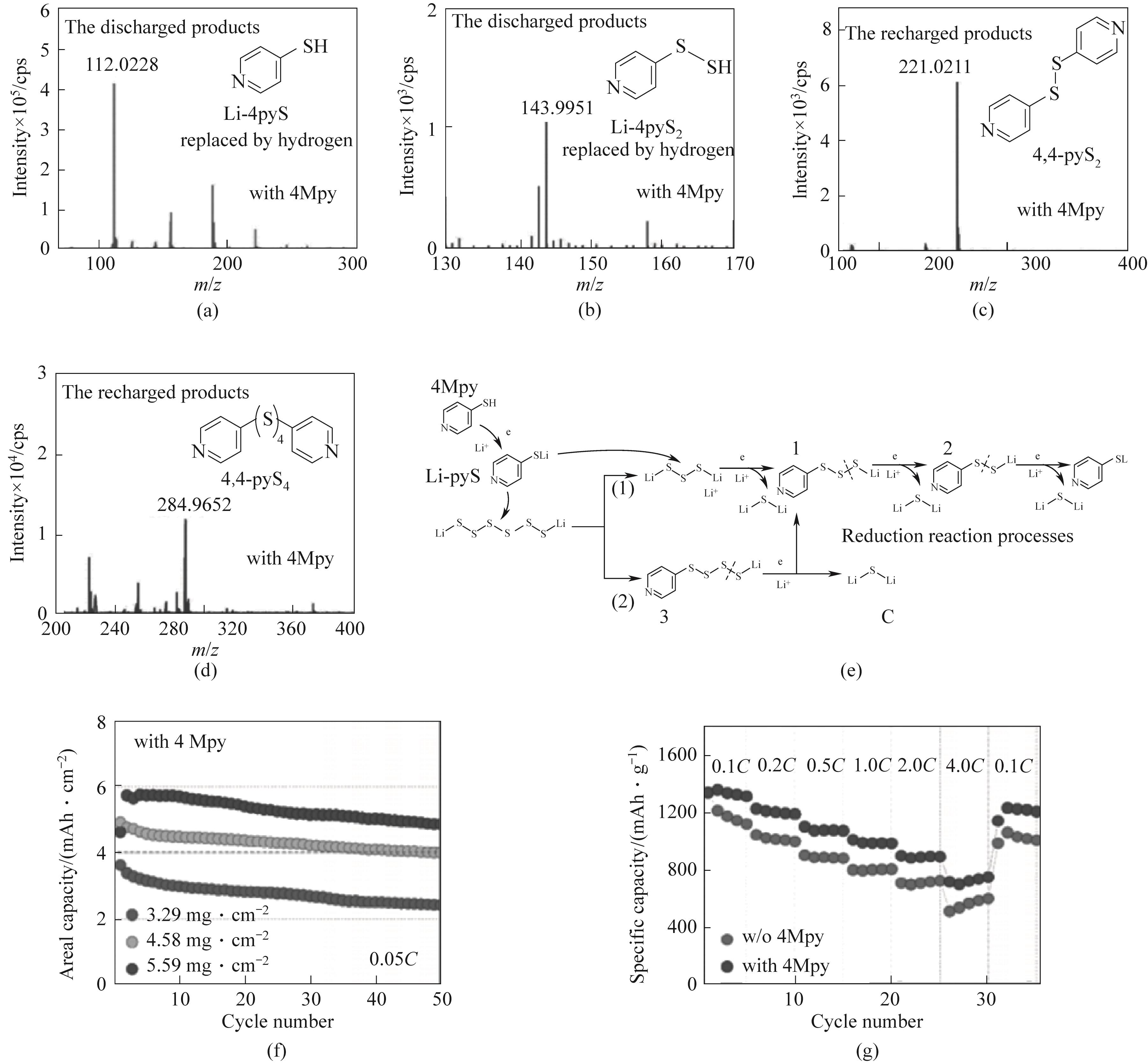
图11 添加4Mpy的锂硫电池的放电产物的液相色谱-质谱(LC-MS)谱图(a)锂-吡啶硫醇盐(Li-pyS)和(b)(Li-pyS2);充电产物的LC-MS谱图(c)4,4-吡啶二硫化物(4,4-pyS2)和(d)4,4-吡啶四硫化物(4,4-pyS4);(e)含有4Mpy添加剂的锂硫电池放电还原过程反应过程;(f)在高硫负载0.05C下的循环性能;(g)0.1C至4C下的倍率性能[73]
Fig.11 LC-MS spectra of discharged products in Li-S batteries with 4Mpy additive: (a) lithium-pyridinethiolate (Li-pyS) and (b) Li-pyS2; LC-MS spectra of recharged products: (c) 4,4-pyridinedisulfide (4,4-pyS2) and (d) 4,4-pyridinetetrasulfide (4,4-pyS4);(e) Discharge reduction process of Li-S batteries with 4Mpy additive; (f) Cycle performance under high sulfur load of 0.05C; (g) Rate performance at 0.1C to 4C[73]
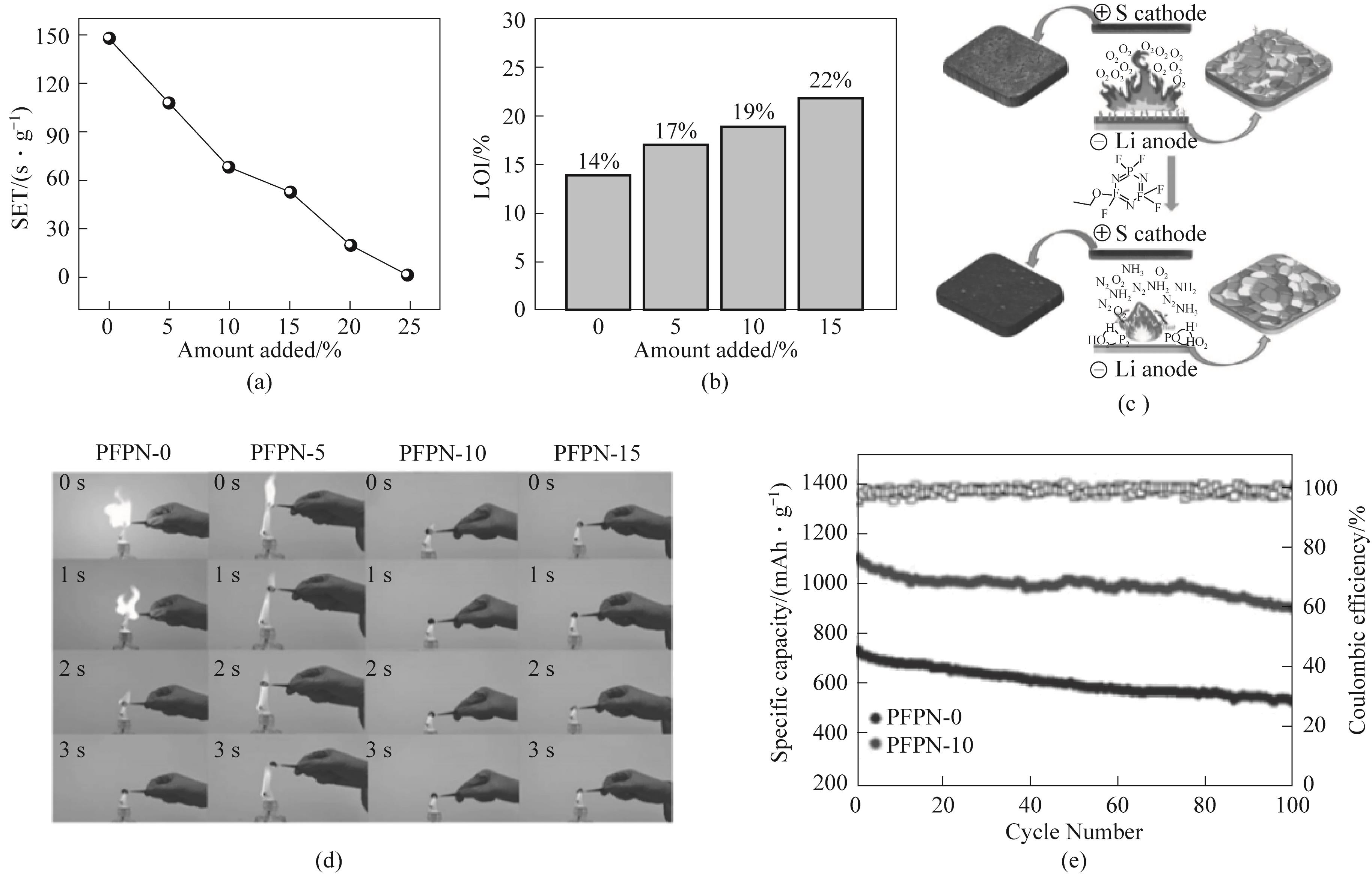
图12 (a)不同电解液的自熄时间测试;(b)不同电解液的极限氧指数;(c)PFPN阻燃机理示意图及其对电极表面SEI的影响;(d)乙醚基电解液中含PFPN的循环硫正极在50次循环后的燃烧测试;(e)0.2C循环性能[74]
Fig.12 (a) Self extinguishing time test of different electrolytes; (b) Limit oxygen index of different electrolytes; (c) Schematic diagram of PFPN flame retardant mechanism and its influence on electrode surface SEI; (d) Combustion test of cyclic sulfur positive electrode containing PFPN in ether based electrolyte after 50 cycles; (e) 0.2C Cycle performance[74]
| 特征原子 | 添加剂 | 面载量/ (mg·cm-2) | 放电比容量/面积容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| N | 卤化季铵盐-四丙基溴化铵(T3Br) | 1.5~2.0 | 1132 mAh·g-1(0.1C) | 1C,700次循环后放电比容量590 mAh·g-1 | [ |
| 三唑(Ta)、四唑(Tta) | 3.4、3.7 | 800 mAh·g-1、约790 mAh·g-1(0.1C) | 0.1C,100次循环后容量保持率96.5%(Ta)87.5%(Tta) | [ | |
| 4-(三氟甲基)硫代苯甲酰胺(TFBCA) | 7.1 | 8.1 mAh cm-2 (0.2C) | 0.1C,50次循环后面积容量7 mAh·cm-2 | [ | |
| F | 三氟甲磺酰胺(TFMSA) | 5.2 | 1000 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量828 mAh·g-1 | [ |
| 三(2,2,2-三氟乙基)硼酸酯(TFEB) | 5.0 | 1084 mAh·g-1(0.1C) | 0.1C,200次循环后容量保持率63.3% | [ | |
| 1,2-双(二氟甲基硅基)乙烷(FSE)(锂离子电池) | — | 约90 mAh (g of LiMn2O4) -1 (60℃ 10 mA (g of LiMn2O4) -1) | 60℃,20次循环后放容量保持率超过62%;从室温升到60℃时,电池的库仑效率立即恢复 | [ | |
| S | 噻吩 | 3.6 | 1016 mAh·g-1(C/40) | C/40,100次循环后容量保持率74% | [ |
| 3,5-双(三氟甲基)苯硫酚(BTB) | 4.5 | 950 mAh·g-1(0.1C) | 0.1C,82次循环后放电比容量700 mAh·g-1 | [ | |
| 联苯-4,4′-二硫醇(BPD) | 0.7~1.2 | 900 mAh·g-1(0.1C) | 0.1C,300次循环后放电比容量575 mAh·g-1 | [ | |
| N、S | 4-巯基吡啶(4Mpy) | 5.59 | 6 mAh·cm-2(0.05C) | 0.05C,50次循环后面积容量4.85 mAh·cm-2 | [ |
| N、F | 乙氧基(五氟)环三磷腈(PFPN) | 2.3 | 1103.4 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量904.6 mAh·g-1;使用PFPN电池循环50次后硫正极点燃后迅速熄灭 | [ |
表1 采用不同电解液添加剂组装锂硫电池的性能参数
Table 1 Performance parameters of lithium sulfur batteries assembled using different electrolyte additives
| 特征原子 | 添加剂 | 面载量/ (mg·cm-2) | 放电比容量/面积容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| N | 卤化季铵盐-四丙基溴化铵(T3Br) | 1.5~2.0 | 1132 mAh·g-1(0.1C) | 1C,700次循环后放电比容量590 mAh·g-1 | [ |
| 三唑(Ta)、四唑(Tta) | 3.4、3.7 | 800 mAh·g-1、约790 mAh·g-1(0.1C) | 0.1C,100次循环后容量保持率96.5%(Ta)87.5%(Tta) | [ | |
| 4-(三氟甲基)硫代苯甲酰胺(TFBCA) | 7.1 | 8.1 mAh cm-2 (0.2C) | 0.1C,50次循环后面积容量7 mAh·cm-2 | [ | |
| F | 三氟甲磺酰胺(TFMSA) | 5.2 | 1000 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量828 mAh·g-1 | [ |
| 三(2,2,2-三氟乙基)硼酸酯(TFEB) | 5.0 | 1084 mAh·g-1(0.1C) | 0.1C,200次循环后容量保持率63.3% | [ | |
| 1,2-双(二氟甲基硅基)乙烷(FSE)(锂离子电池) | — | 约90 mAh (g of LiMn2O4) -1 (60℃ 10 mA (g of LiMn2O4) -1) | 60℃,20次循环后放容量保持率超过62%;从室温升到60℃时,电池的库仑效率立即恢复 | [ | |
| S | 噻吩 | 3.6 | 1016 mAh·g-1(C/40) | C/40,100次循环后容量保持率74% | [ |
