• •
贾玉倩( ), 李佳书, 孙逸, 刘英杰, 叶青, 李进龙(
), 李佳书, 孙逸, 刘英杰, 叶青, 李进龙( )
)
收稿日期:2025-08-18
修回日期:2025-11-04
出版日期:2025-11-05
通讯作者:
李进龙
作者简介:贾玉倩(1999—),女,硕士,jia30807@qq.com
基金资助:
Yuqian JIA( ), Jiashu LI, Yi SUN, Yingjie LIU, Qing YE, Jinlong LI(
), Jiashu LI, Yi SUN, Yingjie LIU, Qing YE, Jinlong LI( )
)
Received:2025-08-18
Revised:2025-11-04
Online:2025-11-05
Contact:
Jinlong LI
摘要:
借助基于二阶微扰理论和PY2近似积分方程建立的变阱宽方阱链流体状态方程(SWCF-VR)和先前获得的18种制冷剂分子参数,预测了纯制冷剂及其二元混合物在饱和相区的热容、焦汤系数、声速等热力学性质,并比较了原始SAFT-VR(SW)、PC-SAFT等模型预测效果。结果表明,SWCF-VR模型可满意预测纯制冷剂的上述各热力学性质,但受二阶导数精度影响,对热容预测偏差较大。和同类模型相比,SWCF-VR在多数热力学性质的预测性能要优于SAFT-VR和PC-SAFT。对二元混合物,引入基于汽液平衡实验数据获得的二元交互参数后,可显著改善模型预测性能。本文结果将为制冷剂的设计、复配及制冷过程设计优化提供参考。
中图分类号:
贾玉倩, 李佳书, 孙逸, 刘英杰, 叶青, 李进龙. 变阱宽方阱链流体状态方程预测制冷剂的热力学性质[J]. 化工学报, DOI: 10.11949/0438-1157.20250932.
Yuqian JIA, Jiashu LI, Yi SUN, Yingjie LIU, Qing YE, Jinlong LI. Predicting thermodynamic properties of refrigerants using an equation of state for square-well chain fluid with variable range[J]. CIESC Journal, DOI: 10.11949/0438-1157.20250932.
| Thermodynamic property | formulae |
|---|---|
| Isochoric heat capacity | |
| Isobaric heat capacity | |
| Speed of sound | |
| Joule-Thomson coefficient | |
| Residual enthalpy | |
| partial derivative of | |
| partial derivative of |
表 1 热力学性质关系式
Table 1 The thermodynamic property formulae.
| Thermodynamic property | formulae |
|---|---|
| Isochoric heat capacity | |
| Isobaric heat capacity | |
| Speed of sound | |
| Joule-Thomson coefficient | |
| Residual enthalpy | |
| partial derivative of | |
| partial derivative of |
| System | T/K | AARD (%)/AAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vapor | Liquid | |||||||||
| Hvap | Cv | Cp | u | Cv | Cp | u | ||||
| Average | 4.14 | 10.1 | 13.9 | 1.93 | 2.50 | 16.5 | 6.60 | 0.007 | 11.7 | |
| R11 | 245~425 | 2.79 | 7.65 | 9.71 | 1.78 | 1.35 | 9.92 | 5.16 | 0.005 | 5.65 |
| R12 | 175~351 | 2.85 | 6.62 | 8.28 | 1.37 | 1.19 | 11.9 | 7.66 | 0.006 | 7.31 |
| R13 | 145~295 | 5.16 | 11.0 | 15.7 | 1.90 | 2.50 | 16.7 | 11.2 | 0.017 | 11.8 |
| R14 | 130~220 | 0.94 | 8.84 | 10.7 | 0.08 | 1.14 | 18.7 | 4.43 | 0.011 | 5.57 |
| R21 | 230~430 | 4.52 | 9.16 | 13.0 | 1.71 | 2.18 | 20.0 | 7.22 | 0.006 | 13.0 |
| R22 | 210~350 | 5.01 | 12.7 | 17.3 | 1.12 | 2.55 | 18.5 | 5.21 | 0.006 | 10.7 |
| R23 | 140~280 | 4.47 | 22.2 | 25.2 | 7.28 | 3.36 | 28.7 | 6.77 | 0.005 | 18.9 |
| R32 | 190~340 | 7.31 | 25.6 | 30.7 | 3.57 | 4.72 | 30.3 | 3.92 | 0.006 | 33.9 |
| R113 | 270~430 | 2.52 | 4.13 | 5.45 | 0.90 | 1.38 | 6.5 | 5.70 | 0.003 | 7.3 |
| R114 | 280~400 | 4.36 | 4.78 | 9.30 | 0.45 | 3.18 | 6.0 | 4.24 | 0.009 | 9.5 |
| R116 | 198~282 | 4.90 | 7.10 | 12.6 | 0.47 | 3.20 | 9.1 | 7.16 | 0.012 | 8.1 |
| R123 | 230~434 | 4.36 | 5.88 | 9.12 | 1.67 | 2.54 | 9.5 | 6.59 | 0.007 | 10.0 |
| R125 | 180~320 | 2.90 | 7.27 | 9.83 | 1.46 | 1.77 | 14.6 | 5.56 | 0.004 | 5.7 |
| R134a | 200~350 | 4.43 | 9.92 | 15.1 | 2.32 | 3.04 | 17.9 | 8.37 | 0.004 | 7.75 |
