• •
靳常洲( ), 尹梦阳, 王昊琛, 宋佳, 王竞侦, 马双龙, 黄岩(
), 尹梦阳, 王昊琛, 宋佳, 王竞侦, 马双龙, 黄岩( )
)
收稿日期:2025-09-26
修回日期:2025-11-24
出版日期:2025-12-08
通讯作者:
黄岩
作者简介:靳常洲(2002年), 男, 本科, 在读研究生, 3306672958@qq.com
基金资助:
Chang'zhou JIN( ), Meng'yang YIN, Hao'chen WANG, Jia SONG, Jing'zhen WANG, Shuang'long MA, Yan HUANG(
), Meng'yang YIN, Hao'chen WANG, Jia SONG, Jing'zhen WANG, Shuang'long MA, Yan HUANG( )
)
Received:2025-09-26
Revised:2025-11-24
Online:2025-12-08
Contact:
Yan HUANG
摘要:
H2S是一种剧毒、强腐蚀性的气体,其高效脱除对工业生产和人居环境具有重要意义。本研究基于过渡金属基氮掺杂炭(TM-NPC)催化剂在脱硫方面的应用前景,以葡萄糖酸盐为碳源和金属前驱体,草酸铵为氮源,碳酸氢钠为活化剂,通过一步热解制备了系列TM-NPC,考察了碳酸氢钠、热解温度、过渡金属类型对脱硫性能的影响,借助扫描电子显微镜(SEM)、X射线光电子能谱(XPS)、比表面积分析仪(BET)和傅里叶变换红外光谱仪(FTIR)对催化剂进行了理化性质分析。结果表明,在葡萄糖酸盐:草酸铵:碳酸氢钠配比为1:2:5、热解温度为800 ℃时,所获得的Cu-NPC脱硫性能最佳,且明显优于Fe-NPC和Mn-NPC,发现与氮气相比,氧气环境对于H2S的脱除具有极大促进作用。脱硫持久性实验表明,催化剂可在常温条件保持高效脱硫能力长达800 min,吸附量可达到430.5 mg/g,并发现Cu位点是Cu-NPC实现高效脱硫的关键活性位。综上,本研究为开发新型高效TM-NPC催化剂提供了新思路,促进了H2S资源化转化及利用。
中图分类号:
靳常洲, 尹梦阳, 王昊琛, 宋佳, 王竞侦, 马双龙, 黄岩. 过渡金属基氮掺杂炭的构筑及其脱硫性能研究[J]. 化工学报, DOI: 10.11949/0438-1157.20251078.
Chang'zhou JIN, Meng'yang YIN, Hao'chen WANG, Jia SONG, Jing'zhen WANG, Shuang'long MA, Yan HUANG. Construction of Transition Metal-based Nitrogen-doped Carbon and Their Desulfurization Performance[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251078.
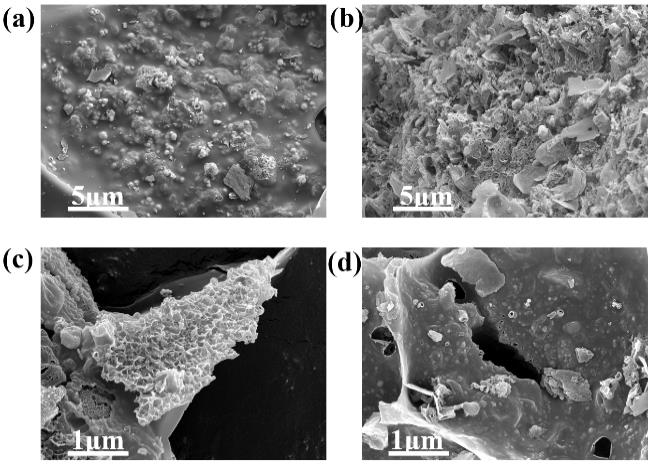
图2 不同配比Cu-NPC的SEM图:1:2:1(a);1:2:3(b);1:2:5(c);1:2:7(d)
Figure 2 SEM images of Cu-NPC with different ratios: 1:2:1 (a); 1:2:3 (b); 1:2:5 (c); 1:2:7 (d)
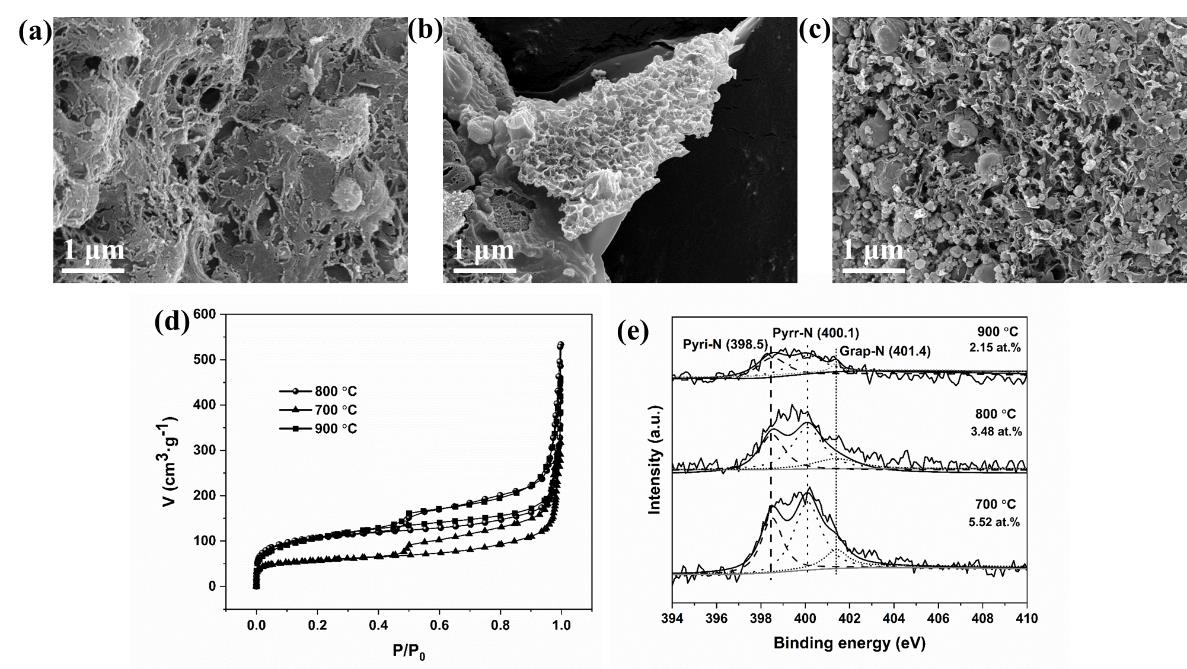
图4 不同热解温度Cu-NPC的SEM图:700 ℃(a);800 ℃(b);900 ℃(c);氮气吸附-脱附等温线(e)和N 1s谱图(f)
Figure 4 SEM images of Cu-NPC obtained at different pyrolysis temperature: 700 ℃ (a); 800 ℃ (b); 900 ℃ (c); Nitrogen adsorption-desorption isotherms (e) and N 1s XPS spectra (f) of Cu-NPC at different pyrolysis temperatures