| 3,5-双(三氟甲基)苯硫酚(BTB) | 4.5 | 950 mAh·g-1(0.1C) | 0.1C,82次循环后放电比容量700 mAh·g-1 | [ | |
| 联苯-4,4′-二硫醇(BPD) | 0.7~1.2 | 900 mAh·g-1(0.1C) | 0.1C,300次循环后放电比容量575 mAh·g-1 | [ | |
| N、S | 4-巯基吡啶(4Mpy) | 5.59 | 6 mAh·cm-2(0.05C) | 0.05C,50次循环后面积容量4.85 mAh·cm-2 | [ |
| N、F | 乙氧基(五氟)环三磷腈(PFPN) | 2.3 | 1103.4 mAh·g-1(0.2C) | 0.2C,100次循环后放电比容量904.6 mAh·g-1;使用PFPN电池循环50次后硫正极点燃后迅速熄灭 | [ |
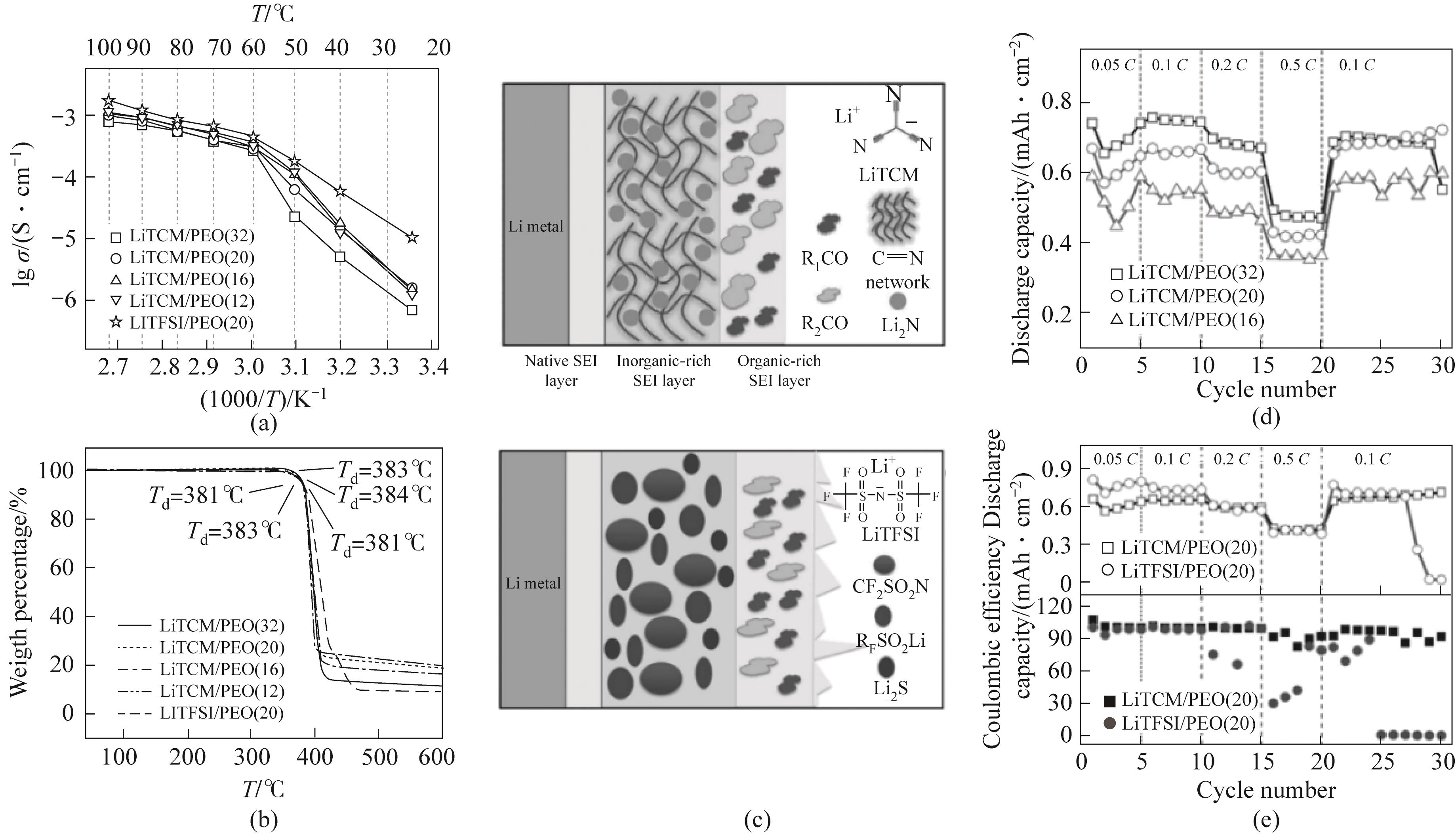
图13 (a)离子电导率的Arrhenius图;(b)电解质的热重(TGA)曲线;(c)LiTCM和LiTFSI电池中锂负极上形成的固体电解质界面膜的示意图;(d)不同盐含量下,LiTCM/PEO电解质的倍率性能;(e)在相同环氧乙烷单元(EO)/Li比为20的情况下,使用LiX/PEO(X = TCM-或TFSI-)电解质的锂硫电池循环性能对比[88]
Fig.13 (a) Arrhenius plot of ion conductivity; (b) Thermogravimetric analysis (TGA) curve of electrolyte; (c) Schematic diagram of solid electrolyte interface facial mask formed on lithium cathode in LiTCM and LiTFSI batteries; (d) The rate performance of LiTCM/PEO electrolyte under different salt contents; (e) Comparison of cycling performance of lithium sulfur batteries using Li X /PEO (X=TCM- or TFSI-) electrolyte under the same ethylene oxide unit (EO) / Li ratio of 20[88]
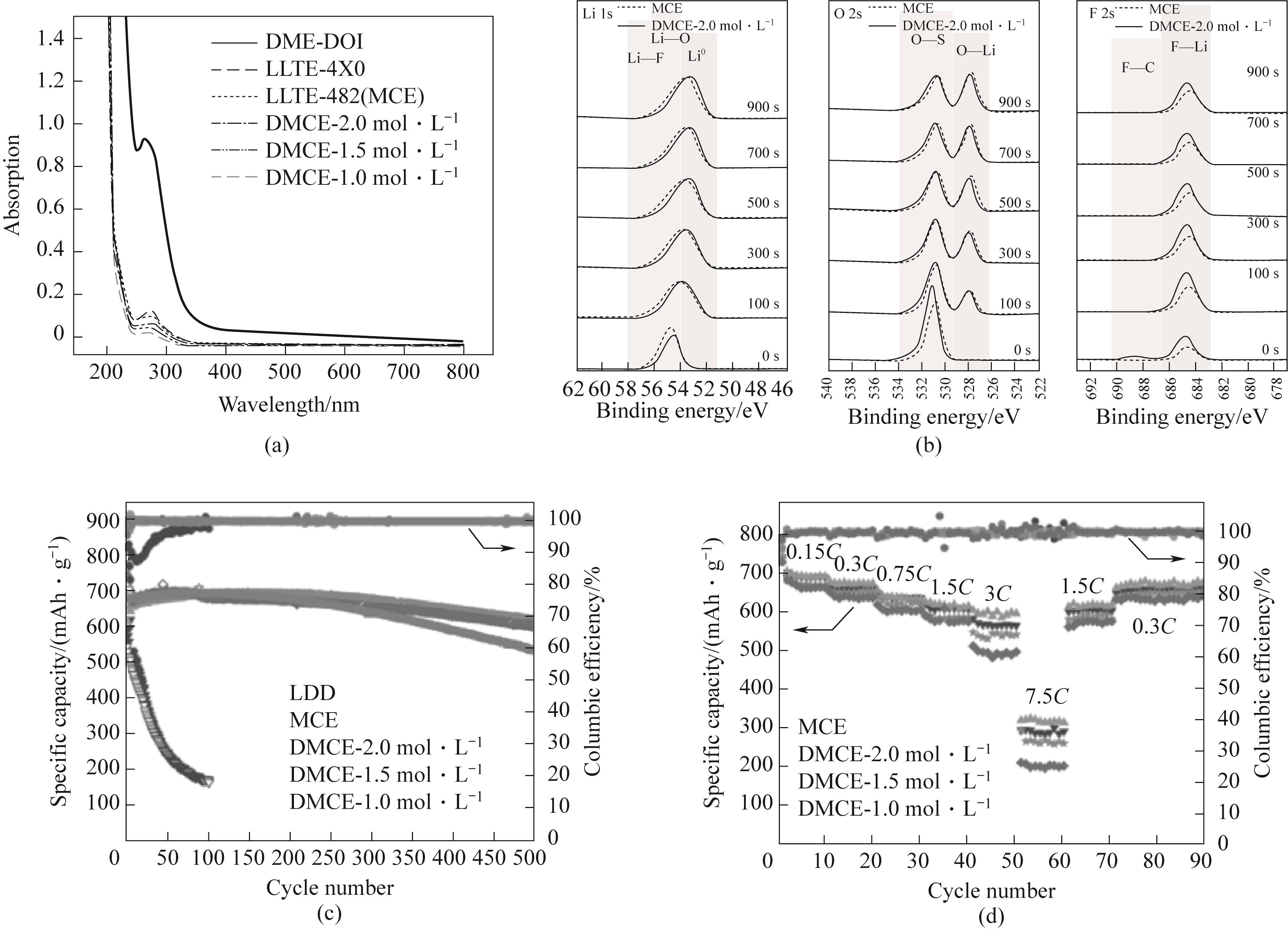
图14 (a)紫外-可见吸收光谱;(b)锂电极表面的Li 1s、O 2s 和F 2s XPS的高分辨率光谱;(c)0.3C,不同电解质下Li-S电池的长期循环性能;(d)使用双盐中浓度电解液(MCE)和新型的稀释中浓度电解液(DMCE)时Li-S电池的倍率性能[89]
Figure 14 (a) UV visible absorption spectrum; High resolution spectra of (b) Li 1s、O 2s and F 2s XPS on the surface of lithium electrode; (c) 0.3C, Long term cycling performance of Li-S batteries under different electrolytes; (d) Rate performance of Li-S batteries using dual salt medium concentration electrolyte (MCE) and a novel diluted medium concentration electrolyte (DMCE) electrolyte[89]
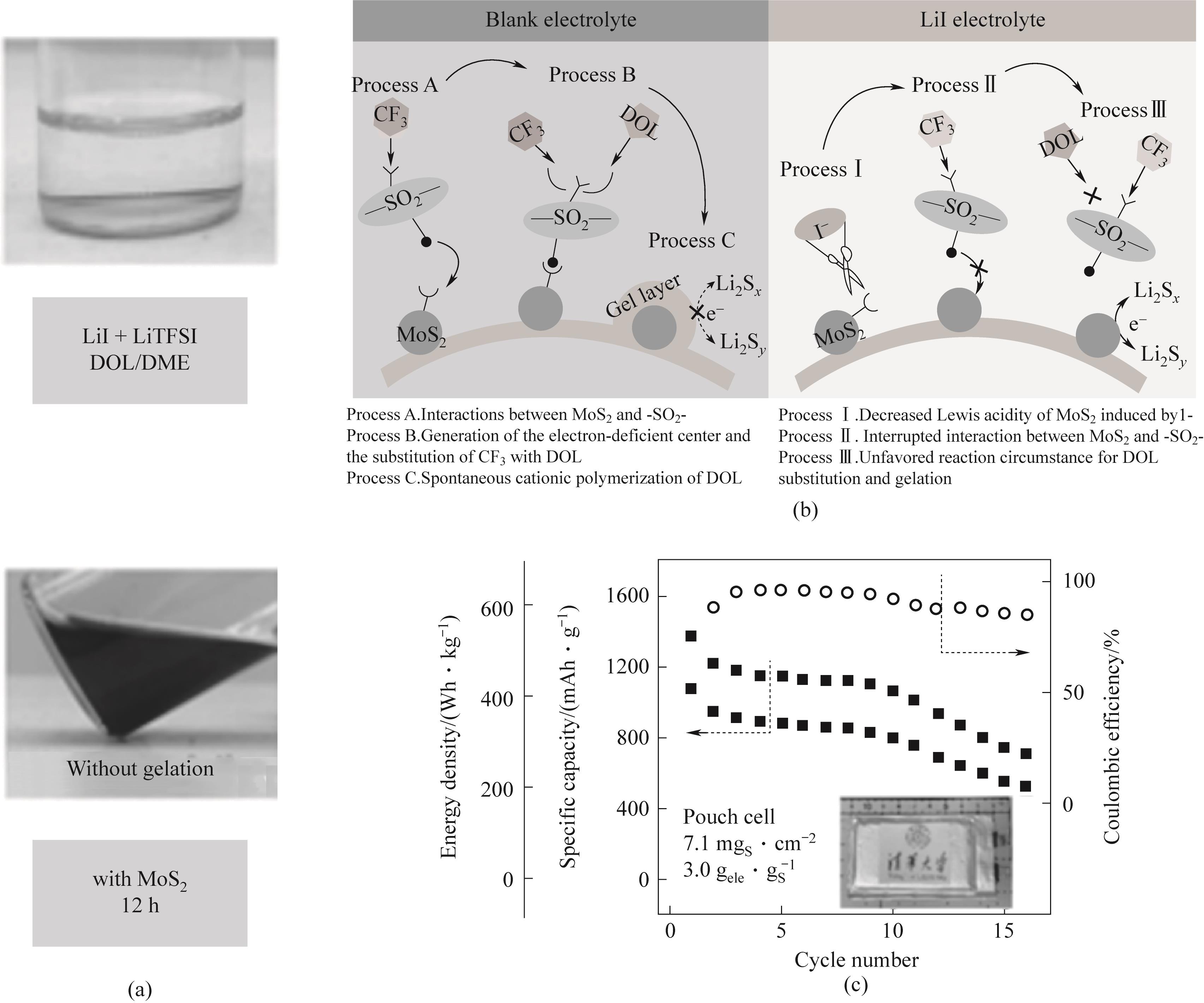
图15 (a)LiI凝胶抑制现象的光学图像;(b)Li-S电池在空白电解液和LiI电解液中的反应途径示意图;(c)0.05C,400 Wh·kg-1级软包电池的循环性能[90]
Fig.15 (a) Optical image of LiI gel suppression phenomenon; (b) Schematic diagram of reaction pathways of Li-S battery in blank electrolyte and LiI electrolyte; (c) 0.05C, Cycle performance of 400 Wh·kg-1 soft pack battery[90]
| 体系 | 锂盐 | 面载量/(mg·cm-2) | 放电比容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| 单盐体系 | 三氰甲烷化物(LiTCM)① | 0.8~1.1 | ≈0.7 mAh·cm -2(0.1C) | 0.2C,库仑效率约100%,保持30次循环 | [ |
| 双盐体系 | LiTFSI-LiFSI② | 2.0 | 682 mAh·g-1(0.3C) | 0.3C,500次循环放电比容量626 mAh·g-1 | [ |
| LiTFSI-LiI③ | 7.1(单面) | 416 Wh·kg-1(0.05C) | 0.05C,16次循环保持稳定的库仑效率 | [ |
表2 运用不同锂盐组装锂硫电池的性能参数
Table 2 Performance parameters of lithium-sulfur batteries assembled with different lithium salts
| 体系 | 锂盐 | 面载量/(mg·cm-2) | 放电比容量 | 循环性能 | 文献 |
|---|---|---|---|---|---|
| 单盐体系 | 三氰甲烷化物(LiTCM)① | 0.8~1.1 | ≈0.7 mAh·cm -2(0.1C) | 0.2C,库仑效率约100%,保持30次循环 | [ |
| 双盐体系 | LiTFSI-LiFSI② | 2.0 | 682 mAh·g-1(0.3C) | 0.3C,500次循环放电比容量626 mAh·g-1 | [ |
| LiTFSI-LiI③ | 7.1(单面) | 416 Wh·kg-1(0.05C) | 0.05C,16次循环保持稳定的库仑效率 | [ |
| [1] | Nasajpour-Esfahani N, Garmestani H, Bagheritabar M, et al. Comprehensive review of lithium-ion battery materials and development challenges[J]. Renewable and Sustainable Energy Reviews, 2024, 203: 114783. |
| [2] | Zhang C F, Chou S L, Guo Z P, et al. Beyond lithium-ion batteries[J]. Advanced Functional Materials, 2024, 34(5): 2308001. |
| [3] | Xue J X, Jia S X, Xiang T Q, et al. Cross-linkable binders for Si anodes in high-energy-density lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2024, 16(29): 38458-38465. |