| R142b | 195~390 | 3.95 | 8.36 | 12.3 | 2.69 | 2.19 | 13.5 | 8.98 | 0.007 | 8.77 |
| R143a | 190~330 | 6.80 | 12.3 | 18.3 | 2.36 | 4.06 | 16.3 | 6.13 | 0.010 | 15.57 |
| R152a | 205~340 | 2.89 | 9.63 | 12.9 | 2.75 | 2.23 | 21.2 | 8.04 | 0.003 | 11.36 |
| R161 | 200~350 | 4.44 | 9.00 | 14.0 | 0.95 | 2.46 | 27.0 | 7.24 | 0.008 | 20.01 |
表 2 纯制冷剂的各种热力学性质预测值与实验值的总体平均偏差
Table 2 AARD and AAD of predicted thermodynamic properties from experimental values for pure refrigerants
| System | T/K | AARD (%)/AAD | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vapor | Liquid | |||||||||
| Hvap | Cv | Cp | u | Cv | Cp | u | ||||
| Average | 4.14 | 10.1 | 13.9 | 1.93 | 2.50 | 16.5 | 6.60 | 0.007 | 11.7 | |
| R11 | 245~425 | 2.79 | 7.65 | 9.71 | 1.78 | 1.35 | 9.92 | 5.16 | 0.005 | 5.65 |
| R12 | 175~351 | 2.85 | 6.62 | 8.28 | 1.37 | 1.19 | 11.9 | 7.66 | 0.006 | 7.31 |
| R13 | 145~295 | 5.16 | 11.0 | 15.7 | 1.90 | 2.50 | 16.7 | 11.2 | 0.017 | 11.8 |
| R14 | 130~220 | 0.94 | 8.84 | 10.7 | 0.08 | 1.14 | 18.7 | 4.43 | 0.011 | 5.57 |
| R21 | 230~430 | 4.52 | 9.16 | 13.0 | 1.71 | 2.18 | 20.0 | 7.22 | 0.006 | 13.0 |
| R22 | 210~350 | 5.01 | 12.7 | 17.3 | 1.12 | 2.55 | 18.5 | 5.21 | 0.006 | 10.7 |
| R23 | 140~280 | 4.47 | 22.2 | 25.2 | 7.28 | 3.36 | 28.7 | 6.77 | 0.005 | 18.9 |
| R32 | 190~340 | 7.31 | 25.6 | 30.7 | 3.57 | 4.72 | 30.3 | 3.92 | 0.006 | 33.9 |
| R113 | 270~430 | 2.52 | 4.13 | 5.45 | 0.90 | 1.38 | 6.5 | 5.70 | 0.003 | 7.3 |
| R114 | 280~400 | 4.36 | 4.78 | 9.30 | 0.45 | 3.18 | 6.0 | 4.24 | 0.009 | 9.5 |
| R116 | 198~282 | 4.90 | 7.10 | 12.6 | 0.47 | 3.20 | 9.1 | 7.16 | 0.012 | 8.1 |
| R123 | 230~434 | 4.36 | 5.88 | 9.12 | 1.67 | 2.54 | 9.5 | 6.59 | 0.007 | 10.0 |
| R125 | 180~320 | 2.90 | 7.27 | 9.83 | 1.46 | 1.77 | 14.6 | 5.56 | 0.004 | 5.7 |
| R134a | 200~350 | 4.43 | 9.92 | 15.1 | 2.32 | 3.04 | 17.9 | 8.37 | 0.004 | 7.75 |
| R142b | 195~390 | 3.95 | 8.36 | 12.3 | 2.69 | 2.19 | 13.5 | 8.98 | 0.007 | 8.77 |
| R143a | 190~330 | 6.80 | 12.3 | 18.3 | 2.36 | 4.06 | 16.3 | 6.13 | 0.010 | 15.57 |
| R152a | 205~340 | 2.89 | 9.63 | 12.9 | 2.75 | 2.23 | 21.2 | 8.04 | 0.003 | 11.36 |
| R161 | 200~350 | 4.44 | 9.00 | 14.0 | 0.95 | 2.46 | 27.0 | 7.24 | 0.008 | 20.01 |
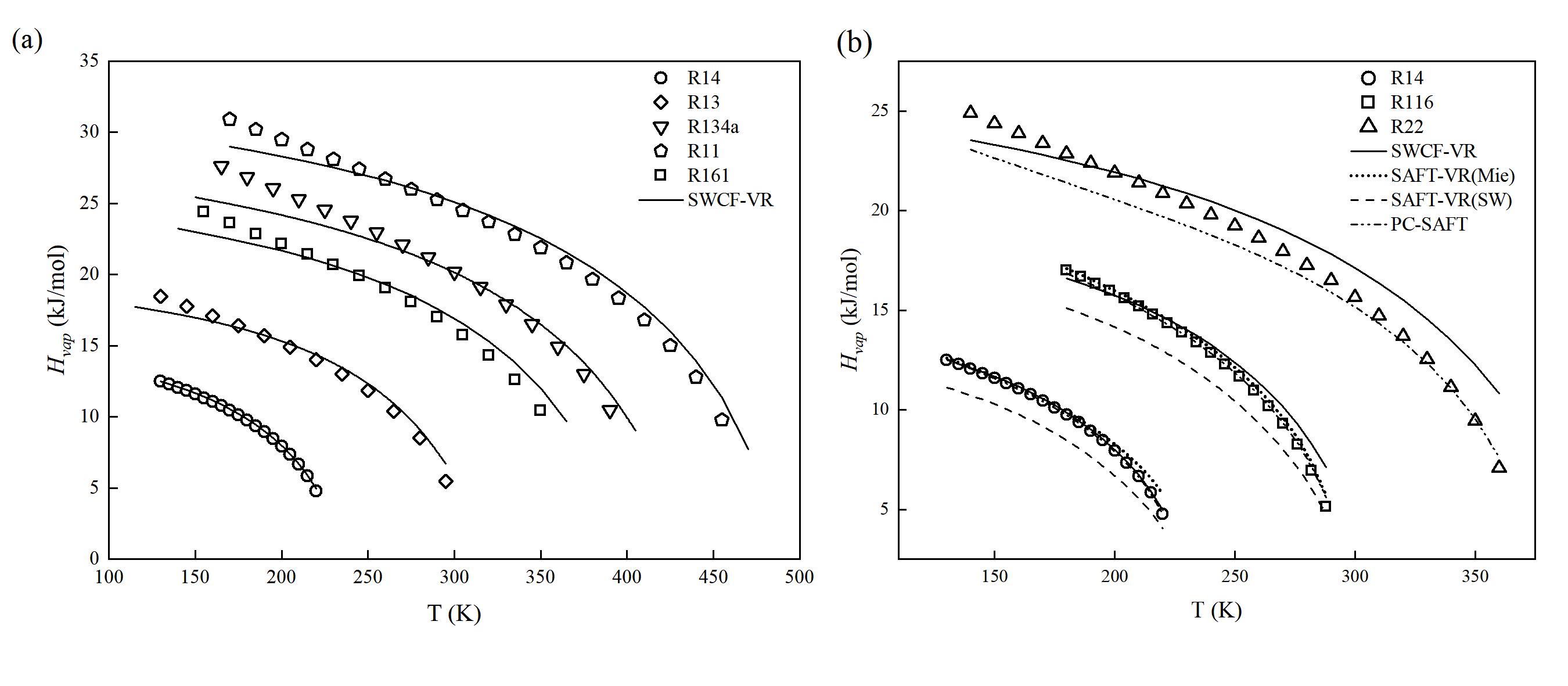
图 1 (a) 制冷剂的蒸发焓 (Hvap ) 实验值和理论值对比;(b) 不同状态方程预测制冷剂蒸发焓 (Hvap ) 的对比
Fig. 1 (a) Comparison of experimental and theoretical values for refrigerant enthalpy of vaporization; (b) Comparison of enthalpy of vaporization predictions for refrigerants using different equations of state (Points represent experimental values provided by the NIST REFPROP 9.1 database, and lines represent prediction curves)
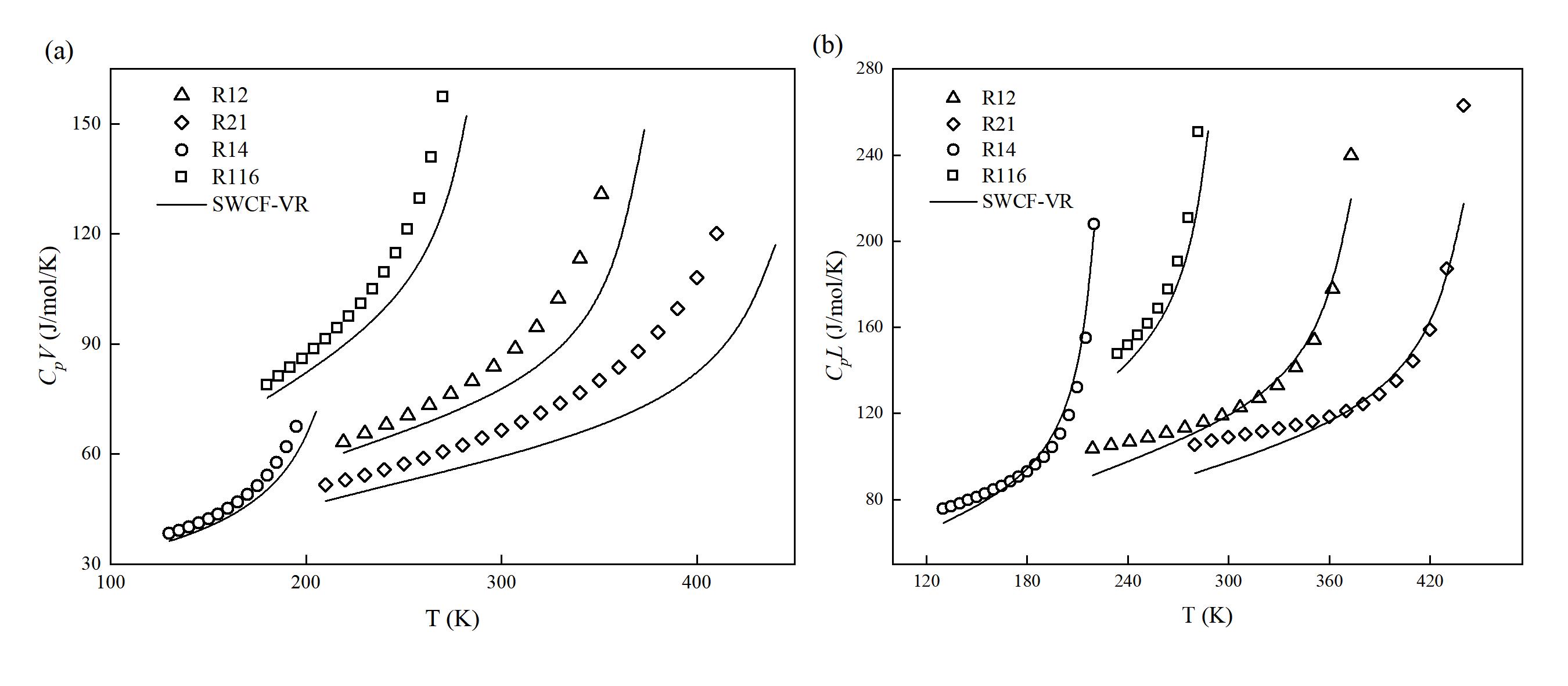
图 2 (a) 制冷剂的饱和气体的等压热容 (CpV) 实验值和理论值对比;(b) 制冷剂的饱和液体的等压热容 (CpL) 实验值和理论值对比
Fig. 2 (a) Comparison of experimental and theoretical values for saturated vapor isobaric heat capacity of refrigerants; (b) Comparison of experimental and theoretical values for saturated liquid isobaric heat capacity of refrigerants (Points represent experimental values provided by the NIST REFPROP 9.1 database, and lines represent prediction curves of SWCF-VR)
