• •
宋刘斌1,2( ), 李澳1,2, 陈涛涛1,2, 陈丽霞1,2, 鄢立祥1,2, 熊逸雨1,2, 匡尹杰1,2, 赵亭亭1,2(
), 李澳1,2, 陈涛涛1,2, 陈丽霞1,2, 鄢立祥1,2, 熊逸雨1,2, 匡尹杰1,2, 赵亭亭1,2( )
)
收稿日期:2025-09-19
修回日期:2025-10-28
出版日期:2025-11-06
通讯作者:
赵亭亭
作者简介:宋刘斌(1981—),男,博士,副教授,liubinsong1981@126.com
基金资助:
LiuBin SONG1,2( ), Ao LI1,2, Taotao CHEN1,2, Lixia CHEN1,2, Lixiang YAN1,2, Yiyu XIONG1,2, Yinjie KUANG1,2, Tingting ZHAO1,2(
), Ao LI1,2, Taotao CHEN1,2, Lixia CHEN1,2, Lixiang YAN1,2, Yiyu XIONG1,2, Yinjie KUANG1,2, Tingting ZHAO1,2( )
)
Received:2025-09-19
Revised:2025-10-28
Online:2025-11-06
Contact:
Tingting ZHAO
摘要:
离子掺杂是一种有效提升单斜相磷酸钒锂Li3V2(PO4)3(LVP)正极材料电化学性能的常用方法。本研究通过喷雾干燥辅助溶胶凝胶法,以2-甲基咪唑(C4H6N2)作为碳源和氮源,实现了氮掺杂碳包覆层的原位合成。并在氮掺杂碳包覆层的基础上,进一步采用Ti4+掺杂对Li₃V₂(PO₄)₃/NC(简称LVP/NC)进行双重修饰改性,制备了不同Ti4+掺杂比例的Li₃V₂-xTix(PO₄)₃/NC(简称LVTxP/NC)。XPS分析在该纳米复合材料中观察到Ti4+特有的2p3/2和2p1/2峰,表明Ti4+已成功掺入晶格并取代部分V3+位置。与未掺杂的 Li₃V₂(PO₄)₃/C(简称LVP/C)相比,所制备的LVTxP/NC具有更高的可逆容量、更优异的高倍率性能和更好的循环性能。特别是LVT0.1P/NC样品,在10C倍率(3.0~4.3 V)下经过1000次循环后,容量下降至99.47 mAh∙g-1,容量保持率为94.02%。而在5C倍率(3.0~4.8 V)下LVT0.1P/NC的初始容量为164.63mAh∙g-1,1000次循环后容量保持率可达94.11%,循环性能稳定。LVT0.1P/NC的良好电化学性能得益于氮掺杂碳包覆与Ti4+共掺杂的协同效应,有效提高了材料的电子导电性和锂离子扩散系数,为高倍率锂离子电池正极材料的设计提供了参考。
中图分类号:
宋刘斌, 李澳, 陈涛涛, 陈丽霞, 鄢立祥, 熊逸雨, 匡尹杰, 赵亭亭. 磷酸钒锂正极材料的氮掺杂碳包覆与Ti4+共掺杂协同改性研究[J]. 化工学报, DOI: 10.11949/0438-1157.20251052.
LiuBin SONG, Ao LI, Taotao CHEN, Lixia CHEN, Lixiang YAN, Yiyu XIONG, Yinjie KUANG, Tingting ZHAO. Study on Synergistic Modification of Nitrogen-Doped Carbon Coating and Ti⁴⁺ Co-Doping for Lithium Vanadium Phosphate Cathode Materials[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251052.
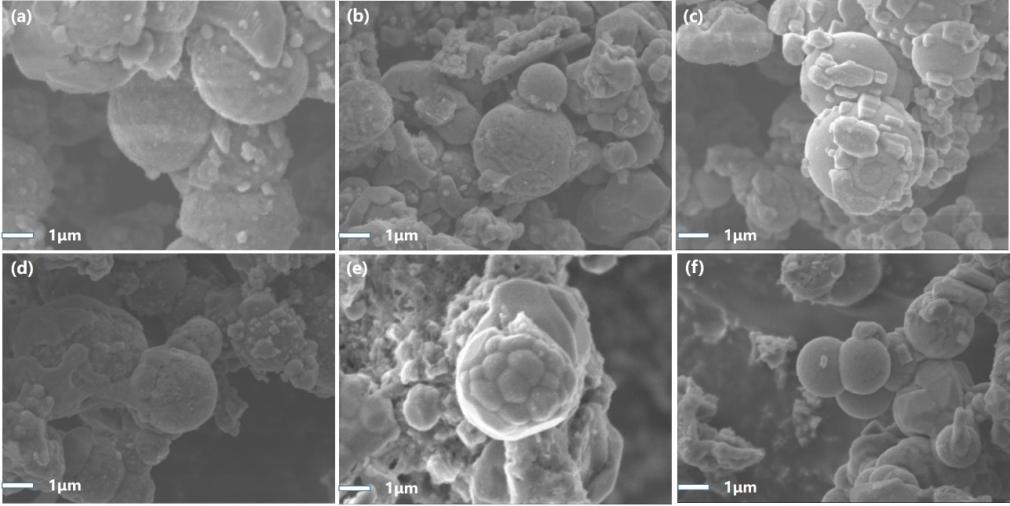