| [4] | Janek J, Zeier W G. A solid future for battery development[J]. Nature Energy, 2016, 1(9): 16141. |
| [5] | Ji L, Jia Y F, Wang X, et al. Strong adsorption, catalysis and lithiophilic modulation of carbon nitride for lithium/sulfur battery[J]. Nanotechnology, 2021, 32(19): 192002. |
| [6] | Peng Y Q, Zhao M, Chen Z X, et al. Boosting sulfur redox kinetics by a pentacenetetrone redox mediator for high-energy-density lithium-sulfur batteries[J]. Nano Research, 2023, 16(6): 8253-8259. |
| [7] | Du M, Shi J K, Geng P B, et al. Nitrogen and sulfur co-doped MXene@FeCoNiP as an efficient catalyst for enhanced lithium-sulfur batteries[J]. Materials Today Chemistry, 2024, 41: 102289. |
| [8] | Chung W J, Griebel J J, Kim E T, et al. The use of elemental sulfur as an alternative feedstock for polymeric materials[J]. Nature Chemistry, 2013, 5(6): 518-524. |
| [9] | Yang S B, Wang B, Lv Q, et al. Recent advances in cathodes for all-solid-state lithium-sulfur batteries[J]. Chinese Chemical Letters, 2023, 34(7): 107783. |
| [10] | Tan J, Liu D N, Xu X, et al. In situ/operando characterization techniques for rechargeable lithium-sulfur batteries: a review[J]. Nanoscale, 2017, 9(48): 19001-19016. |
| [11] | Li Z, Li B Q, Bi C X, et al. A review of lithium-sulfur batteries at different working conditions: the role of ambient temperature, external force, and electromagnetic field[J]. Materials Science and Engineering: R: Reports, 2025, 164: 100955. |
| [12] | Zhang Y Q, Yuan H M, Guo E Y, et al. Facilitating sulfur species capture and bi-directional redox in Li-S batteries with single-atomic Co-O2N2 coordination structure[J]. Journal of Energy Chemistry, 2024, 99: 604-614. |
| [13] | Jeong Y C, Kim J H, Nam S, et al. Rational design of nanostructured functional interlayer/separator for advanced Li-S batteries[J]. Advanced Functional Materials, 2018, 28(38): 1707411. |
| [14] | Lin B X, Zhang Y Y, Li W F, et al. Recent advances in rare earth compounds for lithium-sulfur batteries[J]. eScience, 2024, 4(3): 100180. |
| [15] | Zhang L L, Wang Y J, Niu Z Q, et al. Advanced nanostructured carbon-based materials for rechargeable lithium-sulfur batteries[J]. Carbon, 2019, 141: 400-416. |
| [16] | Zhang S S. Liquid electrolyte lithium/sulfur battery: fundamental chemistry, problems, and solutions[J]. Journal of Power Sources, 2013, 231: 153-162. |
| [17] | Zhao S L, Hao Q Y, Qian X Y, et al. Application of Y-Zn-MOF derived Y2O3/ZnO@C in modification of lithium-sulfur battery separator[J]. Journal of Energy Storage, 2024, 101: 113833. |
| [18] | Tan J, Li X Y, Fang Z, et al. Designing a stable solid electrolyte interphase on lithium metal anodes by tailoring a Mg atom center and the inner Helmholtz plane for lithium-sulfur batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(14): 17893-17903. |
| [19] | Wang L, Wang R R, Liu Q Q, et al. WN nanocubes embedded on carbon mesh towards high-performance Li-S batteries: balancing physical capture, chemical adsorption and catalysis capability[J]. Journal of Energy Storage, 2024, 100: 113591. |
| [20] | Akhtar N, Sun X, Akram M Y, et al. A gelatin-based artificial SEI for lithium deposition regulation and polysulfide shuttle suppression in lithium-sulfur batteries[J]. Journal of Energy Chemistry, 2021, 52: 310-317. |