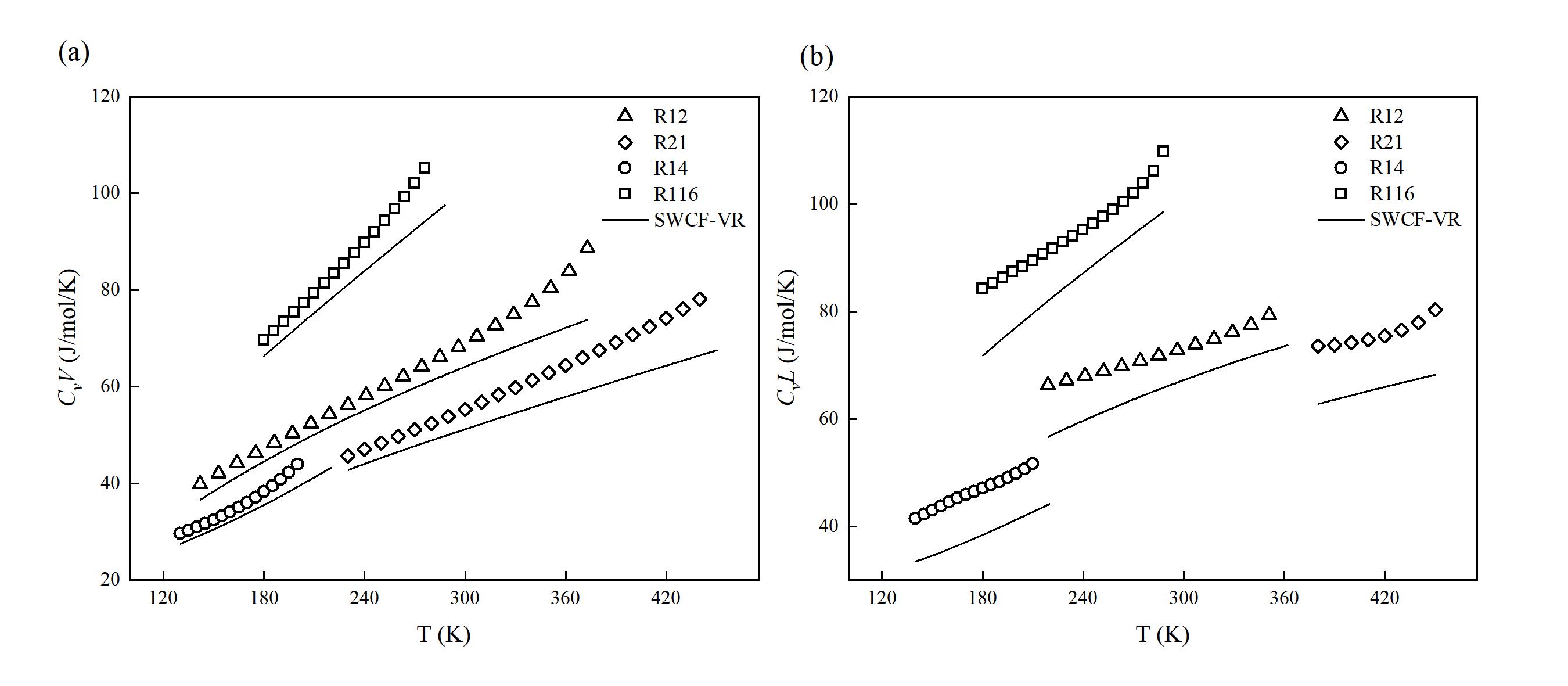
图 3 (a) 制冷剂的饱和气体的等容热容 (CvV) 实验值和理论值对比;(b) 制冷剂的饱和液体的等容热容 (CvL) 实验值和理论值对比
Fig. 3 (a) Comparison of experimental and theoretical values for saturated vapor isochoric heat capacity of refrigerants; (b) Comparison of experimental and theoretical values for saturated liquid isochoric heat capacity of refrigerants (Points represent experimental values provided by the NIST REFPROP 9.1 database, and lines represent prediction curves of SWCF-VR)
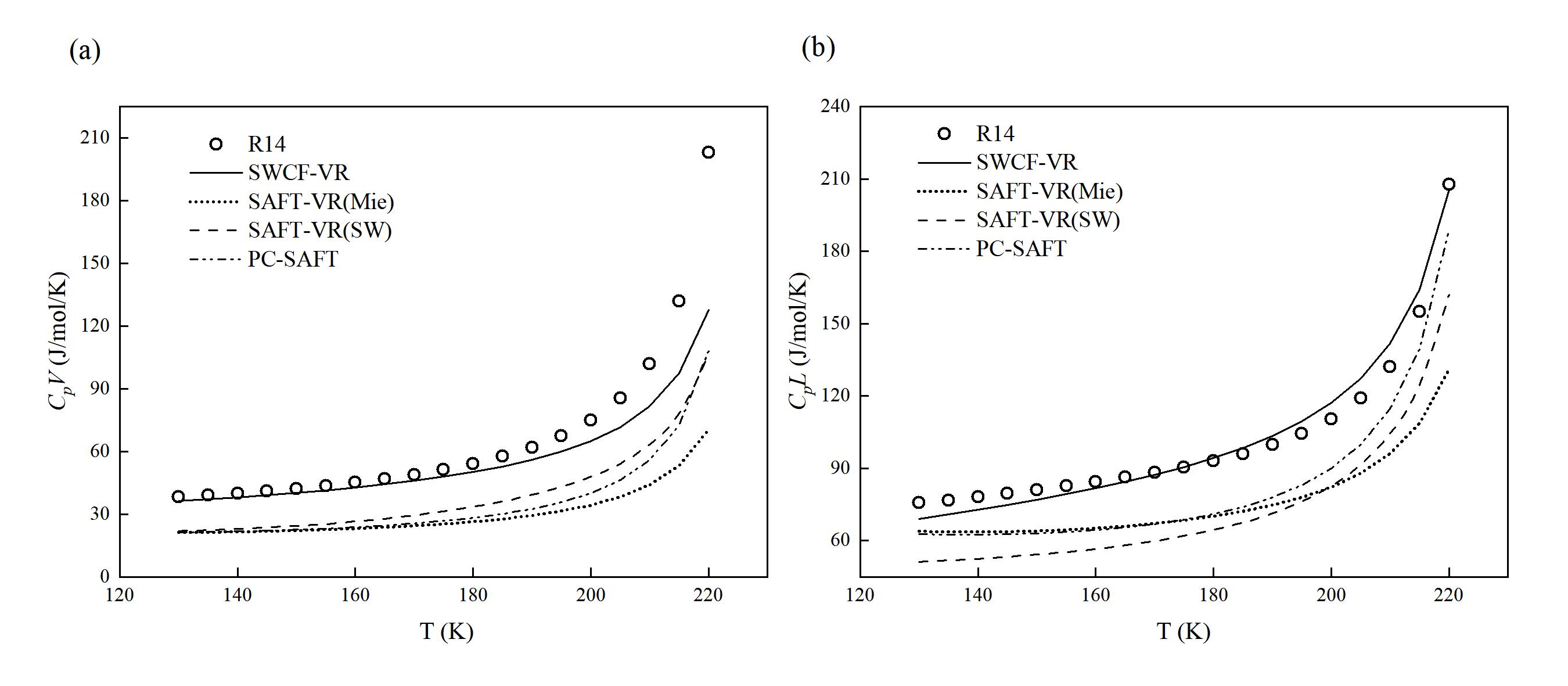
图 4 (a) 不同状态方程预测R14的饱和气体等压热容 (CpV) 的结果;(b) 不同状态方程预测R14的饱和液体等压热容 (CpL) 的结果
Fig. 4 (a) Results of saturated vapor isobaric heat capacity predictions for R14 using different equations of state; (b) Results of saturated liquid isobaric heat capacity predictions for R14 using different equations of state (Points represent experimental values provided by the NIST REFPROP 9.1 database, and lines represent prediction curves)
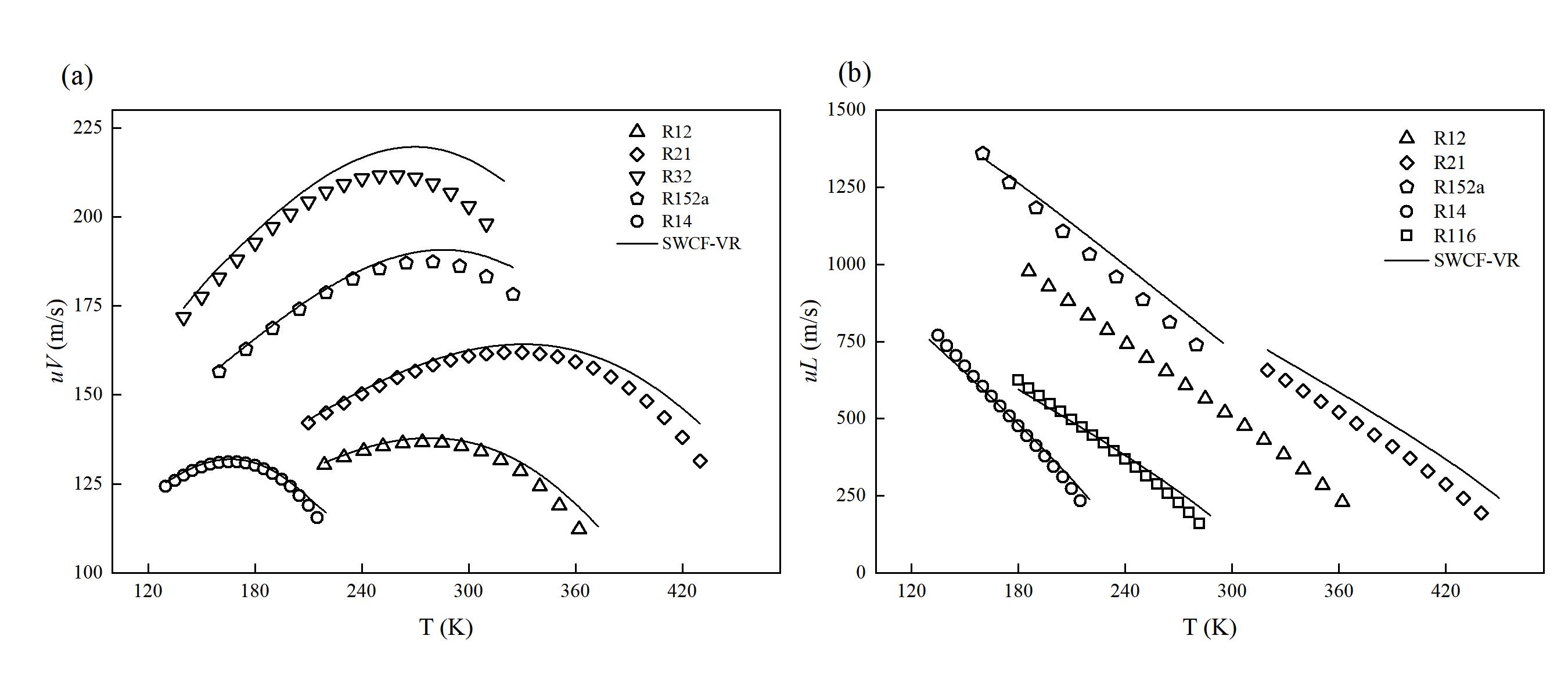
图 5 (a) 饱和气体的声速 (uV) 预测曲线与实验值对比;(b) 饱和液体的声速 (uL) 预测曲线与实验值对比
Fig. 5 (a) Comparison of saturated vapor speed of sound prediction curves and experimental values; (b) Comparison of saturated liquid speed of sound prediction curves and experimental values (Points represent experimental values provided by the NIST REFPROP 9.1 database, and lines represent prediction curves of SWCF-VR)
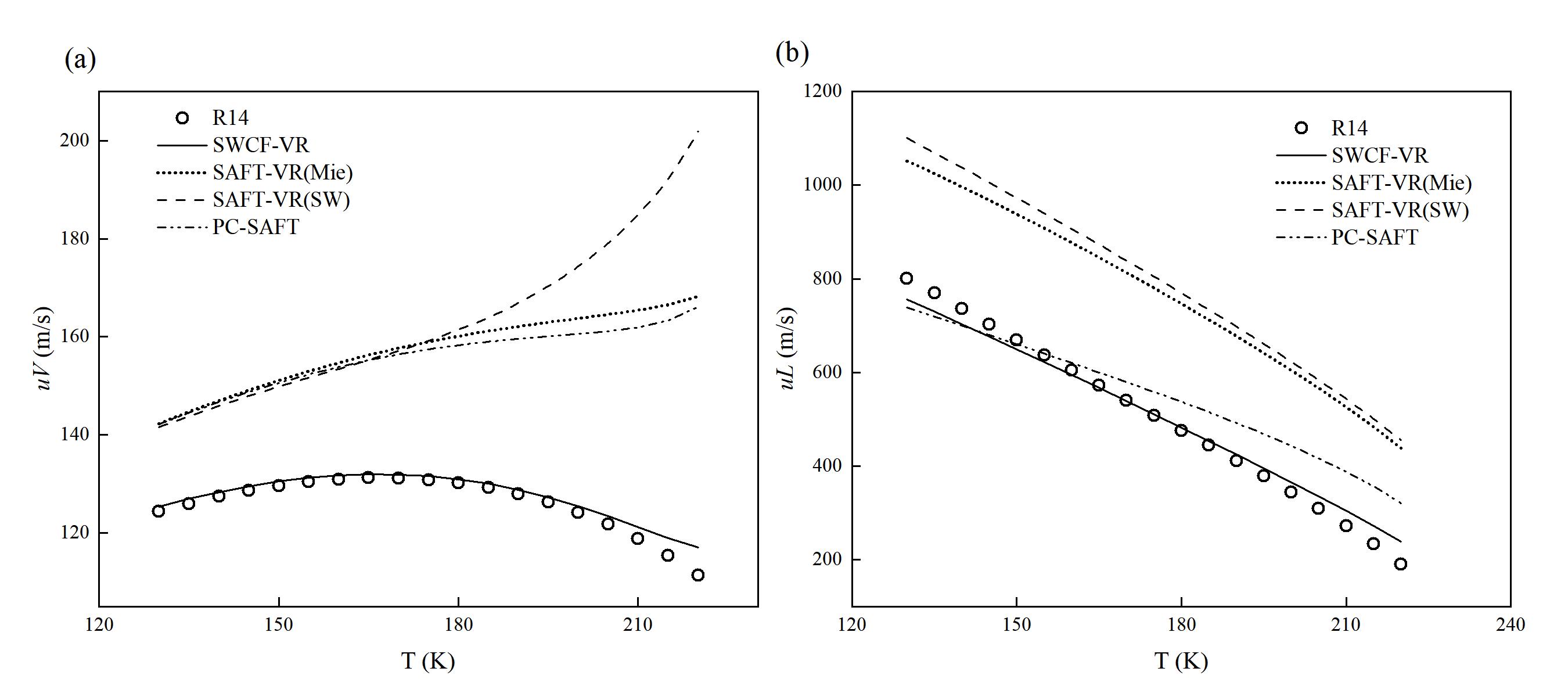
图 6 (a) R14的饱和气体声速 (uV) 预测曲线对比;(b) R14的饱和液体声速 (uL) 预测曲线对比
Fig.6 (a) Comparison of saturated vapor speed of sound prediction curves for R14; (b) Comparison of saturated liquid speed of sound prediction curves for R14 (Points represent experimental values provided by the NIST REFPROP 9.1 database, and lines represent prediction curves)
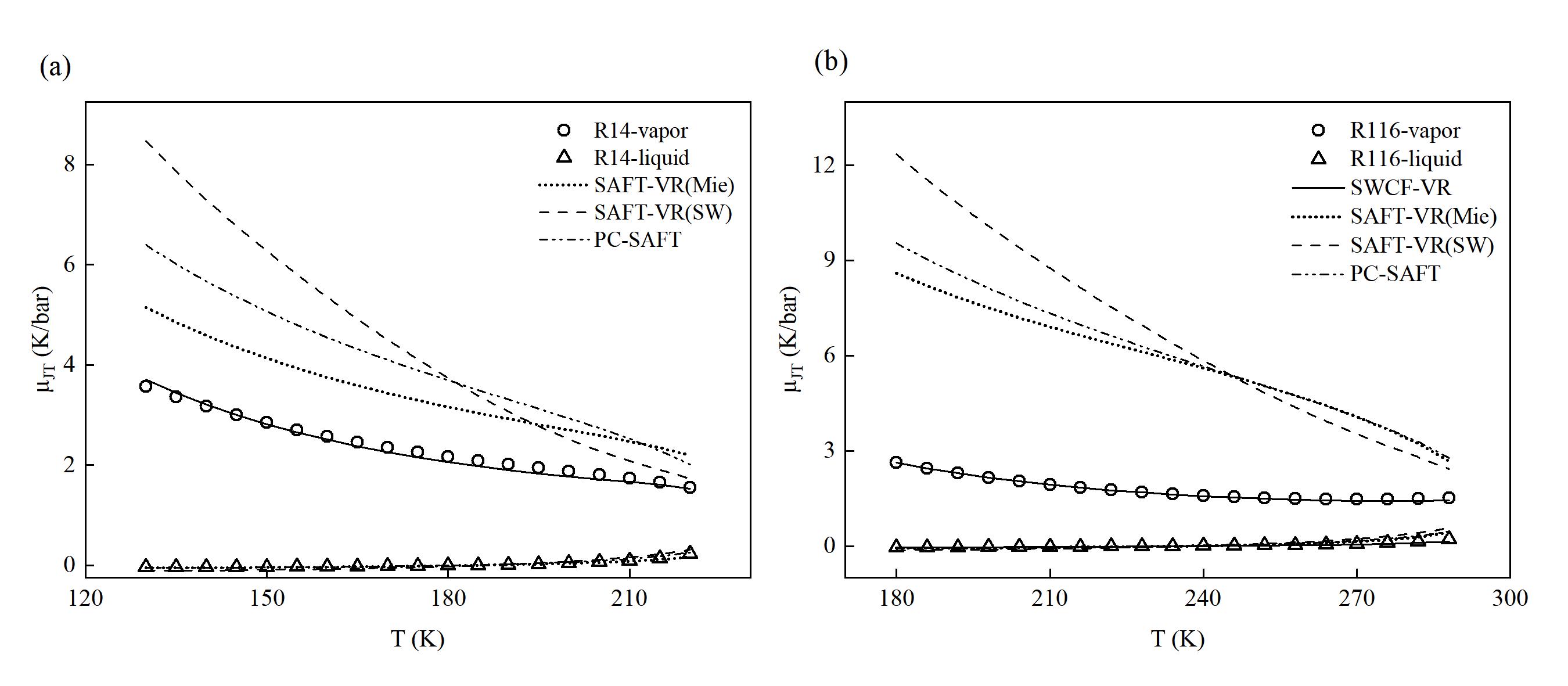
图 7 (a) R14的饱和焦汤系数 (μJT ) 预测曲线对比;(b) R116的饱和焦汤系数 (μJT ) 预测曲线对比
Fig. 7 (a) Comparison of saturated Joule-Thomson coefficient prediction curves for R14; (b) Comparison of saturated Joule-Thomson coefficient prediction curves for R116 (Points represent experimental values provided by the NIST REFPROP 9.1 database, and lines represent prediction curves)
| System | T/K | P/bar | AARD (%)/AAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ypre* | Ppre | Vapor | Liquid | ||||||||||
| Cv | Cp | u | Cv | Cp | u | ||||||||
| Average | 0.0298 | 2.68 | 11.7 | 18.3 | 0.69 | 3.08 | 13.4 | 3.66 | 0.006 | 13.9 | |||
| R123+R134a | 250~300 | 0.97~7.02 | 0.0523 | 0.0264 | 4.65 | 6.65 | 8.33 | 1.64 | 0.72 | 14.2 | 8.53 | 0.004 | 6.54 |
| R22+R12 | 343~350 | 18.7~34.4 | 0.0290 | 0.0255 | 0.75 | 16.4 | 30.0 | 0.27 | 5.50 | 11.9 | 1.85 | 0.002 | 21.6 |
| R22+R123 | 320~350 | 1.92-34.42 | 0.0716 | 0.0248 | 0.66 | 10.6 | 14.8 | 0.43 | 1.62 | 10.6 | 3.29 | 0.008 | 4.23 |
| R23+R116 | 200~250 | 1.64~14.74 | 0.0667 | 0.0978 | 9.00 | 14.5 | 18.7 | 0.86 | 2.24 | 16.1 | 6.27 | 0.005 | 13.3 |
