• •
周骏1( ), 金烁2, 王思远2, 高冲3(
), 金烁2, 王思远2, 高冲3( ), 陈笑非1(
), 陈笑非1( ), 贺志远1
), 贺志远1
收稿日期:2025-10-15
修回日期:2025-11-25
出版日期:2026-01-13
通讯作者:
高冲,陈笑非
作者简介:周骏(2002—),男,硕士研究生,3120241071@bit.edu.cn
基金资助:
Jun ZHOU1( ), Shuo JIN2, Siyuan WANG2, Chong GAO3(
), Shuo JIN2, Siyuan WANG2, Chong GAO3( ), Xiaofei CHEN1(
), Xiaofei CHEN1( ), Zhiyuan HE1
), Zhiyuan HE1
Received:2025-10-15
Revised:2025-11-25
Online:2026-01-13
Contact:
Chong GAO, Xiaofei CHEN
摘要:
乳液法与微流控技术在仿生细胞构建中具有关键作用和广阔应用前景。乳液法(包括 Pickering 乳液)以操作简便、设备依赖低及易于宏量制备的优势,成为封装酶、药物等功能组分的高效平台,尤其适用于对尺寸均一性要求不高的大规模应用。微流控技术则利用微米级通道精确操控流体,能够生成尺寸高度均一、结构可控的液滴,实现仿生细胞组成、尺寸与功能的精确编程,是高通量筛选、基础研究及定制化人工细胞构建的理想工具。两者互为补充,共同推动了仿生细胞在限域催化、底物筛选、信号传递及药物递送等领域的创新应用。尽管在构建精度、安全性和功能复杂性方面仍存在挑战,但随着多学科交叉发展,基于乳液与微流控的仿生细胞已成为探索生命本质与开发创新生物技术的强大平台。
中图分类号:
周骏, 金烁, 王思远, 高冲, 陈笑非, 贺志远. 乳液与微流控工艺在构建仿生细胞中的应用[J]. 化工学报, DOI: 10.11949/0438-1157.20251141.