| 煅烧温度 | 比表面积 (m2/g) | 总孔体积 (cm3/g) | 微孔体积 (cm3/g) | 孔径 (nm) | N (at.%) | Cu (at.%) |
|---|---|---|---|---|---|---|
| 700 ℃ | 197.38 | 0.3892 | 0.0483 | 7.8878 | 5.52 | 3.42 |
| 800 ℃ | 380.79 | 0.4732 | 0.1309 | 4.9710 | 3.48 | 2.11 |
| 900 ℃ | 378.26 | 0.4670 | 0.1552 | 4.9383 | 2.15 | 0.71 |
表1 不同热解温度Cu-NPC的比表面积、N含量和Cu含量分布
Table 1 Specific surface area, N and Cu contents of Cu-NPC obtained at different pyrolysis temperatures
| 煅烧温度 | 比表面积 (m2/g) | 总孔体积 (cm3/g) | 微孔体积 (cm3/g) | 孔径 (nm) | N (at.%) | Cu (at.%) |
|---|---|---|---|---|---|---|
| 700 ℃ | 197.38 | 0.3892 | 0.0483 | 7.8878 | 5.52 | 3.42 |
| 800 ℃ | 380.79 | 0.4732 | 0.1309 | 4.9710 | 3.48 | 2.11 |
| 900 ℃ | 378.26 | 0.4670 | 0.1552 | 4.9383 | 2.15 | 0.71 |
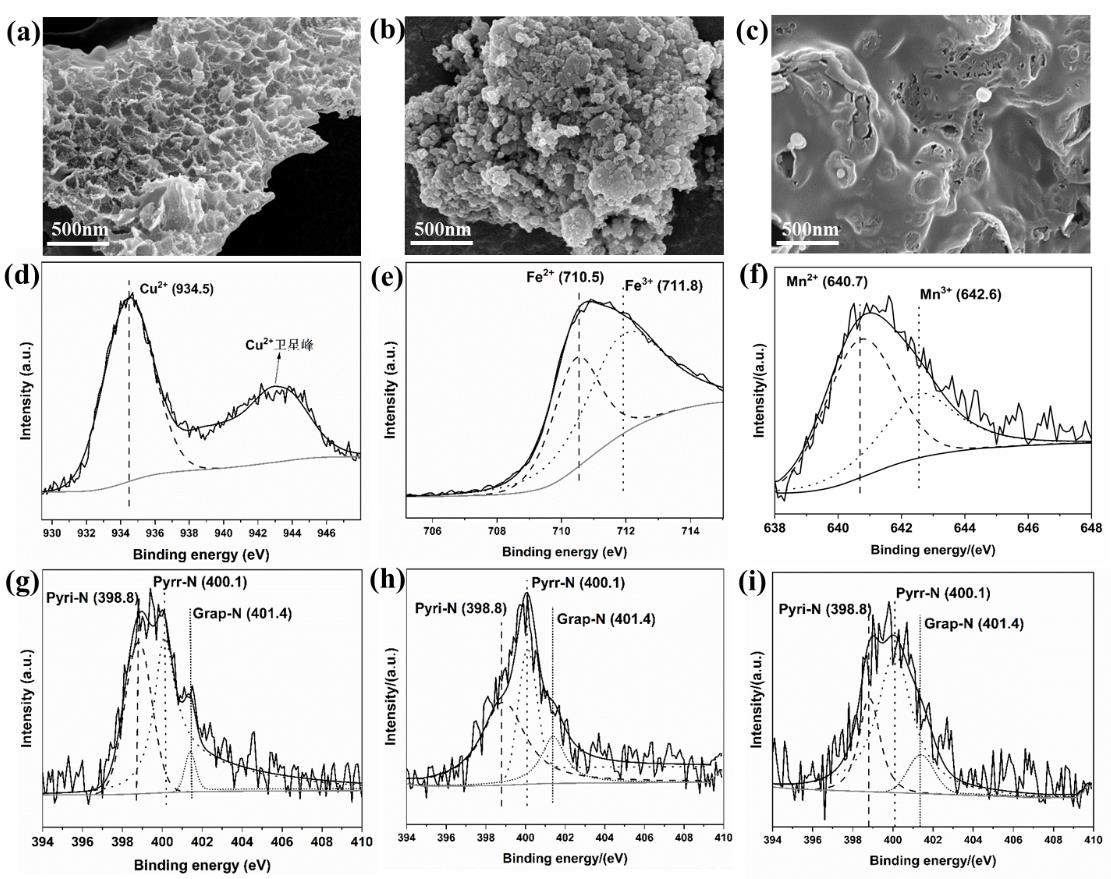
图6 不同金属掺杂TM-NPC的SEM图:Cu-NPC(a);Fe-NPC(b);Mn-NPC(c);不同金属掺杂TM-NPC的XPS谱图:Cu-NPC的 Cu 2p(d)及N 1s(g)谱图;Fe-NPC的Fe 2p(e)及N 1s(h)谱图;Mn-NPC的Mn 2p(f)及N 1s(i)谱图
Figure 6 SEM images of TM-NPC with different metal doping: (a) Cu-NPC; (b) Fe-NPC; (c) Mn-NPC; XPS spectra of TM-NPC with different metal doping: Cu 2p (d) and N 1s (g) spectra of Cu-NPC; Fe 2p (e) and N 1s (h) spectra of Fe-NPC; Mn 2p (f) and N 1s (i) spectra of Mn-NPC
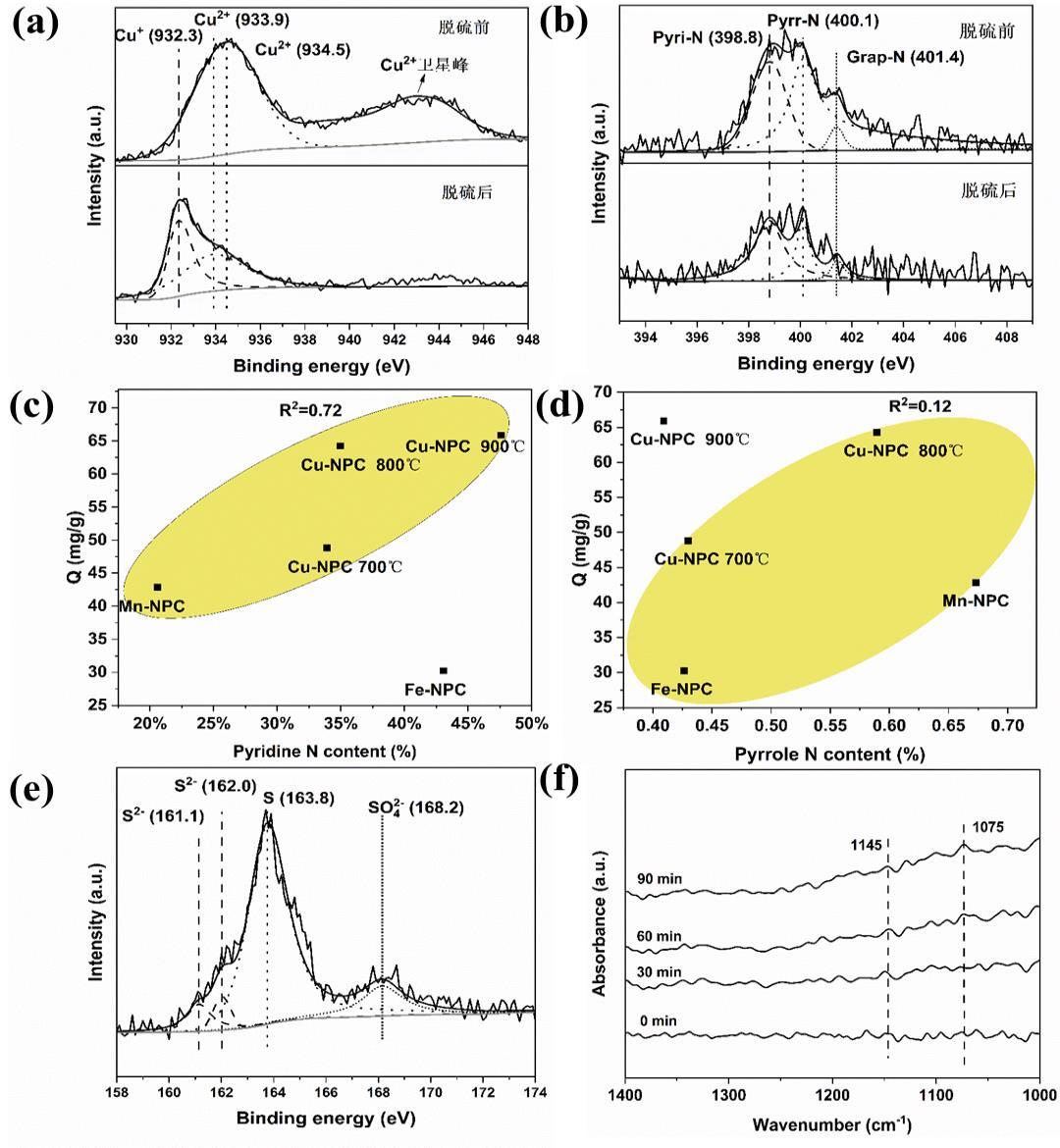
图11 Cu-NPC脱硫前后Cu 2p谱图(a)、N 1s谱图(b);N物种与硫容量相关性分析(c,d);注:Cu-NPC脱硫后S 2p谱图(e)和in-situ DRIFT谱图(f)
Figure 11 Cu 2p (a) and N 1s (b) spectra for Cu-NPC catalyst before and after desulfurization; Relationships between H2S capacity and N species (c, d); S 2p spectrum (e) and in-situ DRIFT spectra (f) of Cu-NPC after desulfurization
| [1] | Choi Y S, Hassani S, Vu T N, et al. Strategies for corrosion inhibition of carbon steel pipelines under supercritical CO2/H2S environments[J]. Corrosion, 2019, 75(10): 1156-1172. |