| R125+R134a | 290~320 | 4.7~23.6 | 0.0071 | 0.0292 | 2.65 | 11.5 | 19.2 | 0.57 | 3.68 | 13.4 | 2.58 | 0.006 | 9.84 |
| R143a+R152a | 300~320 | 6.29~21.4 | 0 | 0.0146 | 0.36 | 14.2 | 22.7 | 0.72 | 5.21 | 14.7 | 2.21 | 0.006 | 21.7 |
| R125+R143a | 290-320 | 10.42~22.8 | 0 | 0.0089 | 1.80 | 14.1 | 25.4 | 0.56 | 5.86 | 13.0 | 1.75 | 0.014 | 21.5 |
| R125+R161 | 290~320 | 7.35~23.6 | 0 | 0.0335 | 2.73 | 11.1 | 18.5 | 0.52 | 1.90 | 16.6 | 1.39 | 0.006 | 20.4 |
| R11+R12 | 250~350 | 0.13~21.96 | 0.0124 | 0.0077 | 1.56 | 6.0 | 7.30 | 0.68 | 0.95 | 9.83 | 5.10 | 0.005 | 5.74 |
表 3 混合物制冷剂的各种热力学性质预测值与实验值的总体平均偏差
Table 3 AARD and AAD of predicted thermodynamic properties from experimental values for refrigerant mixture
| System | T/K | P/bar | AARD (%)/AAD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ypre* | Ppre | Vapor | Liquid | ||||||||||
| Cv | Cp | u | Cv | Cp | u | ||||||||
| Average | 0.0298 | 2.68 | 11.7 | 18.3 | 0.69 | 3.08 | 13.4 | 3.66 | 0.006 | 13.9 | |||
| R123+R134a | 250~300 | 0.97~7.02 | 0.0523 | 0.0264 | 4.65 | 6.65 | 8.33 | 1.64 | 0.72 | 14.2 | 8.53 | 0.004 | 6.54 |
| R22+R12 | 343~350 | 18.7~34.4 | 0.0290 | 0.0255 | 0.75 | 16.4 | 30.0 | 0.27 | 5.50 | 11.9 | 1.85 | 0.002 | 21.6 |
| R22+R123 | 320~350 | 1.92-34.42 | 0.0716 | 0.0248 | 0.66 | 10.6 | 14.8 | 0.43 | 1.62 | 10.6 | 3.29 | 0.008 | 4.23 |
| R23+R116 | 200~250 | 1.64~14.74 | 0.0667 | 0.0978 | 9.00 | 14.5 | 18.7 | 0.86 | 2.24 | 16.1 | 6.27 | 0.005 | 13.3 |
| R125+R134a | 290~320 | 4.7~23.6 | 0.0071 | 0.0292 | 2.65 | 11.5 | 19.2 | 0.57 | 3.68 | 13.4 | 2.58 | 0.006 | 9.84 |
| R143a+R152a | 300~320 | 6.29~21.4 | 0 | 0.0146 | 0.36 | 14.2 | 22.7 | 0.72 | 5.21 | 14.7 | 2.21 | 0.006 | 21.7 |
| R125+R143a | 290-320 | 10.42~22.8 | 0 | 0.0089 | 1.80 | 14.1 | 25.4 | 0.56 | 5.86 | 13.0 | 1.75 | 0.014 | 21.5 |
| R125+R161 | 290~320 | 7.35~23.6 | 0 | 0.0335 | 2.73 | 11.1 | 18.5 | 0.52 | 1.90 | 16.6 | 1.39 | 0.006 | 20.4 |
| R11+R12 | 250~350 | 0.13~21.96 | 0.0124 | 0.0077 | 1.56 | 6.0 | 7.30 | 0.68 | 0.95 | 9.83 | 5.10 | 0.005 | 5.74 |
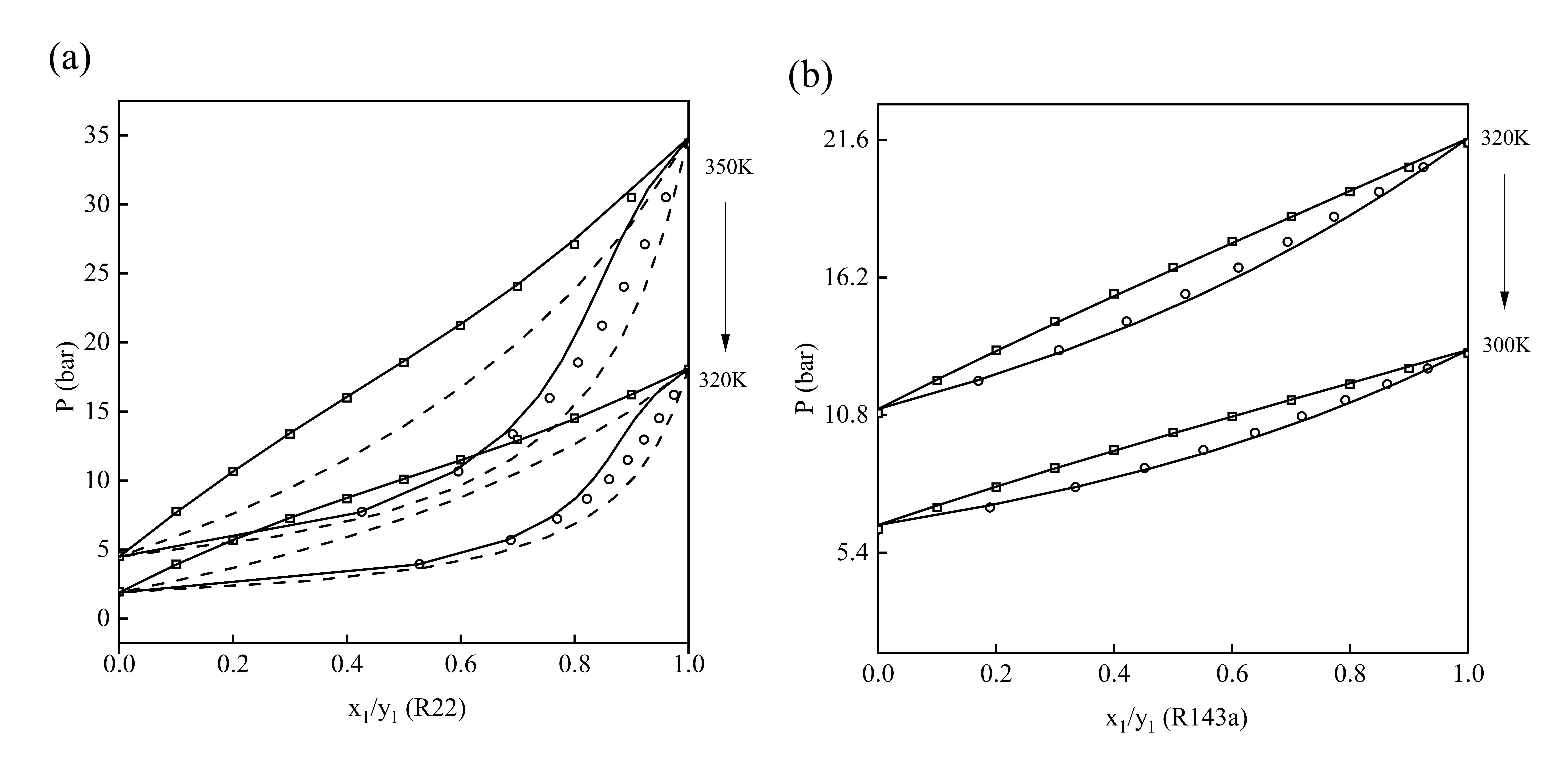
图 8(a) R22+R123体系的二元汽液平衡相图;(b) R143a+R152a体系的二元汽液平衡相图
Fig. 8(a) Vapor-liquid equilibrium (VLE) phase diagram for the R22 + R123 system (The solid line represents predictions with fitted binary interaction parameters, the dashed line denotes predictions without fitted binary interaction parameters, and the scattered points are experimental data); (b) Vapor-liquid equilibrium (VLE) phase diagram for the R143a + R152a system
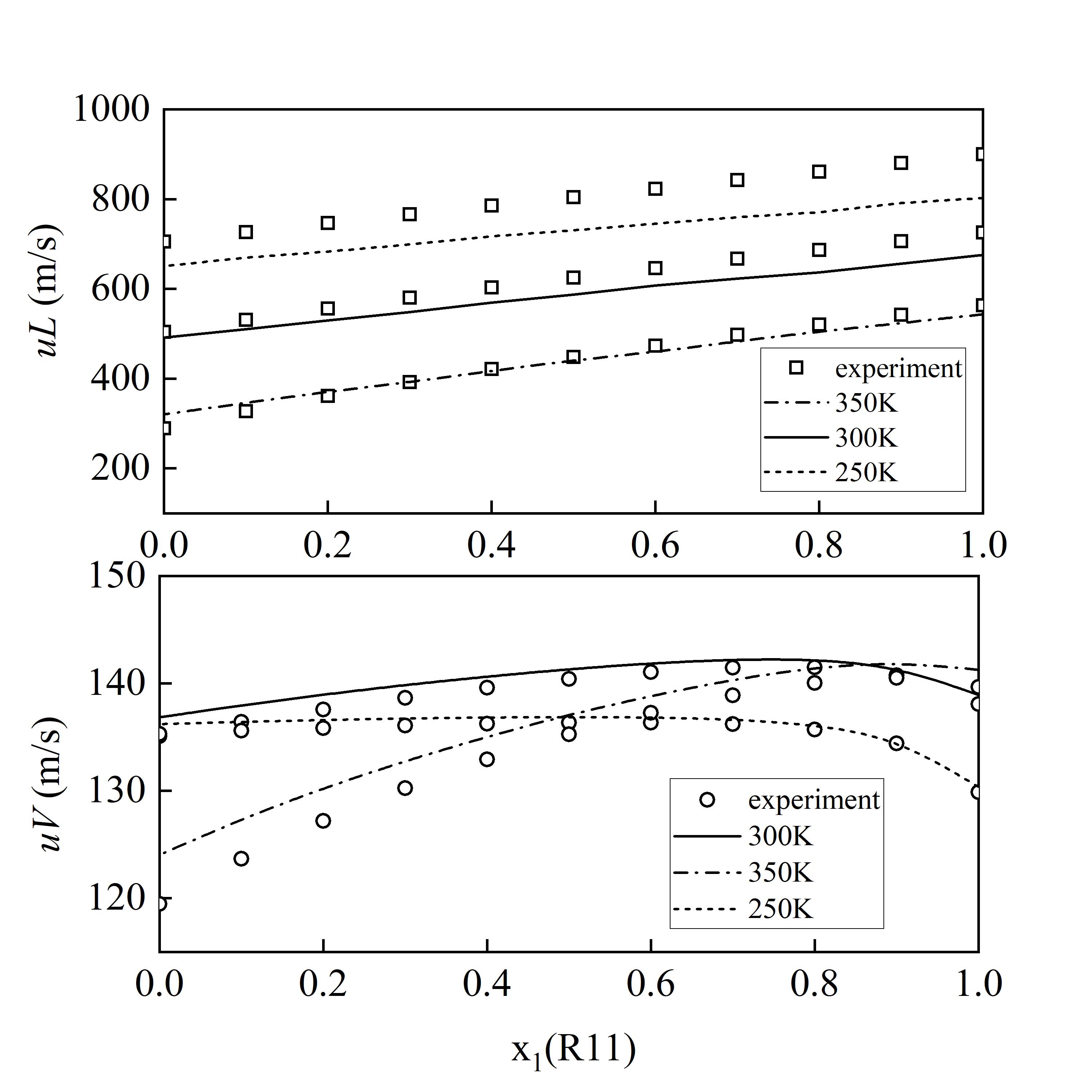
图 9 R11+R12体系的混合液体声速 (uL) 与混合气体声速 (uV) 预测结果
Fig. 9 Predicted mixing saturated speed of sound for the R11+R12 (Solid lines represent liquid-phase results, while dashed lines represent vapor-phase results. The three lines correspond to temperatures of 250 K, 300 K, and 350 K, respectively)
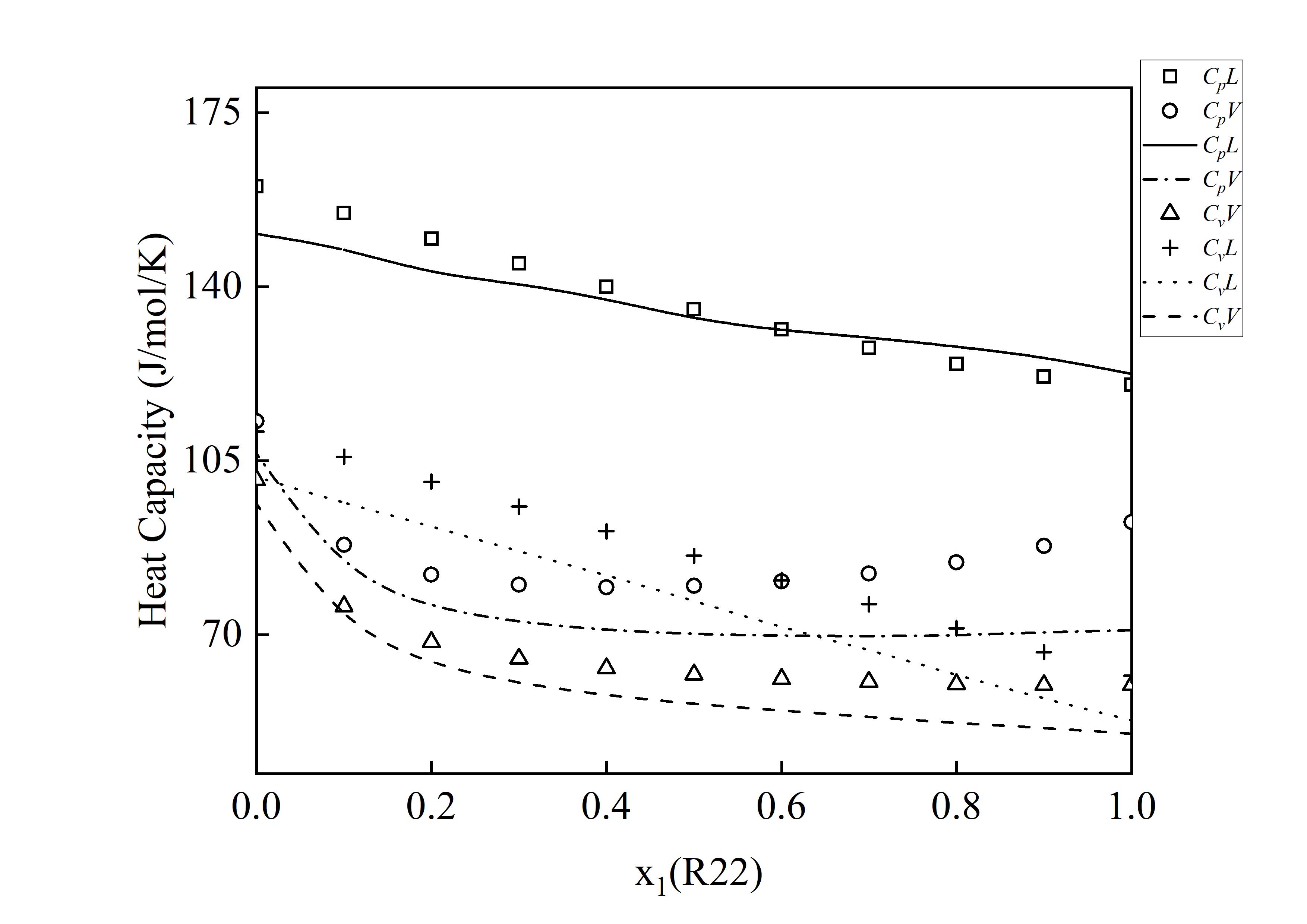
图 10 R22+R123体系在320 K下的混合热容预测结果