图3 不同包覆量材料的SEM图:(a)NC-1; (b)NC-2; (c)NC-3; (d)NC-4;(e)NC-5;(f)LVP/C注:(d) NC-4; (e) NC-5;(f)LVP/C
Fig.3 SEM images of materials with different amounts of coating: (a) NC-1; (b) NC-2; (c) NC-3;
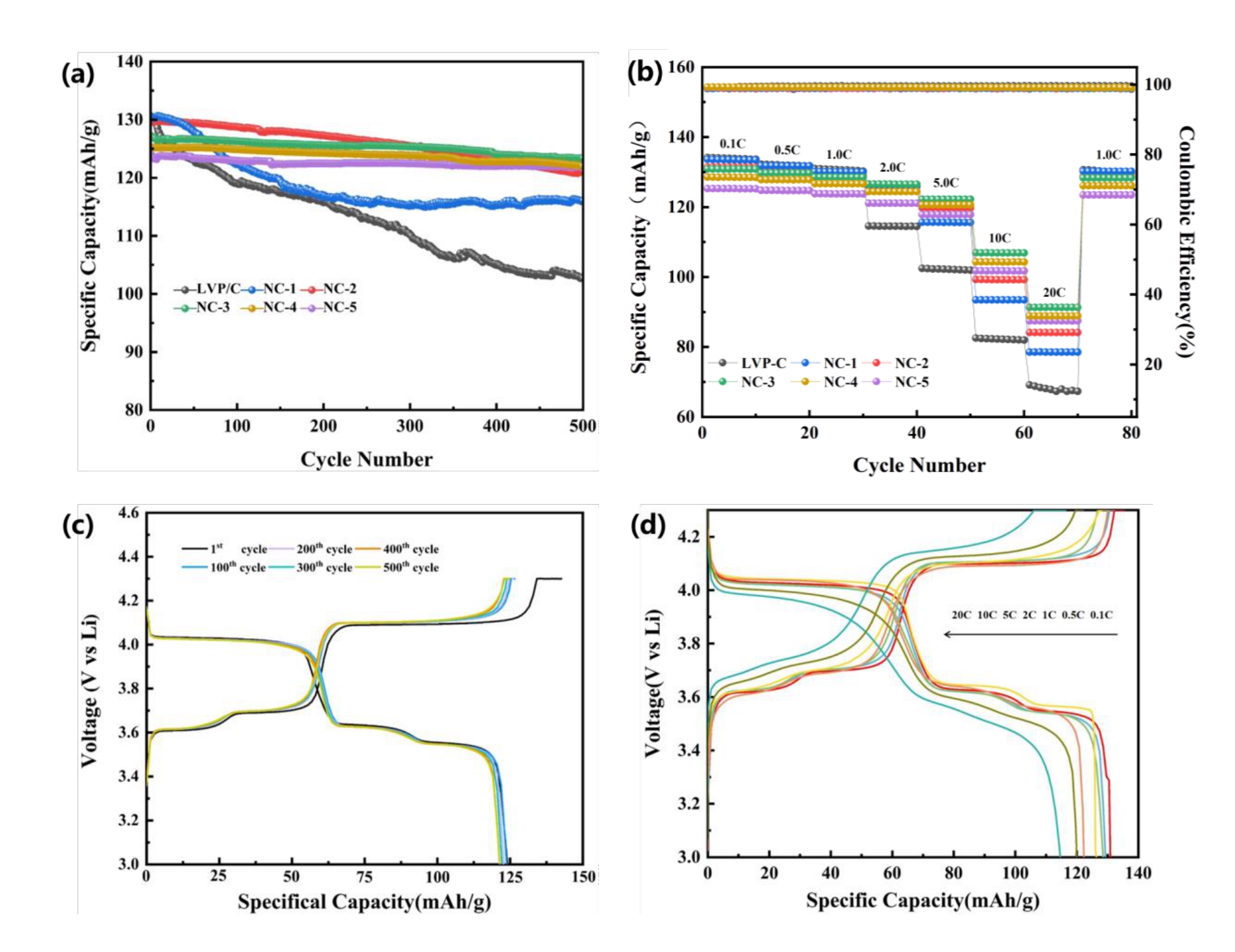
图6 (a)不同碳包覆材料的循环性能曲线;(b)不同碳复合材料的倍率性能曲线注:(c)NC-3在1c倍率下的充放电曲线;(d)NC-3在不同倍率下的充放电曲线
Fig.6 (a)Cycling Performance Curves of Different Carbon Coated Materials;(b) Rate performance curves of different carbon composites;(c)Charge-discharge curve of NC-3 at 1c rate;(b)Charge/discharge curves of NC-3 at different rates
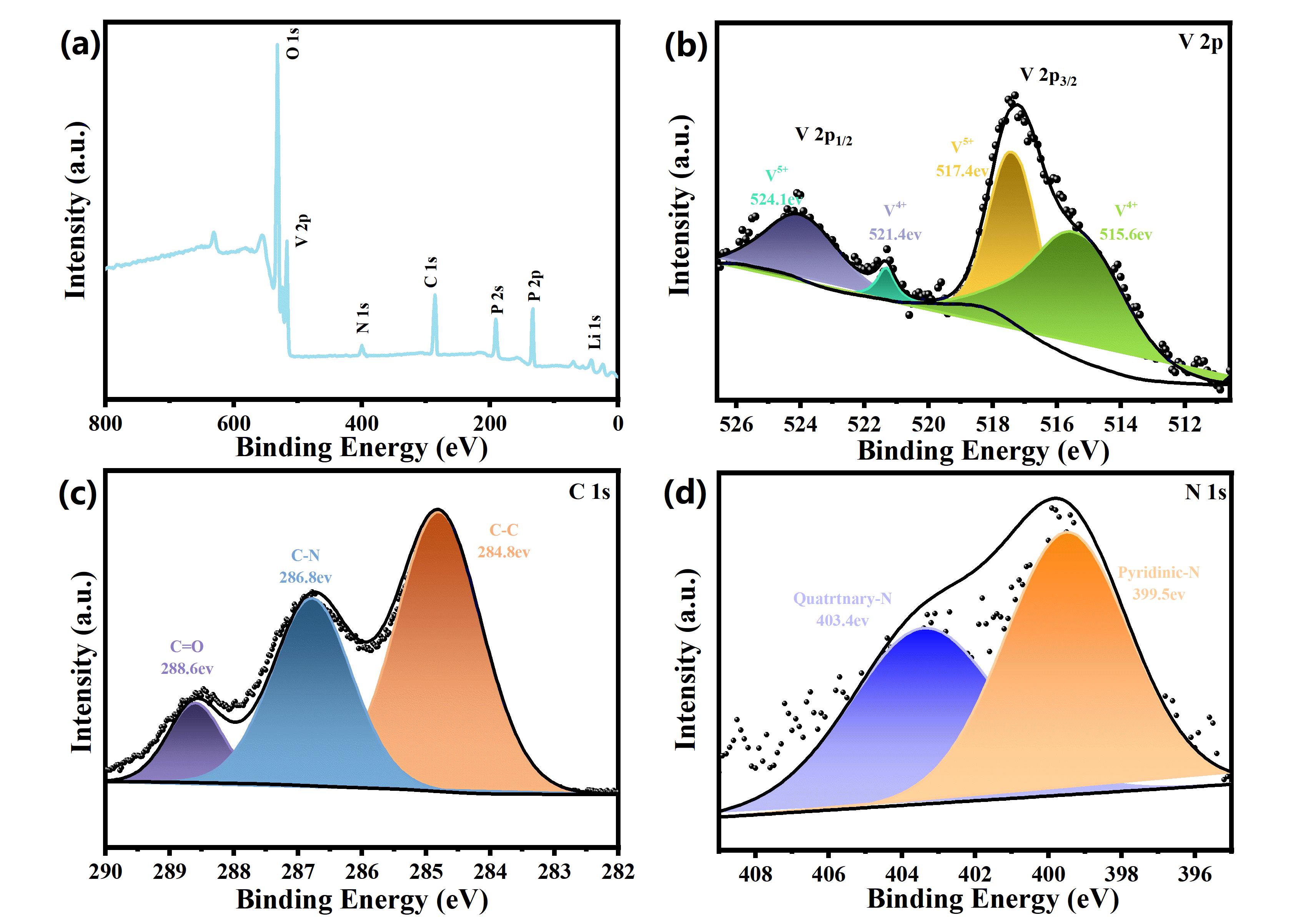
图7 (a) NC-3的XPS谱图:(a)全谱图;注:(b) V 2p精细谱;(c) C 1s精细谱;(d) N 1s精细谱
Fig 7 XPS spectra of NC-3: (a)full spectrum; (b) V 2p refined spectrum; (c) C 1s refined spectrum; (d) N 1s refined spectrum
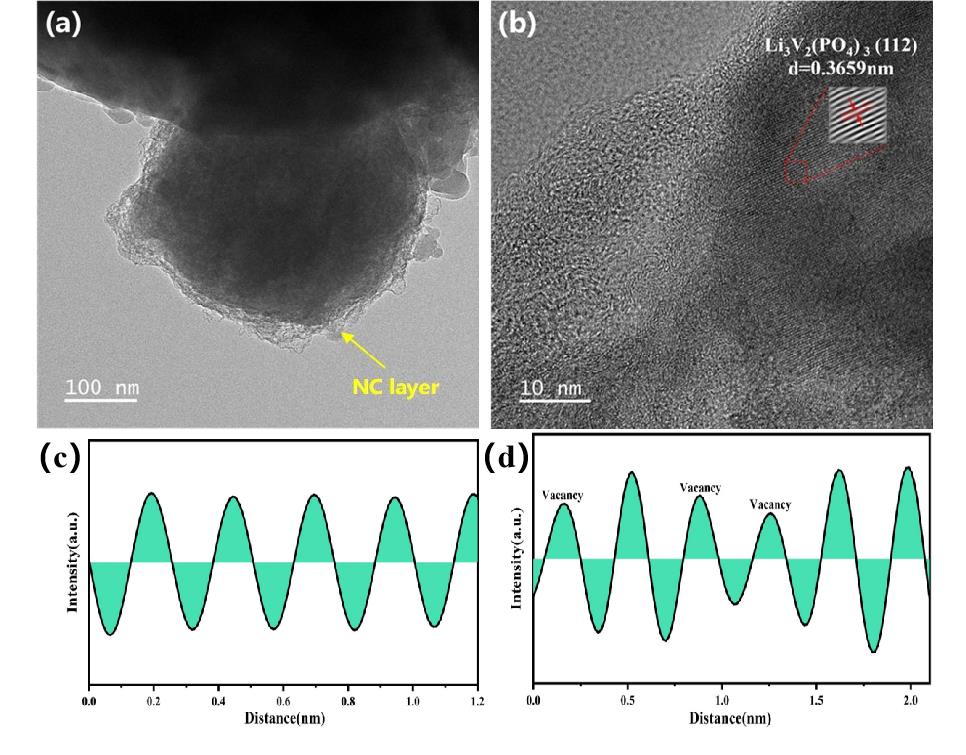
图11 (a)、(b)LVT0.1P/NC的TEM图像;(c)LVP/NC的晶格条纹强度曲线;(d) LVT0.1P/NC的晶格条纹强度曲线
Fig 11 (a), (b) TEM images of LVT0.1P/NC;(c) Lattice stripe intensity curve of LVP/NC; (d) Lattice stripe intensity curve of LVT0.1P/NC
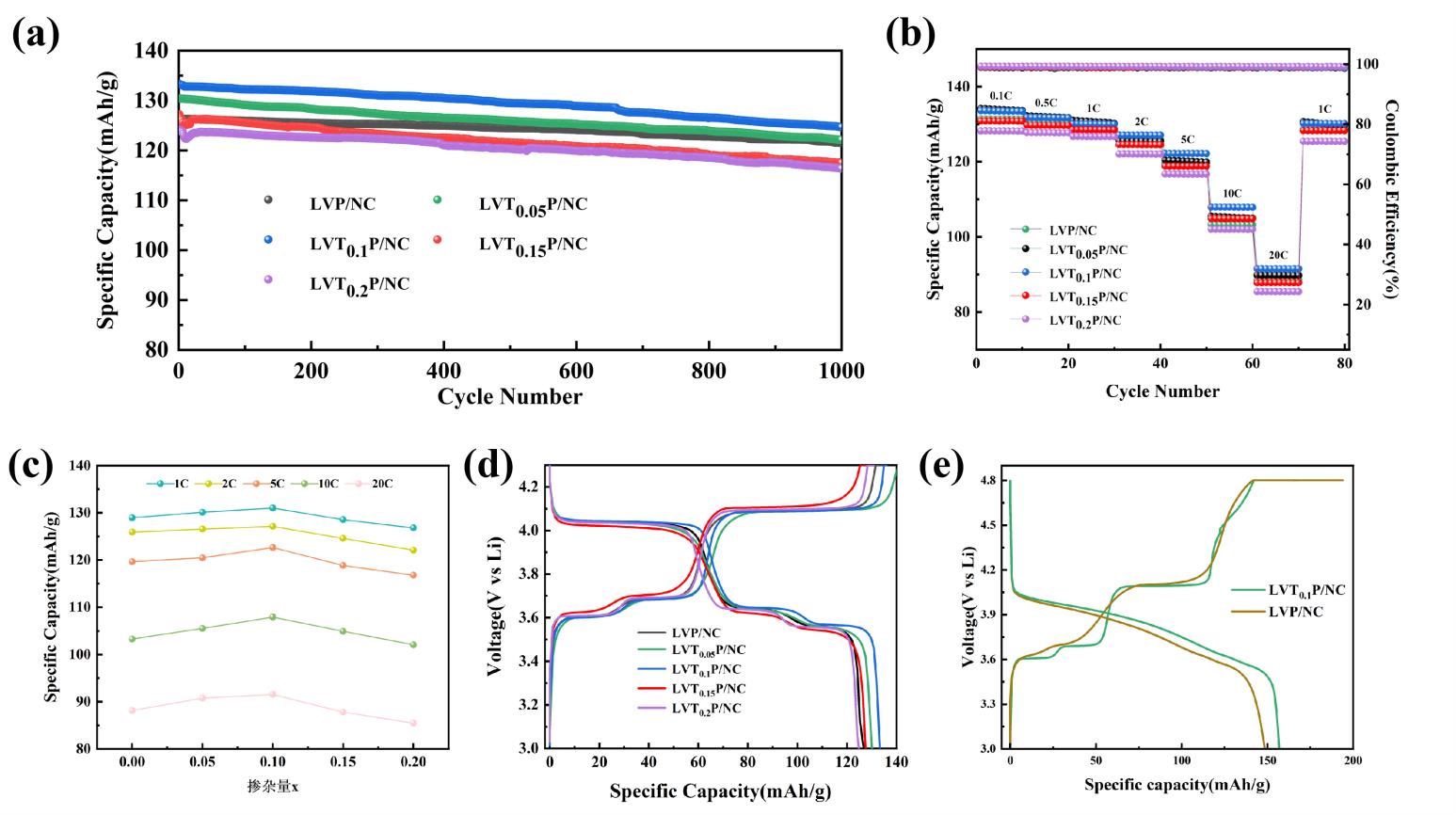
图12 (a) LVTxP/NC(x=0、0.05、0.1、0.15、0.2)样品在1C下循环性能图;(b)LVTxP/NC倍率性能图;(c)LVTxP/NC容量变化趋势图;(d)首次充放电曲线LVTxP/NC(4.3V);(e)LVT0.1P/NC与LVP/NC首次充放电曲线对比图(4.8V)
Fig 12 (a) Cycling performance plot of LVTxP/NC (x=0, 0.05, 0.1, 0.15, 0.2) samples at 1C; (b) LVTxP/NC Rate performance plot; (c) LVTxP/NC Capacity trend plotrve; (d) First charge/discharge curve(4.3V); (e)Comparison of Initial Charge-Discharge Curves for LVT0.1P/NC and LVP/NC(4.8V)
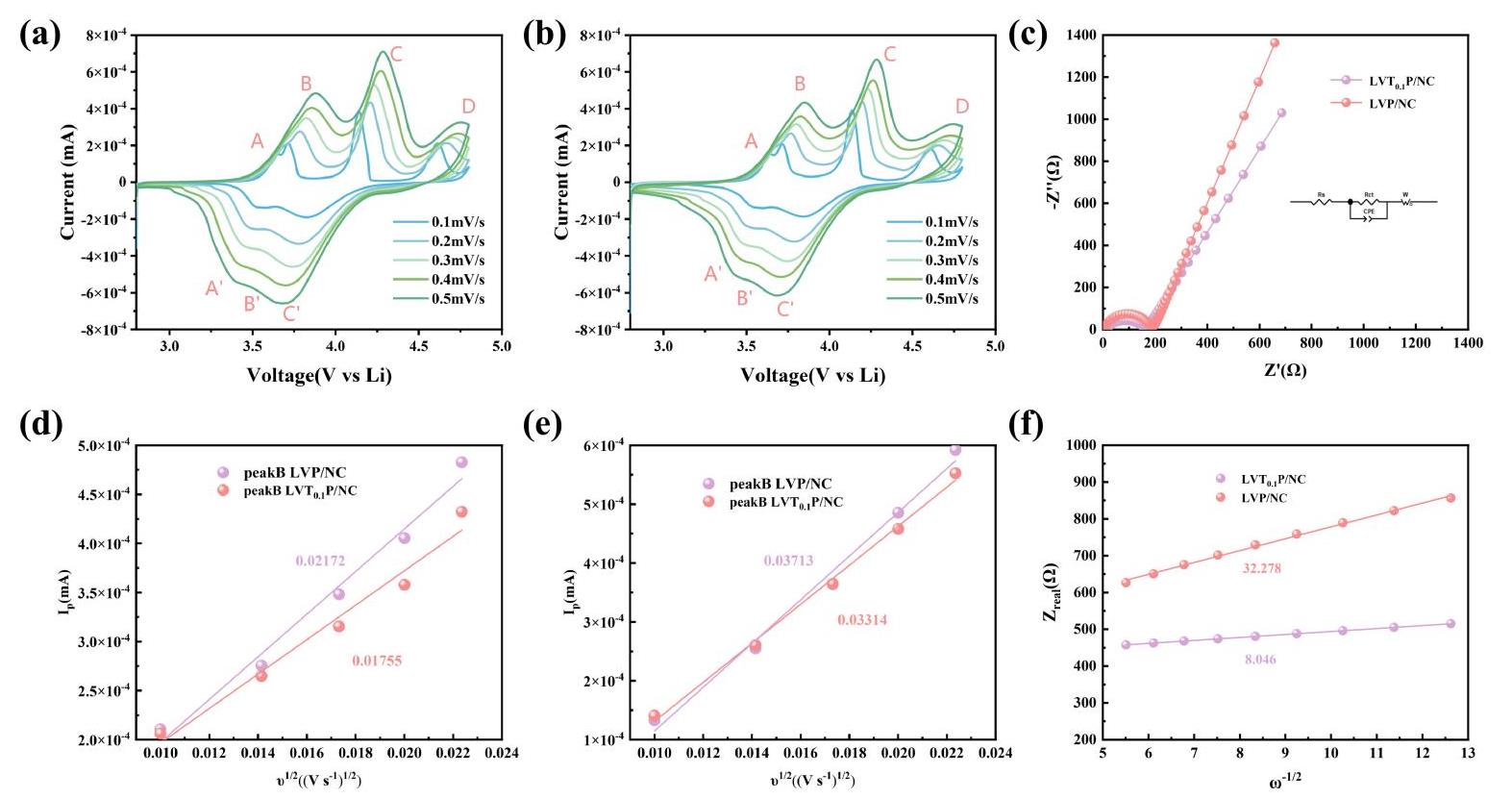
图13 CV曲线(0.1~0.5 mV∙s-1):(a) LVT0.1P/NC;(b) LVP/NC;(c)LVT0.1P/NC和LVP/NC的EIS曲线及等效电路;Ip与v1/2关系的线性拟合:(d) 氧化过程;(e) 还原过程;(f)Zreal与ω-1/2的线性拟合