| [21] | Eshetu D G G, Judez X, Li D C, et al. Lithium azide as an electrolyte additive for all-olid-state lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2017, 56(48): 15368-15372. |
| [22] | 贾铭勋, 吴桐, 杨道通, 等. 锂硫电池电解液多功能添加剂:作用机制及先进表征[J]. 储能科学与技术, 2024, 13(1): 36-47. |
| Jia M X, Wu T, Yang D T, et al. Electrolyte multifunctional additives of lithium-sulfur battery: mechanism of action and advanced characterization[J]. Energy Storage Science and Technology, 2024, 13(1): 36-47. | |
| [23] | Ma X K, Song X M, Wang Y D, et al. Polyhalogenated heterocycle additive induced in situ 3D gelatinous polymerization with polysulfides for shuttle effect inhibited lithium-sulfur batteries[J]. Chemical Engineering Journal, 2025, 513: 162921. |
| [24] | Jan W, Khan A D, Iftikhar F J, et al. Electrolyte design for lithium-sulfur batteries: progress and challenges[J]. Renewable and Sustainable Energy Reviews, 2025, 221: 115916. |
| [25] | Yu Z H, Huang X H, Zheng M T, et al. Self-assembled macrocyclic copper complex enables homogeneous catalysis for high-loading lithium-sulfur batteries[J]. Advanced Materials, 2023, 35(26): 2300861. |
| [26] | Kautz D J, Cao X, Gao P Y, et al. Designing moderately-solvating electrolytes for high-performance lithium-sulfur batteries[J]. Advanced Materials, 2025: 2503365. |
| [27] | Zhang J H, Fan X Z, Zhou X H, et al. Charging lithium polysulfides by cationic lithium nitrate species for low-temperature lithium-sulfur batteries[J]. Energy Storage Materials, 2024, 73: 103786. |
| [28] | Aurbach D, Pollak E, Elazari R, et al. On the surface chemical aspects of very high energy density, rechargeable Li-sulfur batteries[J]. Journal of the Electrochemical Society, 2009, 156 (8): A694. |
| [29] | Xiong S Z, Regula M, Wang D H, et al. Toward better lithium-sulfur batteries: functional non-aqueous liquid electrolytes[J]. Electrochemical Energy Reviews, 2018, 1(3): 388-402. |
| [30] | Song Y W, Shen L, Yao N, et al. Anion-involved solvation structure of lithium polysulfides in lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2024, 63(19): e202400343. |
| [31] | Cai L C, Ying H J, He C W, et al. Polymorph interface strategy presents avenues for kinetics-enhanced and dendrite-free lithium sulfur batteries[J]. Chemical Engineering Journal, 2024, 498: 155856. |
| [32] | Li X Y, Feng S, Song Y W, et al. Kinetic Evaluation on lithium polysulfide in weakly solvating electrolyte toward practical lithium-sulfur batteries[J]. Journal of the American Chemical Society, 2024, 146(21): 14754-14764. |
| [33] | Kilic A, Abdelaty O, Zeeshan M, et al. Selection of ionic liquid electrolytes for high-performing lithium-sulfur batteries: an experiment-guided high-throughput machine learning analysis[J]. Chemical Engineering Journal, 2024, 490: 151562. |
| [34] | Guo J L, Yang Q, Dou Y, et al. Shelf life of lithium-sulfur batteries under lean electrolytes: status and challenges[J]. Energy & Environmental Science, 2024, 17(5): 1695-1724. |
| [35] | Zhou T, Liang J N, Ye S H, et al. Fundamental, application and opportunities of single atom catalysts for Li-S batteries[J]. Energy Storage Materials, 2023, 55: 322-355. |
| [36] | Cui M N, Zheng Z H, Wang J C, et al. Rational design of lithium-sulfur battery cathodes based on differential Atom Electronegativity[J]. Energy Storage Materials, 2021, 35: 577-585. |