| [2] | Yu X L, Tao X, Gao Y F, et al. Oxygen vacancy-mediated selective H2S oxidation over Co-doped LaFe x Co1– x O3 perovskite[J]. Catalysts, 2022, 12(2): 236. |
| [3] | 马云倩, 王睿. 有机胺型铁基离子液体的H2S吸收和再生性能[J]. 高等学校化学学报, 2014, 35(4): 760-765. |
| Ma Y Q, Wang R. H2S absorption capacity and regeneration performance of amine Fe-based ionic liquid [J]. Chemical Journal of Chinese Universities, 2014, 35(4): 760-765. | |
| [4] | Zhang P, Luo Q, Wang R L, et al. Hydrogen sulfide toxicity inhibits primary root growth through the ROS-NO pathway[J]. Scientific Reports, 2017, 7: 868. |
| [5] | Atelge M R, Senol H, Djaafri M, et al. A critical overview of the state-of-the-art methods for biogas purification and utilization processes[J]. Sustainability, 2021, 13(20): 11515. |
| [6] | Shah M S, Tsapatsis M, Siepmann J I. Hydrogen sulfide capture: from absorption in polar liquids to oxide, zeolite, and metal-organic framework adsorbents and membranes [J]. Chemical Reviews, 2017, 117(14): 9755-9803. |
| [7] | Pudi A, Rezaei M, Signorini V, et al. Hydrogen sulfide capture and removal technologies: a comprehensive review of recent developments and emerging trends[J]. Separation and Purification Technology, 2022, 298: 121448. |
| [8] | Ruiz-Rodríguez L, Blasco T, Rodríguez-Castellón E, et al. Partial oxidation of H2S to sulfur on V-Cu-O mixed oxides bronzes[J]. Catalysis Today, 2019, 333: 237-244. |
| [9] | Chen L, Yuan J, Li T B, et al. A regenerable N-rich hierarchical porous carbon synthesized from waste biomass for H2S removal at room temperature[J]. Science of the Total Environment, 2021, 768: 144452. |