Fig 13 CV curves (0.1~0.5 mV∙s-1) for (a) LVT0.1P/NC; (b) LVP/NC; (c) EIS curves and equivalent circuits for LVT0.1P/NC and LVP/NC; Linear fitting between Ip and v1/2: (d) oxidize process; (e) reduce process;(f) linear fit of Zreal to ω-1/2
| LVT0.1P/NC | 氧化过程 | 还原过程 | LVP/NC | 氧化过程 | 还原过程 |
|---|---|---|---|---|---|
| 0.1 mV∙s-1 | 1.765×10-9 | 0.902×10-9 | 0.1 mV∙s-1 | 4.137×10-10 | 2.128×10-10 |
| 0.2 mV∙s-1 | 1.076×10-9 | 1.492×10-9 | 0.2 mV∙s-1 | 3.939×10-10 | 3.647×10-10 |
| 0.3 mV∙s-1 | 1.341×10-9 | 1.745×10-9 | 0.3 mV∙s-1 | 3.886×10-10 | 4.764×10-10 |
| 0.4 mV∙s-1 | 1.406×10-9 | 2.325×10-9 | 0.4 mV∙s-1 | 3.854×10-10 | 5.624×10-10 |
| 0.5 mV∙s-1 | 2.002×10-9 | 3.053×10-9 | 0.5 mV∙s-1 | 4.003×10-10 | 6.553×10-10 |
表1 LVT0.1P/NC和LVP/NC的锂离子扩散系数DLi+
Table 1 Diffusion coefficients (DLi+) of the LVT0.1P/NC & LVP/NCsamples cm2 s-1Unit: cm2 s-1
| LVT0.1P/NC | 氧化过程 | 还原过程 | LVP/NC | 氧化过程 | 还原过程 |
|---|---|---|---|---|---|
| 0.1 mV∙s-1 | 1.765×10-9 | 0.902×10-9 | 0.1 mV∙s-1 | 4.137×10-10 | 2.128×10-10 |
| 0.2 mV∙s-1 | 1.076×10-9 | 1.492×10-9 | 0.2 mV∙s-1 | 3.939×10-10 | 3.647×10-10 |
| 0.3 mV∙s-1 | 1.341×10-9 | 1.745×10-9 | 0.3 mV∙s-1 | 3.886×10-10 | 4.764×10-10 |
| 0.4 mV∙s-1 | 1.406×10-9 | 2.325×10-9 | 0.4 mV∙s-1 | 3.854×10-10 | 5.624×10-10 |
| 0.5 mV∙s-1 | 2.002×10-9 | 3.053×10-9 | 0.5 mV∙s-1 | 4.003×10-10 | 6.553×10-10 |
| 样品 | Rs (Ω) | Rct (Ω) | σ (Ω∙s-1/2) | DLi+ (cm2 s-1) |
|---|---|---|---|---|
| LVT0.1P/NC | 14.79 | 163.28 | 8.046 | 1.710×10-9 |
| LVP/NC | 3.43 | 190.58 | 32.278 | 1.063×10-10 |
表2 LVP/NC和LVT0.1P/NC的韦伯因子σ和锂离子扩散系数DLi+
Table 2 Warburg coefficient (σ) and Diffusion coefficient (DLi+) for LVP/NC and LVT0.1P/NC