Fig. 10 Predicted results of mixing saturated heat capacity for the R22 + R123 system at 320 K (The curves represent predicted values, while the scattered points denote experimental data. Cp is the isobaric heat capacity, Cv is the isochoric heat capacity, V denotes vapor phase, and L denotes liquid phase)
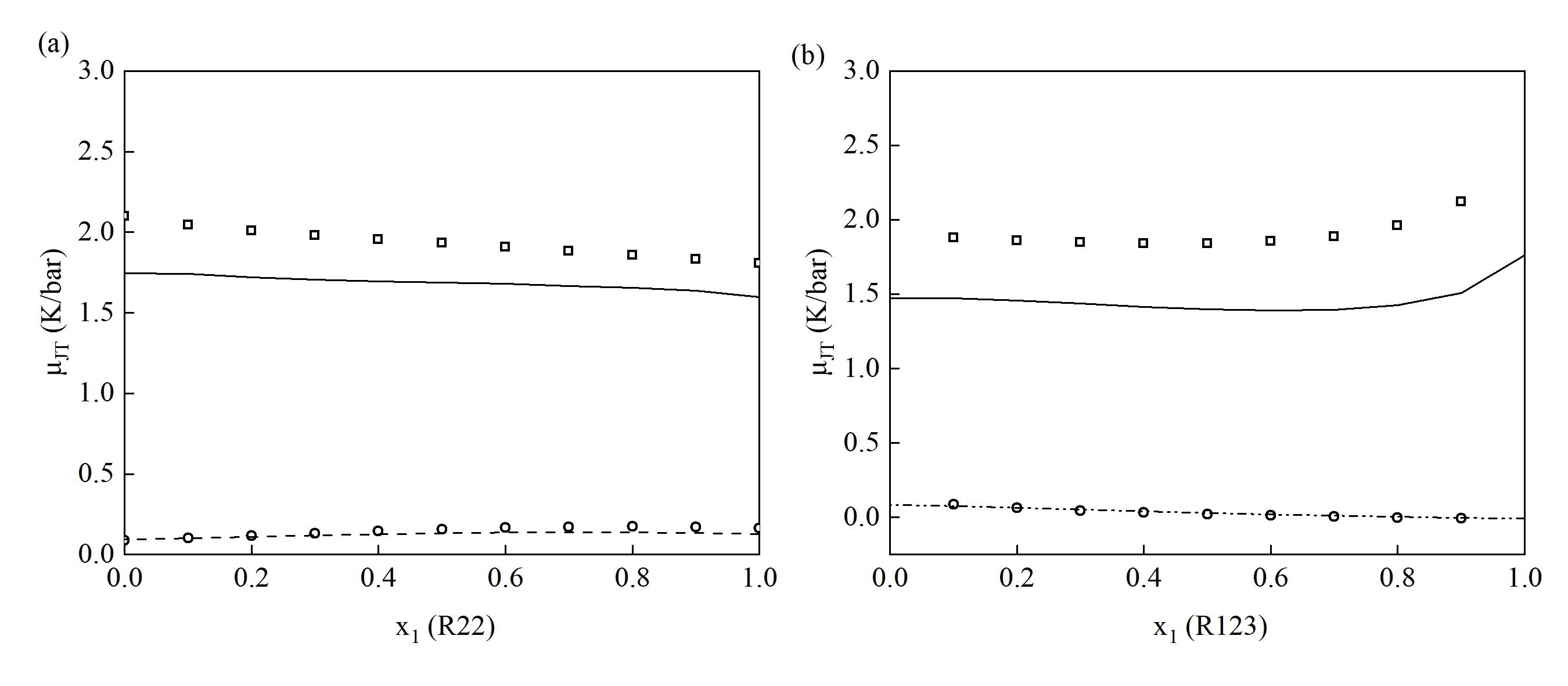
图 11(a) R22+R12体系在350 K下的混合焦汤系数 (μJT ) 预测结果;(b) R123+R134a体系在350 K下的混合焦汤系数(μJT ) 预测结果
Fig. 11(a) Predicted mixing saturated Joule-Thomson coefficient for the R22+R12 at 350 K; (b) Predicted mixing saturated Joule-Thomson coefficient for the R123+R134a at 350 K (Solid lines represent gas-phase results, while dashed lines represent liquid-phase results)
| [1] | 李政杰, 宫子怡, 魏嘉成, 等. 疫苗在冷链运输中的稳定性分析[J]. 管理科学与工程, 2021, 10(4): 365-374. |
| Li Z J, Gong Z Y, Wei J C, et al. Stability analysis of vaccine in cold chain transportation[J]. Management Science and Engineering, 2021, 10(4): 365-374. | |
| [2] | Kasi P, Cheralathan M. Review of cascade refrigeration systems for vaccine storage[J]. Journal of Physics: Conference Series, 2021, 2054(1): 012041. |
| [3] | Chen X T, Zhou L Y, Zhang C B, et al. Research status and future development of cooling technologies for green and energy-efficient data centers[J]. Chinese Journal of Engineering Science, 2022, 24(4): 94. |
| [4] | Ebrahimi K, Jones G F, Fleischer A S. A review of data center cooling technology, operating conditions and the corresponding low-grade waste heat recovery opportunities[J]. Renewable and Sustainable Energy Reviews, 2014, 31: 622-638. |
| [5] | 蒋翘羽, 张仕达, 刘艳, 等. 基于流体PRG EOS常用制冷剂饱和热力学性质的预测[J]. 山东化工, 2021, 50(20): 146-148, 151. |
| Jiang Q Y, Zhang S D, Liu Y, et al. Prediction of saturated thermodynamic properties of common refrigerants based on fluid PRG EOS[J]. Shandong Chemical Industry, 2021, 50(20): 146-148, 151. | |
| [6] | 康凯, 李雪莉, 杨树, 等. 状态方程参数化耦合制冷工质热导率性质预测模型[J]. 西安交通大学学报, 2024, 58(4): 148-157. |
| Kang K, Li X L, Yang S, et al. A prediction model of refrigerant thermal conductivity coupled with the standardized equation of the state parameterization scheme[J]. Journal of Xi'an Jiaotong University, 2024, 58(4): 148-157. | |
| [7] | 邱琳祯, 谷波, 缪梦华. R32热力学性质计算模型及其分析[J]. 化工学报, 2019, 70(6): 2075-2082. |
| Qiu L Z, Gu B, Miao M H. Calculation model and analysis of thermodynamic properties of R32 refrigerant[J]. CIESC Journal, 2019, 70(6): 2075-2082. | |
| [8] | 张永旺, 贾涛. R1234ze(E)环保制冷剂CPA状态方程研究[J]. 化学工程, 2022, 50(3): 57-61. |
| Zhang Y W, Jia T. Research on CPA equation of state of environmentally-friendly refrigerant R1234ze(E)[J]. Chemical Engineering (China), 2022, 50(3): 57-61. | |
| [9] | Tanaka K, Takahashi G, Higashi Y. Measurements of the vapor pressures and pρT properties for trans-1, 3, 3, 3-tetrafluoropropene (HFO-1234ze(E))[J]. Journal of Chemical & Engineering Data, 2010, 55(6): 2169-2172. |
| [10] | Gong M Q, Zhang H Y, Li H Y, et al. Vapor pressures and saturated liquid densities of HFO1234ze(E) at temperatures from 253.343 to 293.318 K[J]. International Journal of Refrigeration, 2016, 64: 168-175. |