| [37] | Jia M X, Li T N, Yang D T, et al. Polymer electrolytes for lithium-sulfur batteries: progress and challenges[J]. Batteries, 2023, 9(10): 488. |
| [38] | Li S, Luo Z, Tu H Y, et al. N, S-codoped carbon dots as deposition regulating electrolyte additive for stable lithium metal anode[J]. Energy Storage Materials, 2021, 42: 679-686. |
| [39] | Wang Y M, Zhang X X, Liu C S, et al. Remarkable improvement of MOF-based triboelectric nanogenerators with strong electron-withdrawing groups[J]. Nano Energy, 2023, 107: 108149. |
| [40] | Wu P, Dong M X, Tan J, et al. Revamping lithium-sulfur batteries for high cell-level energy density by synergistic utilization of polysulfide additives and artificial solid-electrolyte interphase layers[J]. Advanced Materials, 2021, 33(48): 2104246. |
| [41] | Santos É A, Policano M C, Pinzón M J, et al. operando FTIR study on water additive in lithium-sulfur batteries to mitigate shuttle effect[J]. Journal of Energy Chemistry, 2024, 98: 702-713. |
| [42] | Zhong J, Ren L L, Ying C H, et al. Amino acid as a multifunctional electrolyte additive for enhancing Li-S battery performance[J]. Journal of Energy Storage, 2025, 109: 115251. |
| [43] | Zhang W, Ma F F, Wu Q, et al. Bifunctional fluorinated anthraquinone additive for improving kinetics and interfacial chemistry in rechargeable Li-S batteries[J]. ACS Applied Energy Materials, 2022, 5(12): 15719-15728. |
| [44] | Lin L P, Yang K, Tan R, et al. Effect of sulfur-containing additives on the formation of a solid-electrolyte interphase evaluated by in situ AFM and ex situ characterizations[J]. Journal of Materials Chemistry A, 2017, 5(36): 19364-19370. |
| [45] | Meng D R, He D X, Ong D S J H, et al. A radical pathway and stabilized Li anode enabled by halide quaternary ammonium electrolyte additives for lithium-sulfur batteries[J]. Angewandte Chemie International Edition, 2023, 62(38): e202309046. |
| [46] | Wang D Y, Wang W M, Li F L, et al. Nitrogen-rich azoles as trifunctional electrolyte additives for high-performance lithium-sulfur battery[J]. Journal of Energy Chemistry, 2022, 71: 572-579. |
| [47] | Rao J P, Yu T, Zhou Y S, et al. A bifunctional thiobenzamide additive for improvement of cathode kinetics and anode stability in lithium-sulfur batteries[J]. Chemical Engineering Journal, 2024, 498: 155807. |
| [48] | Wu T, Jia M X, Lu Y, et al. Fluorinated imine modulating efficient sulfur redox kinetics and a stable solid electrolyte interphase in lithium-sulfur batteries[J]. Journal of Materials Chemistry A, 2025, 13(10): 7196-7206. |
| [49] | Chen X, Ji H, Chen W, et al. In situ protection of a sulfur cathode and a lithium anode via adopting a fluorinated electrolyte for stable lithium-sulfur batteries[J]. Science China-Materials, 2021, 64(9): 2127-2138. |
| [50] | Liu F Y, Zong C X, He L, et al. Improving the electrochemical performance of lithium-sulfur batteries by interface modification with a bifunctional electrolyte additive[J]. Chemical Engineering Journal, 2022, 443: 136489. |
| [51] | Gao S Y, Li B M, Zhu Q J, et al. A fluorinated lewis acidic organoboron tunes polysulfide complex structure for high-performance lithium-sulfur batteries[J]. Advanced Energy Materials, 2025, 15(6): 2403439. |