| [10] | Liu W J, Li W W, Jiang H, et al. Fates of chemical elements in biomass during its pyrolysis[J]. Chemical Reviews, 2017, 117(9): 6367-6398. |
| [11] | 郑皓鸣, 朱文富, 罗颖鸿, 等. 掺氮石油焦基活性炭常温催化氧化硫化氢研究[J]. 中国环境科学, 2023, 43(9): 4550-4560. |
| Zheng H M, Zhu W F, Luo Y H, et al. Preparation of nitrogen-doped petroleum coke based activated carbon and its performance in catalytic oxidation of hydrogen sulfide at room temperature[J]. China Environmental Science, 2023, 43(9): 4550-4560. | |
| [12] | Dong L Y, Hu X, Du Y Z, et al. Marked enhancement of electrocatalytic activities for gas-consuming reactions by bimodal mesopores[J]. Journal of Materials Chemistry A, 2021, 9(33): 17821-17829. |
| [13] | Wang T T, Yang J, Chen J Y, et al. Nitrogen-doped carbon nanotube-encapsulated nickel nanoparticles assembled on graphene for efficient CO2 electroreduction[J]. Chinese Chemical Letters, 2020, 31(6): 1438-1442. |
| [14] | Sun T, Mitchell S, Li J, et al. Design of local atomic environments in single-atom electrocatalysts for renewable energy conversions[J]. Advanced Materials, 2021, 33(5): 2003075. |
| [15] | Ju W, Bagger A, Hao G P, Varela A S, Sinev I, Bon V, Cuenya B, Kaskel S, Rossmeisl J, Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2 [J]. Nature Communications, 2017, 8(1) 1-9. |
| [16] | Ma H F, Zheng X H, Zhang H, et al. Atomic Cu-N-P-C active complex with integrated oxidation and chlorination for improved ethylene oxychlorination[J]. Advanced Science, 2023, 10(8): 2205635. |
| [17] | Sun M H, Wang X Z, Li Y, et al. Selective catalytic oxidation of pollutant H2S over co-decorated hollow N-doped carbon nanofibers for high-performance Li-S batteries[J]. Applied Catalysis B: Environmental, 2022, 317: 121763. |