| 样品 | Rs (Ω) | Rct (Ω) | σ (Ω∙s-1/2) | DLi+ (cm2 s-1) |
|---|---|---|---|---|
| LVT0.1P/NC | 14.79 | 163.28 | 8.046 | 1.710×10-9 |
| LVP/NC | 3.43 | 190.58 | 32.278 | 1.063×10-10 |
| [1] | Stambouli A B, Traversa E. Solid oxide fuel cells (SOFCs): a review of an environmentally clean and efficient source of energy [J]. Renewable and Sustainable Energy Reviews, 2002, 6(5): 433-455. |
| [2] | Li Y, Bai Y, Yang Z, et al. Reorganizing electronic structure of Li3V2(PO4)3 using polyanion(BO3)3-:towards better electrochemical performances [J]. Rare Metals, 2017, 36(05): 397-402. |
| [3] | Dunn B, Kamath H, Tarascon J M. Electrical energy storage for the grid: a battery of choices [J]. Science, 2011, 334(6058): 928-935. |
| [4] | Lu L G, Han X B, Li J Q, et al. A review on the key issues for lithium-ion battery management in electric vehicles[J]. Journal of Power Sources, 2013, 226: 272-288. |
| [5] | Cho J, Jeong S, Kim Y. Commercial and research battery technologies for electrical energy storage applications [J]. Progress in Energy and Combustion Science, 2015, 48: 84-101. |
| [6] | Xia Y, Zhang W K, Huang H, et al. Synthesis and electrochemical properties of Nb-doped Li3V2(PO4)3/C cathode materials for lithium-ion batteries [J]. Materials Science and Engineering: B, 2011, 176(8): 633-639. |
| [7] | Barker J, Gover R K B, Burns P, et al. TThe effect of Al substitution on the electrochemical insertion properties of the lithium vanadium phosphate, Li3V2(PO4)3 [J]. Journal of The Electrochemical Society, 2007, 154(4): A307. |
| [8] | Dong Y Z, Zhao Y M, Duan H. The effect of doping Mg2+ on the structure and electrochemical properties of Li3V2(PO4)3 cathode materials for lithium-ion batteries [J]. Journal of Electroanalytical Chemistry, 2011, 660(1): 14-21. |