| [11] | Katsuyuki T. Measurements of vapor pressure and saturated liquid density for HFO–1234ze(E) and HFO–1234ze(Z)[J]. Journal of Chemical & Engineering Data, 2016, 61(4): 1645-1648. |
| [12] | 张永旺. R1234ze(E)制冷剂PC-SAFT状态方程[J]. 化工装备技术, 2023, 44(6): 47-50. |
| Zhang Y W. PC-SAFT equation of state for R1234ze(E)refrigerant[J]. Chemical Equipment Technology, 2023, 44(6): 47-50. | |
| [13] | 宋有强, 张秀平, 贾磊, 等. PR状态方程的二元混合制冷剂热力性质计算[J]. 低温与超导, 2013, 41(8): 72-77. |
| Song Y Q, Zhang X P, Jia L, et al. Calculation of thermodynamic properties of binary-mixture refrigerants using PR EOS[J]. Cryogenics & Superconductivity, 2013, 41(8): 72-77. | |
| [14] | 田镇, 谷波, 王婷, 等. HFC-32制冷剂饱和液体热力性能参数计算模型[J]. 制冷学报, 2013, 34(2): 28-32. |
| Tian Z, Gu B, Wang T, et al. Calculation model of thermodynamic properties of saturated liquid for HFC-32 refrigerant[J]. Journal of Refrigeration, 2013, 34(2): 28-32. | |
| [15] | 巩玉发, 高珊. RKS方程计算制冷工质的热力学性质的应用[J]. 中国新技术新产品, 2009(21): 80-81. |
| Gong Y F, Gao S. Application of RKS equation in calculating thermodynamic properties of refrigeration working fluids[J]. China New Technologies and Products, 2009(21): 80-81. | |
| [16] | 赵佳美. 新型吸收制冷工质对CO2-[emim] |
| Tf 2N]热力学性质理论研究[D]. 包头:内蒙古科技大学,2014.ZhaoJ M. Theoretic study on thermodynamic performance of new absorption working pair CO 2-[emim][Tf2N][D]. Baotou: Inner Mongolia University of Science and Technology, 2014. | |
| [17] | Moslehi H, Hosseini S M, Alavianmehr M M. Density gradient theory study of surface tension of pure and mixture of refrigerant fluids with the help of perturbed-hard-chain equation of state[J]. Fluid Phase Equilibria, 2023, 568: 113751. |
| [18] | Coquelet C, El Abbadi J, Houriez C. Prediction of thermodynamic properties of refrigerant fluids with a new three-parameter cubic equation of state[J]. International Journal of Refrigeration, 2016, 69: 418-436. |
| [19] | Alavianmehr M M, Pahlavan F, Moghadasi J, et al. Modeling thermodynamic properties of refrigerants from new version of Tao-Mason equation of state[J]. International Journal of Refrigeration, 2014, 45: 100-106. |
| [20] | Mimoune Z, Anoune I, Madani H. Implementation of PC-SAFT for Predicting thermodynamic properties of pure refrigerants and vapor-liquid equilibria of refrigerants binary mixtures[J]. Fluid Phase Equilibria, 2023, 573: 113868. |
| [21] | Ghoderao P N P, Narayan M, Dalvi V H, et al. Predictions of thermodynamic properties of pure fluids, refrigerants, and binary mixtures using modified Peng-Robinson equation of state[J]. Korean Journal of Chemical Engineering, 2022, 39(12): 3452-3463. |
| [22] | Rykov S V, Popov P V, Kudryavtseva I V, et al. Thermodynamic properties of the trans-1, 3, 3, 3-tetrafluoropropene refrigerant: a method for constructing the equation of state and the tabulated data[J]. Measurement Techniques, 2024, 66(10): 765-775. |
| [23] | Akasaka R, Lemmon E W. A Helmholtz energy equation of state for calculations of thermodynamic properties of trans-1, 2-difluoroethene [R-1132(E)][J]. International Journal of Thermophysics, 2024, 45(12): 174. |