| [52] | Chen Y Q, Kang Y Q, Zhao Y, et al. A review of lithium-ion battery safety concerns: the issues, strategies, and testing standards[J]. Journal of Energy Chemistry, 2021, 59: 83-99. |
| [53] | Yamagiwa K, Morita D, Yabuuchi N, et al. Improved high-temperature performance and surface chemistry of graphite/LiMn2O4 Li-ion cells by fluorosilane-based electrolyte additive[J]. Electrochimica Acta, 2015, 160: 347-356. |
| [54] | Ming J, Cao Z, Wu Y Q, et al. New insight on the role of electrolyte additives in rechargeable lithium ion batteries[J]. ACS Energy Letters, 2019, 4(11): 2613-2622. |
| [55] | Wu T, Sun G R, Lu W, et al. A polypyrrole/black-TiO2/S double-shelled composite fixing polysulfides for lithium-sulfur batteries[J]. Electrochimica Acta, 2020, 353: 136529. |
| [56] | Liu G, Cao Z, Zhou L, et al. Additives engineered nonflammable electrolyte for safer potassium ion batteries[J]. Advanced Functional Materials, 2020, 30(43): 2001934. |
| [57] | Li G R, Chen Z W, Lu J. Lithium-sulfur batteries for commercial applications[J]. Chem, 2018, 4(1): 3-7. |
| [58] | Song Y W, Shen L, Li X Y, et al. Phase equilibrium thermodynamics of lithium-sulfur batteries[J]. Nature Chemical Engineering, 2024, 1: 588-596. |
| [59] | Fan L L, Deng N P, Yan J, et al. The recent research status quo and the prospect of electrolytes for lithium sulfur batteries[J]. Chemical Engineering Journal, 2019, 369: 874-897. |
| [60] | Li J Y, Gao L, Pan F Y, et al. Engineering strategies for suppressing the shuttle effect in lithium-sulfur batteries[J]. Nano-Micro Letters, 2023, 16(1): 12. |
| [61] | Liu X, Sun X R, Yan R, et al. Structural and functional optimization of lithium‐sulfur battery separators by sulfur‐containing of covalent organic frameworks[J]. Advanced Functional Materials, 2025: 2505986. |
| [62] | Lee S, Sim K, Cho K Y, et al. Regulating the interfacial reaction pathway by controlling the disproportionation of lithium polysulfides to improve the performance of lithium-sulfur batteries[J]. Journal of Power Sources, 2023, 582: 233517. |
| [63] | Wei J Y, Zhang X Q, Hou L P, et al. Shielding polysulfide intermediates by an organosulfur‐containing solid electrolyte interphase on the lithium anode in lithium-sulfur batteries[J]. Advanced Materials, 2020, 32(37): 2003012. |
| [64] | Wu H L, Shin M, Liu Y M, et al. Thiol-based electrolyte additives for high-performance lithium-sulfur batteries[J]. Nano Energy, 2017, 32: 50-58. |
| [65] | Ding Y, Li X, Chen Y M, et al. Hit two birds with one stone: a bi-functional selenium-substituted organosulfur polymer additive for high-performance lithium-sulfur batteries[J]. Chemical Engineering Journal, 2024, 482: 148803. |
| [66] | Geng C N, Qu W J, Han Z Y, et al. Superhigh coulombic efficiency lithium-sulfur batteries enabled by in situ coating lithium sulfide with polymerizable electrolyte additive[J]. Advanced Energy Materials, 2023, 13(15): 2204246. |
| [67] | Nie K L, Fu Q Q, Gao R L, et al. Dual-functional chloropyrazine additives for enhanced performance of lithium-sulfur batteries[J]. Energy Storage Materials, 2023, 63: 103011. |
| [68] | Wang Z, Al Alwan B, Simon Ng K Y. Multi-functions of amino thiophenol additives for shielding lithium metal anode in advanced Li-S battery[J]. Materials Science and Engineering: B, 2023, 297: 116727. |