| [9] | Han D W, Lim S J, Kim Y I, et al. ChemInform abstract: facile lithium ion transport through superionic pathways formed on the surface of Li3V2(PO4)3/C for high power Li ion battery [J]. ChemInform, 2014, 45(33): 201433017. |
| [10] | Zhang Y, Nie P, Shen L F, et al. Rhombohedral NASICON-structured Li2NaV2(PO4)3 with single voltage plateau for superior lithium storage [J]. RSC Advances, 2014, 4(17): 8627-8631. |
| [11] | Kuang Q, Zhao Y M, Liang Z Y. Synthesis and electrochemical properties of Na-doped Li3V2(PO4)3 cathode materials for Li-ion batteries [J]. Journal of Power Sources, 2011, 196(23): 10169-10175. |
| [12] | Zhang K X, Dou Y H, Wang Q H, et al. Accelerated lithium ion interchange and enhanced electrochemical performance of lithium vanadium phosphate cathodes by Cr3+ doping [J]. Electrochimica Acta, 2025, 524: 146054. |
| [13] | Chen R J, Lai J N, Li Y J, et al. β-Cyclodextrin coated lithium vanadium phosphate as novel cathode material for lithium ion batteries [J]. RSC Advances, 2016, 6(105): 103364-103371. |
| [14] | Tong J S, Su A Y, Ma T, et al. Boosting low temperature performance of lithium ion batteries at -40°С using a binary surface coated Li3V2(PO4)3 cathode material [J]. Advanced Functional Materials, 2024, 34(10): 2310934. |
| [15] | Zhang R Y, Zhang Y Q, Zhu K, et al. Carbon and RuO2 binary surface coating for the Li3V2(PO4)3 cathode material for lithium-ion batteries [J]. ACS Applied Materials & Interfaces, 2014, 6(15): 12523-12530. |