| [18] | Pan Y K, Chen M Q, Su Z, et al. Two-dimensional CaO/carbon heterostructures with unprecedented catalytic performance in room-temperature H2S oxidization[J]. Applied Catalysis B: Environmental, 2021, 280: 119444. |
| [19] | Li K L, Chen X Y, Zhang J H, et al. One-step synthesis of flower-like MgO/Carbon materials for efficient H2S oxidation at room temperature[J]. Chemical Engineering Journal, 2023, 465: 142871. |
| [20] | 徐颂, 彭佳, 陈海军, 等. 干法碳酸氢钠脱硫过程及影响因素分析[J]. 环境工程, 2019, 37(6): 102-106. |
| Xu S, Peng J, Chen H J, et al. Desulfurization process of dry sodium bicarbonate and analysis of its influencing factors[J]. Environmental Engineering, 2019, 37(6): 102-106. | |
| [21] | Velu S, Suzuki K, Vijayaraj M, et al. In situ XPS investigations of Cu1- x Ni x ZnAl-mixed metal oxide catalysts used in the oxidative steam reforming of bio-ethanol[J]. Applied Catalysis B: Environmental, 2005, 55(4): 287-299. |
| [22] | Li H B, Kang W J, Wang L, et al. Synthesis of three-dimensional flowerlike nitrogen-doped carbons by a copyrolysis route and the effect of nitrogen species on the electrocatalytic activity in oxygen reduction reaction[J]. Carbon, 2013, 54: 249-257. |
| [23] | 李文涛, 傅国志, 黄婷, 等. 热解炭基载锰脱硝催化剂的制备及其低温脱硝性能[J]. 化工进展, 2024, 43(12): 7025-7032. |
| Li W T, Fu G Z, Huang T, et al. Preparation of pyrolytic carbon-based denitrification catalyst modified by Mn and its low-temperature denitrification performance[J]. Chemical Industry and Engineering Progress, 2024, 43(12): 7025-7032. | |
| [24] | 刘芬, 陈萦. 含铁化合物的Fe2p和Fe3s电子能谱研究[J]. 分析测试技术与仪器, 2001, 7(3): 166-169. |
| Liu F, Chen Y. XPS study of Fe2p and Fe3s for Fe-containing compounds[J]. Analysis and Testing Technology and Instruments, 2001, 7(3): 166-169. | |