| [69] | Li J, He L, Qin F R, et al. Dual-enhancement on electrochemical performance with thioacetamide as an electrolyte additive for lithium-sulfur batteries[J]. Electrochimica Acta, 2021, 376: 138041. |
| [70] | Gu J H, Yang D, Wang X Y, et al. Ammonium benzenesulfonate as an electrolyte additive to relieve the irreversible accumulation of lithium sulfide for high-energy density lithium-sulfur battery[J]. Journal of Colloid and Interface Science, 2023, 629: 368-376. |
| [71] | Lu H, Liu M, Zhang X L, et al. Catalytic effect of ammonium thiosulfate as a bifunctional electrolyte additive for regulating redox kinetics in lithium-sulfur batteries by altering the reaction pathway[J]. ACS Applied Materials & Interfaces, 2024, 16(11): 13640-13650. |
| [72] | Han W C, Hou J Y, Wang F, et al. Dual functional coordination interactions enable fast polysulfide conversion and robust interphase for high-loading lithium-sulfur batteries[J]. Materials Horizons, 2025, 12(5): 1473-1485. |
| [73] | Deng T, Wang J, Zhao H Y, et al. Dynamically regulating polysulfide degradation via organic sulfur electrolyte additives in lithium-sulfur batteries[J]. Advanced Energy Materials, 2024, 14(47): 2402319. |
| [74] | Li N, Zhang Y, Zhang S, et al. Insight into the probability of ethoxy(pentafluoro) cyclotriphosphazene (PFPN) as the functional electrolyte additive in lithium-sulfur batteries[J]. RSC Advances, 2024, 14(18): 12754-12761. |
| [75] | Li J, Hu H, Fang W, et al. Impact of fluorine‐based lithium salts on SEI for all‐solid‐state PEO‐based lithium metal batteries[J]. Advanced Functional Materials, 2023, 33(38): 2303718. |
| [76] | Liu Y H, Chang W, Qu J, et al. A polymer organosulfur redox mediator for high-performance lithium-sulfur batteries[J]. Energy Storage Materials, 2022, 46: 313-321. |
| [77] | Kuai D C, Wang S, Beltran S P, et al. Interfacial electrochemical lithiation and dissolution mechanisms at a sulfurized polyacrylonitrile cathode surface[J]. ACS Energy Letters, 2024, 9(3): 810-818. |
| [78] | Dong Y Y, Wu M Q, Cai D, et al. Confined biomimetic catalysts boost LiNO3-free lithium-sulfur batteries via enhanced LiTFSI decomposition[J]. Energy Storage Materials, 2025, 74: 103937. |
| [79] | Schedlbauer T, Rodehorst U C, Schreiner C, et al. Blends of lithium bis(oxalato)borate and lithium tetrafluoroborate: useful substitutes for lithium difluoro(oxalato)borate in electrolytes for lithium metal based secondary batteries[J]. Electrochimica Acta, 2013, 107: 26-32. |
| [80] | Wu F, Chen R J, Wu F, et al. Binary room-temperature complex electrolytes based on LiClO4 and organic compounds with acylamino group and its characterization for electric double layer capacitors[J]. Journal of Power Sources, 2008, 184(2): 402-407. |
| [81] | Chu H, Noh H, Kim Y J, et al. Achieving three-dimensional lithium sulfide growth in lithium-sulfur batteries using high-donor-number anions[J]. Nature Communications, 2019, 10(1): 188. |
| [82] | Xiang H F, Shi P C, Bhattacharya P, et al. Enhanced charging capability of lithium metal batteries based on lithium bis(trifluoromethanesulfonyl)imide-lithium bis(oxalate)borate dual-salt electrolytes[J]. Journal of Power Sources, 2016, 318: 170-177. |