| [16] | Wu J G, Xu M W, Tang C, et al. F-Doping effects on carbon-coated Li3V2(PO4)3 as a cathode for high performance lithium rechargeable batteries: combined experimental and DFT studies [J]. Physical Chemistry Chemical Physics, 2018, 20(22): 15192-15202. |
| [17] | Wang Z Y, He W, Zhang X D, et al. Multilevel structures of Li3V2(PO4)3/phosphorus-doped carbon nanocomposites derived from hybrid V-MOFs for long-life and cheap lithium ion battery cathodes [J]. Journal of Power Sources, 2017, 366: 9-17. |
| [18] | Li X P, Du X Y, Xu Y L, et al. Three-dimensional holey graphene enwrapped Li3V2(PO4)3/N-doped carbon cathode for high-rate and long-life Li-ion batteries [J]. ChemSusChem, 2022, 15(21): e202201459. |
| [19] | Zhang L, Xiang H F, Li Z, et al. Porous Li3V2(PO4)3/C cathode with extremely high-rate capacity prepared by a sol–gel-combustion method for fast charging and discharging [J]. Journal of Power Sources, 2012, 203: 121-125. |
| [20] | Wu J G, Zhong C Y, Chen X F, et al. Li3V2(PO4)3 particles embedded in a N and S co-doped porous carbon cathode for high performance lithium storage: an experimental and DFT study [J]. Inorganic Chemistry Frontiers, 2025, 12(1): 217-230. |
| [21] | Hsiang H I, Cai B R, Chung S H, et al. Preparation and electrochemical properties of Li3V2(PO4)3 glass-ceramic materials [J]. Ceramics International, 2023, 49(21): 34155-34163. |
| [22] | Inagaki M, Toyoda M, Soneda Y, et al. Nitrogen-doped carbon materials [J]. Carbon, 2018, 132: 104-140. |
| [23] | Kang T, Meng Y S, Liu X Z. Optimizing lithium-ion diffusion in LiFePO4: the impact of Ti4+ doping on high-rate capability and electrochemical stability [J]. Ionics, 2025, 31(3): 2419-2428. |