| [24] | 李进龙, 彭昌军, 刘洪来. 变阱宽方阱链流体状态方程模拟制冷剂的汽液平衡[J]. 化工学报, 2009, 60(3): 545-552. |
| Li J L, Peng C J, Liu H L. Modeling vapor-liquid equilibrium of refrigerants using an equation of state for square-well chain fluid with variable range[J]. Journal of Chemical Industry and Engineering (China), 2009, 60(3): 545-552. | |
| [25] | Li J S, Wang S L, He Q C, et al. Equation of state with quantum effects for square-well chain fluid with variable range[J]. Scientia Sinica Chimica, 2024, 54(7): 1135-1142. |
| [26] | Li J L, He H H, Peng C J, et al. A new development of equation of state for square-well chain-like molecules with variable width 1.1≤ λ ≤3[J]. Fluid Phase Equilibria, 2009, 276(1): 57-68. |
| [27] | Li J L, Tong M, Peng C J, et al. Equation of state for square-well chain molecules with variable range II. Extension to mixtures[J]. Fluid Phase Equilibria, 2009, 287(1): 50-61. |
| [28] | Li J L, He H H, Peng C J, et al. Equation of state for square-well chain molecules with variable range. I: Application for pure substances[J]. Fluid Phase Equilibria, 2009, 286(1): 8-16. |
| [29] | Carnahan N F, Starling K E. Equation of state for nonattracting rigid spheres[J]. The Journal of Chemical Physics, 1969, 51(2): 635-636. |
| [30] | Barker J A, Henderson D. Perturbation theory and equation of state for fluids: the square-well potential[J]. The Journal of Chemical Physics, 1967, 47(8): 2856-2861. |
| [31] | Linstrom P J, Mallard W G. The NIST Chemistry WebBook: a chemical data resource on the Internet[J]. Journal of Chemical & Engineering Data, 2001, 46(5): 1059-1063. |
| [32] | Zhang Z Y, Gao M, Chen X P, et al. The Joule–Thomson effect of (CO2 + H2) binary system relevant to gas switching reforming with carbon capture and storage (CCS)[J]. Chinese Journal of Chemical Engineering, 2023, 54: 215-231. |
| [33] | Lafitte T, Apostolakou A, Avendaño C, et al. Accurate statistical associating fluid theory for chain molecules formed from Mie segments[J]. The Journal of Chemical Physics, 2013, 139(15): 154504. |
| [34] | Dufal S, Lafitte T, Haslam A J, et al. The A in SAFT: developing the contribution of association to the Helmholtz free energy within a Wertheim TPT1 treatment of generic Mie fluids[J]. Molecular Physics, 2015, 113(9/10): 948-984. |
| [35] | Gil-Villegas A, Galindo A, Whitehead P J, et al. Statistical associating fluid theory for chain molecules with attractive potentials of variable range[J]. The Journal of Chemical Physics, 1997, 106(10): 4168-4186. |
| [36] | Gross J, Sadowski G. Perturbed-chain SAFT: an equation of state based on a perturbation theory for chain molecules[J]. Industrial & Engineering Chemistry Research, 2001, 40(4): 1244-1260. |
| [37] | Gross J, Sadowski G. Application of the perturbed-chain SAFT equation of state to associating systems[J]. Industrial & Engineering Chemistry Research, 2002, 41(22): 5510-5515. |