| [83] | Park H, Kang H, Kim H, et al. Strategy for high-energy Li-S battery coupling with a Li metal anode and a sulfurized polyacrylonitrile cathode[J]. ACS Applied Materials & Interfaces, 2023, 15(39): 45876-45885. |
| [84] | Liu J, Zhang J P, Zhu J, et al. A lithium sulfonylimide COF-modified separator for high-performance Li-S batteries[J]. Nano Research, 2023, 16(11): 12601-12607. |
| [85] | Song Z Y, Zheng L P, Cheng P F, et al. Taming the chemical instability of lithium hexafluorophosphate-based electrolyte with lithium fluorosulfonimide salts[J]. Journal of Power Sources, 2022, 526: 231105. |
| [86] | Rohde D M, Eiden D P, Leppert V, et al. Li[B(OCH2CF3)4]: synthesis, characterization and electrochemical application as a conducting salt for Li-S batteries[J]. ChemPhysChem, 2015, 16(3): 666-675. |
| [87] | Zhu Z, Li X, Qi X, et al. Demystifying the salt-induced Li loss: a universal procedure for the electrolyte design of lithium-metal batteries[J]. Nano-Micro Letters, 2023, 15(1): 234. |
| [88] | Zhang H, Judez X, Santiago A, et al. Fluorine-free noble salt anion for high-performance all-solid-state lithium-sulfur batteries[J]. Advanced Energy Materials, 2019, 9(25): 1900763. |
| [89] | Kong X R, Kong Y C, Zheng Y Y, et al. Hydrofluoroether diluted dual-salts-based electrolytes for lithium-sulfur batteries with enhanced lithium anode protection[J]. Small, 2022, 18(52): 2205017. |
| [90] | Wang J N, Yi S S, Liu J W, et al. Suppressing the shuttle effect and dendrite growth in lithium-sulfur batteries[J]. ACS Nano, 2020, 14(8): 9819-9831. |
| [91] | Zhou L, Danilov D L, Qiao F, et al. Sulfur reduction reaction in lithium-sulfur batteries: mechanisms, catalysts, and characterization[J]. Advanced Energy Materials, 2022, 12(44): 2202094. |
| [92] | Li X Y, Feng S, Zhao C X, et al. Regulating lithium salt to inhibit surface gelation on an electrocatalyst for high-energy-density lithium-sulfur batteries[J]. Journal of the American Chemical Society, 2022, 144(32): 14638-14646. |
| [1] | 吴馨, 龚建英, 李祥宇, 王宇涛, 杨小龙, 蒋震. 超声波激励疏水表面液滴运动的实验研究[J]. 化工学报, 2025, 76(S1): 133-139. |
| [2] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [3] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [4] | 刘卓龙, 甘云华, 屈可扬, 陈宁光, 潘铭晖. 均匀电场对生物柴油小尺度射流扩散燃烧特性影响研究[J]. 化工学报, 2025, 76(9): 4800-4808. |
| [5] | 曾宁, 郭振江, 陈建华, 张子轩, 曾玉娇, 肖炘, 刘松林, 薛绍秀, 周智武, 卢振明, 王利民. 二水湿法磷酸工艺中非水溶磷的分子动力学模拟[J]. 化工学报, 2025, 76(9): 4539-4550. |
| [6] | 徐佳琪, 张文君, 余燕萍, 苏宝根, 任其龙, 杨启炜. 热等离子体重整炼厂气制合成气过程数值模拟与实验研究[J]. 化工学报, 2025, 76(9): 4462-4473. |
| [7] | 李相海, 赖德林, 孔纲, 周健. 双仿生表面水下疏油协同机制的分子动力学模拟研究[J]. 化工学报, 2025, 76(9): 4551-4562. |
| [8] | 叶鑫煌, 薛嘉豪, 赵玉来. 可聚型Gemini表面活性剂的制备、表征及其稳定高内相乳液的研究[J]. 化工学报, 2025, 76(8): 4331-4340. |
| [9] | 田宇红, 杜壮壮, 徐慧芳, 祝自强, 王宇聪. ZIF-8基多孔液体制备及其SO2吸附性能[J]. 化工学报, 2025, 76(8): 4284-4296. |
| [10] | 李云昊, 徐纯刚, 李小森, 付骏, 王屹, 陈朝阳. 固液复配型促进剂对盐水体系CO2水合物形成影响研究[J]. 化工学报, 2025, 76(8): 4228-4238. |
| [11] | 刘璐, 杨莹, 杨浩文, 王太, 王腾, 董新宇, 闫润. 星形亲水区组合表面冷凝液滴脱落特性实验研究[J]. 化工学报, 2025, 76(8): 3905-3914. |
| [12] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [13] | 何晨, 陆明飞, 王令金, 许晓颖, 董鹏博, 赵文涛, 隆武强. 氨-甲醇高压混合气稀燃层流实验与模拟研究[J]. 化工学报, 2025, 76(8): 4248-4258. |
| [14] | 常心泉, 张克学, 王军, 夏国栋. 自由分子区内不规则颗粒的热泳力计算[J]. 化工学报, 2025, 76(8): 3944-3953. |
| [15] | 佘海龙, 胡光忠, 崔晓钰, 柳忠彬, 彭帝, 李航. 不同节流工质下叠层微通道分布式节流制冷器性能研究[J]. 化工学报, 2025, 76(8): 4017-4029. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号