| [24] | Huang Y D, Chen Y J, Li P Y, et al. Improved cycling stability of LFP by W-Ti co-doping strategy for Li-ion batteries [J]. Journal of The Electrochemical Society, 2024, 171(5): 050516. |
| [25] | Lee S H, Ryu K S. Effects of Ti doping on the structural stability and enhanced electrochemical performance of α-LiVOPO4 [J]. Bulletin of the Korean Chemical Society, 2018, 39(11): 1266-1272. |
| [26] | Harada Y, Okita N, Fukuyama M, et al. Ultralong lifespan of SuperRedox Capacitor using Ti-doped Li3V2(PO4)3/C cathode with suppressed vanadium dissolution [J]. Journal of Materials Chemistry A, 2024, 12(3): 1703-1713. |
| [27] | Zhang L L, Li Z, Yang X L, et al. Binder-free Li3V2(PO4)3/C membrane electrode supported on 3D nitrogen-doped carbon fibers for high-performance lithium-ion batteries [J]. Nano Energy, 2017, 34: 111-119. |
| [28] | Zhao T Y, Mahandra H, Marthi R, et al. An overview on the life cycle of lithium iron phosphate: synthesis, modification, application, and recycling [J]. Chemical Engineering Journal, 2024, 485: 149923. |
| [29] | Gao A L, Sun Z H, Li S P, et al. Self-assembled layered lithium manganese oxide shows ultra-large adsorption capacity and high selectivity for lithium [J]. Chemical Engineering Journal, 2023, 471: 144287. |
| [30] | Manthiram A, Goodenough J B. Layered lithium cobalt oxide cathodes [J]. Nature Energy, 2021, 6(3): 323. |
| [31] | Xing X, Li Y J, Wang S, et al. Graphite-based lithium-free 3D hybrid anodes for high energy density all-solid-state batteries [J]. ACS Energy Letters, 2021, 6(5): 1831-1838. |