| [25] | Chubar N, Szlachta M, Gerda V. EXAFS/XPS analysis of the arsenite removal mechanism on the Fe/Mn oxide-based composite: pH effect of the long-term oxidative-precipitative sorption[J]. Journal of Environmental Chemical Engineering, 2025, 13(2): 115748. |
| [26] | Wang Z L, Ke X X, Zhou K L, et al. Engineering the structure of ZIF-derived catalysts by revealing the critical role of temperature for enhanced oxygen reduction reaction[J]. Journal of Materials Chemistry A, 2021, 9(34): 18515-18525. |
| [27] | 韩伟, 赵加民, 张乐, 等. Ni2P催化剂电子结构对4, 6-DMDBT加氢脱硫活性和选择性的影响[J]. 石油学报(石油加工), 2022, 38(4): 745-759. |
| Han W, Zhao J M, Zhang L, et al. Effect of electronic structure of Ni2P catalyst on hydrodesulfurization activity and selectivity of 4, 6-DMDBT[J]. ACTA PETROLEI SINICA (PETROLEUM PROCESSING SECTION), 2022, 38(4): 745-759. | |
| [28] | 肖永厚, 王树东, 袁权. 浸渍活性炭脱除硫化氢研究进展[J]. 化工进展, 2006, 25(9): 1025-1030. |
| Xiao Y H, Wang S D, Yuan Q. Research progress of hydrogen sulfide removal by impregnated activated carbon[J]. CHEMICAL INDUSTRY AND ENGINEERING PROGRESS, 2006, 25(9): 1025-1030. | |
| [29] | Xu J, Tang S Y, Zhong S, et al. Solution combustion synthesis of CoS x, MoS x, CoS x -MoS x and their catalytic activity in H2 production from H2S decomposition[J]. International Journal of Hydrogen Energy, 2023, 48(24): 8807-8818. |
| [30] | Lin B L, Chen H X, Wei W Y, et al. Enriched oxygen vacancies of copper(I) oxide particles for enhanced removal of hydrogen sulfide at room temperature[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110113. |
| [31] | Zhang Y L, Yang Y X, Han H X, et al. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO: The effect of ZnO particle size on the adsorption performance[J]. Applied Catalysis B: Environmental, 2012, 119/120: 13-19. |