| [38] | Yu D H, Chen Z. A theoretical analysis on enthalpy of vaporization: Temperature-dependence and singularity at the critical state[J]. Fluid Phase Equilibria, 2020, 516: 112611. |
| [39] | Lafitte T, Bessieres D, Piñeiro M M, et al. Simultaneous estimation of phase behavior and second-derivative properties using the statistical associating fluid theory with variable range approach[J]. The Journal of Chemical Physics, 2006, 124(2): 024509. |
| [40] | Polishuk I, Katz M, Levi Y, et al. Implementation of PC-SAFT and SAFT+Cubic for modeling thermodynamic properties of haloalkanes. I. 11 halomethanes[J]. Fluid Phase Equilibria, 2012, 316: 66-73. |
| [41] | Polishuk I, Assor E, Cohen N, et al. Implementation of PC-SAFT and SAFT + Cubic for modeling thermodynamic properties of haloalkanes. II. 7 Haloethanes and their mixtures[J]. International Journal of Refrigeration, 2013, 36(3): 980-991. |
| [42] | Tarom N, Hossain M M, Rohi A. A new practical method to evaluate the Joule–Thomson coefficient for natural gases[J]. Journal of Petroleum Exploration and Production Technology, 2018, 8(4): 1169-1181. |
| [43] | Li M X, Bai Y F, Zhang C Z, et al. Review on the research of hydrogen storage system fast refueling in fuel cell vehicle[J]. International Journal of Hydrogen Energy, 2019, 44(21): 10677-10693. |
| [44] | Llovell F, Peters C J, Vega L F. Second-order thermodynamic derivative properties of selected mixtures by the soft-SAFT equation of state[J]. Fluid Phase Equilibria, 2006, 248(2): 115-122. |
| [1] | 杨语晴, 李银龙, 晏刚. 采用低GWP制冷剂的级联加热自复叠高温热泵循环热力学分析[J]. 化工学报, 2025, 76(S1): 43-53. |
| [2] | 冯彪, 张昭, 李思琪, 王秉睿, 吴红颖, 史淼, 王丹, 马素霞. 适配环保制冷剂R290的阻燃剂性能研究[J]. 化工学报, 2025, 76(S1): 462-468. |
| [3] | 段浩磊, 陈浩远, 梁坤峰, 王林, 陈彬, 曹勇, 张晨光, 李硕鹏, 朱登宇, 何亚茹, 杨大鹏. 纯电动车热管理系统低GWP工质替代方案性能分析与综合评价[J]. 化工学报, 2025, 76(S1): 54-61. |
| [4] | 刘豪, 王林, 丁昊, 耿嘉怡. R1150+R1234ze(E)二元体系223.15~253.15 K汽液相平衡研究[J]. 化工学报, 2025, 76(S1): 1-8. |
| [5] | 臧子晴, 李修真, 谈莹莹, 刘晓庆. 分凝器对两级分离自复叠制冷循环特性影响研究[J]. 化工学报, 2025, 76(S1): 17-25. |
| [6] | 朱腾飞, 刘晔. 低GWP制冷剂在新能源汽车空调应用性能分析[J]. 化工学报, 2025, 76(S1): 343-350. |
| [7] | 韩光泽, 张佩璇. 静电场作用下液体凝固点变化的热力学机理[J]. 化工学报, 2025, 76(6): 2544-2548. |
| [8] | 陈建兵, 常昊, 高明, 邢兵, 张磊, 刘奇磊. 基于反应模板与分子动力学的胺基相变吸收剂分相预测方法[J]. 化工学报, 2025, 76(5): 2387-2396. |
| [9] | 刘淑丽, 周文豪, 张少良, 沈永亮. 太阳能直接吸收相变集-蓄热器的放热特性研究[J]. 化工学报, 2025, 76(4): 1722-1730. |
| [10] | 尤潇楠, 范小强, 杨遥, 王靖岱, 阳永荣. 超临界乙烯和高压聚乙烯混合物的减压分离过程建模方法[J]. 化工学报, 2025, 76(2): 695-706. |
| [11] | 黄鑫, 李逸龙, 李卫东, 施鸿翔, 尹鹏博, 李臻超, 滕霖, 江莉龙. 液氨-成品油混合体系相平衡及减压相变规律研究[J]. 化工学报, 2025, 76(1): 71-80. |
| [12] | 杨明军, 宋维, 张磊, 凌铮, 陈兵兵, 宋永臣. CO2-海水水合物生成强化方法研究[J]. 化工学报, 2024, 75(8): 2939-2948. |
| [13] | 赵志星, 姚智豪, 于雪峰, 杨游胜, 曾英, 于旭东. 锂钠镁共存硫酸盐体系多温相图及其应用[J]. 化工学报, 2024, 75(6): 2123-2133. |
| [14] | 李新泽, 张双星, 杨洪海, 杜文静. 基于电池冷却用新型脉动热管性能的实验研究[J]. 化工学报, 2024, 75(6): 2222-2232. |
| [15] | 陈好奇, 史博会, 彭琪, 康琦, 宋尚飞, 姚海元, 陈海宏, 吴海浩, 宫敬. 基于稳定性分析的含酸/醇烃水体系相平衡计算[J]. 化工学报, 2024, 75(3): 789-800. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号