| [32] | Zhang Y, Hao S P, Pei F, et al. Operando chemo-mechanical evolution in LiNi0.8Co0.1Mn0.1O2 cathodes [J]. National Science Review, 2024, 11(9): nwae254. |
| [33] | Chen J, Zou G Q, Deng W T, et al. Pseudo-bonding and electric-field harmony for Li-rich Mn-based oxide cathode [J]. Advanced Functional Materials, 2020, 30(46): 2004302. |
| [1] | 赵婧, 董书辰, 李高洋, 黄友科, 石浩森, 缪舒文, 谭辰妍, 朱唐琦, 李永帅, 潘慧, 凌昊. 基于电化学模型的电池性能模拟与优化[J]. 化工学报, 2025, 76(9): 4922-4932. |
| [2] | 娄岚浩, 杨立鹏, 杨晓光. 锂离子电池电化学机理模型参数辨识研究综述[J]. 化工学报, 2025, 76(9): 4369-4382. |
| [3] | 李欣然, 常龙娇, 罗绍华, 李永兵, 杨瑞芬, 侯增磊, 邹杰. Ho掺杂诱导NCM622局域电子重构抑制阳离子混排的改性机制研究[J]. 化工学报, 2025, 76(7): 3733-3741. |
| [4] | 吴鹂霄, 燕溪溪, 张素娜, 徐一鸣, 钱佳颖, 乔永民, 王利军. 磷掺杂微晶石墨的制备及其在锂离子电池负极材料中的电化学性能研究[J]. 化工学报, 2025, 76(7): 3615-3625. |
| [5] | 孙传付, 胡桂林, 曹俊杰, 左启斌, 陈媚, 夏玉珍. 梯度孔分布ZnO-GA锂离子电池负极材料研究[J]. 化工学报, 2025, 76(7): 3710-3718. |
| [6] | 孙文浩, 田君, 张锟, 刘娜, 曹宝森, 梁晓嫱. 锂离子电池用高热稳定性新型隔膜的研究新进展[J]. 化工学报, 2025, 76(6): 2524-2543. |
| [7] | 李坤, 黄锐, 丛君, 马海涛, 常龙娇, 罗绍华. NCM622正极材料结构形态和储锂特性的同步演变[J]. 化工学报, 2025, 76(4): 1831-1840. |
| [8] | 吴德威, 汪郑鹏, 周玥, 李晓宁, 陈招, 李卓, 刘成伟, 李学刚, 肖文德. 固定床法制备锂离子电池硅碳负极材料及其储锂性能研究[J]. 化工学报, 2024, 75(S1): 300-308. |
| [9] | 彭丹, 卢俊杰, 倪文静, 杨媛, 汪靖伦. 高电压钴酸锂电池电解液研究进展[J]. 化工学报, 2024, 75(9): 3028-3040. |
| [10] | 郭邦军, 贾理男, 张希. 全固态硫化物锂电池中NCM正极及其界面研究[J]. 化工学报, 2024, 75(3): 743-759. |
| [11] | 刘邦金, 汪林威, 吴月月, 刘永超, 钟国彬, 项宏发. 锂离子电池热管理研究进展[J]. 化工学报, 2024, 75(12): 4413-4431. |
| [12] | 胡成志, 王国贤, 唐伟建, 李阿飞, 陈章贤, 杨则恒, 张卫新. 高比能锂离子电池高镍正极材料的表面包覆改性研究进展[J]. 化工学报, 2024, 75(11): 4020-4036. |
| [13] | 闻文, 王慧艳, 周静红, 曹约强, 周兴贵. 石墨负极颗粒对锂离子电池容量衰减及SEI膜生长影响的模拟研究[J]. 化工学报, 2024, 75(1): 366-376. |
| [14] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [15] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号