| [32] | Darmstadt H, Roy C, Kaliaguine S. Characterization of pyrolytic carbon blacks from commercial tire pyrolysis plants[J]. Carbon, 1995, 33(10): 1449-1455. |
| [33] | Kataoka S, Lee E, Tejedor-Tejedor M I, et al. Photocatalytic degradation of hydrogen sulfide and in situ FT-IR analysis of reaction products on surface of TiO2 [J]. Applied Catalysis B: Environmental, 2005, 61(1/2): 159-163. |
| [1] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [2] | 王钰, 冯英楠, 王涛, 赵之平. 原位生长构筑纳米复合纳滤膜:膜制备与应用[J]. 化工学报, 2025, 76(9): 4723-4736. |
| [3] | 佟丽丽, 陈英, 艾敏华, 舒玉美, 张香文, 邹吉军, 潘伦. ZnO/WO3异质结光催化环烯烃[2+2]环加成制备高能量密度燃料[J]. 化工学报, 2025, 76(9): 4882-4892. |
| [4] | 张茹, 朱传强, 张栋, 黄政, 肖雨果, 李明, 李长明. 采用高分子非催化还原脱硝的垃圾焚烧工艺伴生固废含氮污染物特征研究[J]. 化工学报, 2025, 76(9): 4944-4959. |
| [5] | 钱慧慧, 王文婕, 陈文尧, 周兴贵, 张晶, 段学志. 聚丙烯定向转化制芳烃:金属-分子筛协同催化机制[J]. 化工学报, 2025, 76(9): 4838-4849. |
| [6] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [7] | 杨敏, 段新伟, 吴俊宏, 米杰, 王建成, 武蒙蒙. Sm2O3/γ-Al2O3催化剂的COS催化水解性能及失活机制[J]. 化工学报, 2025, 76(8): 4061-4070. |
| [8] | 周媚, 曾浩桀, 蒋火炎, 蒲婷, 曾星星, 刘宝玉. 二次晶化法改性合成MTW分子筛及其在苯和环己烯烷基化反应中的催化性能[J]. 化工学报, 2025, 76(8): 4071-4080. |
| [9] | 吴阿强, 诸葛祥群, 刘通, 王明星, 罗鲲. 纳米普鲁士蓝悬浮电解液对锂氧电池性能的影响[J]. 化工学报, 2025, 76(8): 4310-4317. |
| [10] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [11] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [12] | 杨文毅, 由长福, 王海名. 燃煤机组变负荷运行下的脱硫废水零排放流程模拟分析[J]. 化工学报, 2025, 76(7): 3468-3476. |
| [13] | 李秋英, 花亦怀, 程昊, 张涵玮, 刘文睿, 白昊川, 王凯, 邱利民. 集成ORC系统的高效氢液化流程设计研究[J]. 化工学报, 2025, 76(7): 3651-3658. |
| [14] | 李姿睿, 齐凯, 王军, 夏国栋. 基于Janus纳米通道的脱盐过程分子动力学模拟研究[J]. 化工学报, 2025, 76(7): 3531-3538. |
| [15] | 唐羽丰, 陶春珲, 王永正, 李印辉, 段然, 赵泽一, 马和平. 超高比表面积碳基多孔吸附剂制备及其Kr气存储性能研究[J]. 化工学报, 2025, 76(7): 3339-3349. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号