Jun ZHOU, Shuo JIN, Siyuan WANG, Chong GAO, Xiaofei CHEN, Zhiyuan HE. Processes and applications of emulsions and microfluidics in biomimetic cell construction[J]. CIESC Journal, DOI: 10.11949/0438-1157.20251141.
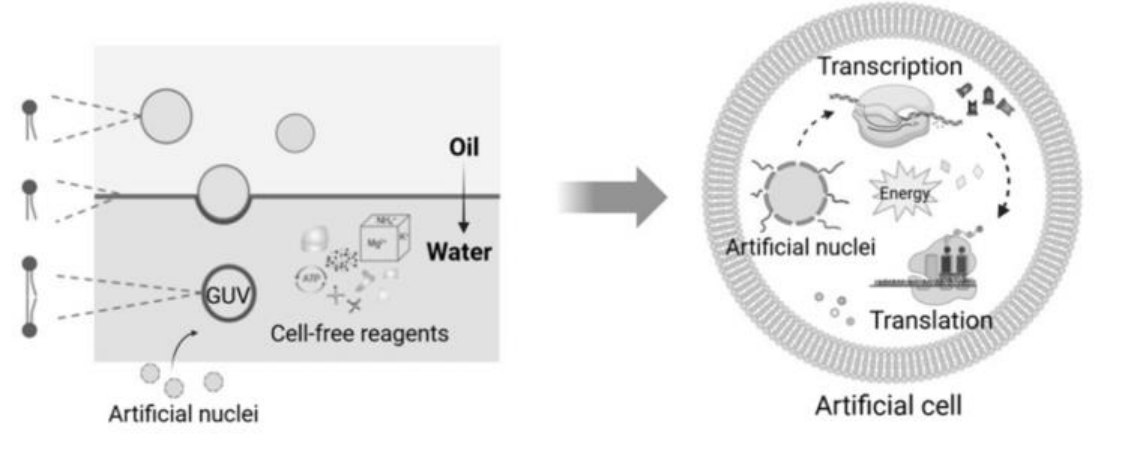
图1 采用油包水乳液法制备封装核结构的仿生细胞巨型单层囊泡(GUVs)[44]
Fig.1 Biomimetic cells with nuclear structures encapsulated within giant unilamellar vesicles (GUVs) via water-in-oil (W/O) emulsion method[44]
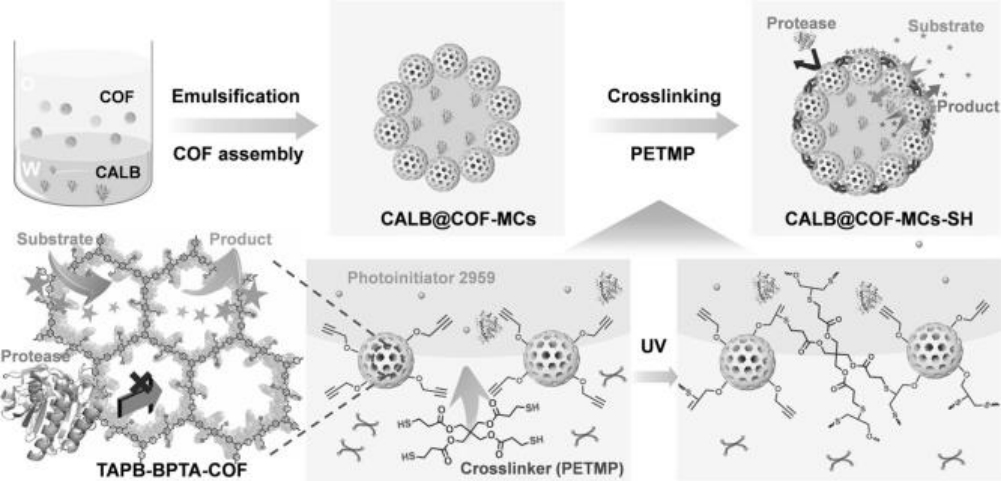
图2 基于皮克林乳液的含交联外壳的COF基多孔微反应器(CALB@COF-MCs-SH)的制备[51]
Fig.2 Fabrication of COF-based porous microreactors with cross-linked shells (CALB@COF-MCs-SH) via Pickering emulsion[51]
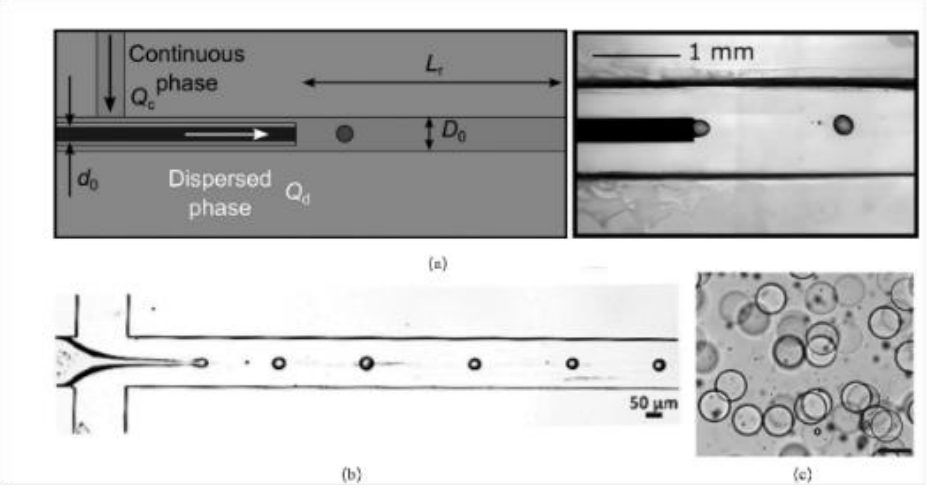
图3 微流控芯片及液滴产生过程:(a)T字型微流控芯片液滴生成示意图;(b)流动聚焦型微流控芯片液滴生成示意图;(c)微流控产生的分散均匀的微液滴[57-60]
Fig.3 Microfluidic Chip and Droplet Generation Process: (a) Schematic diagram of droplet generation in a T-junction microfluidic chip; (b) Schematic diagram of droplet generation in a flow-focusing microfluidic chip; (c) Uniformly dispersed microdroplets produced by microfluidics [57-60]
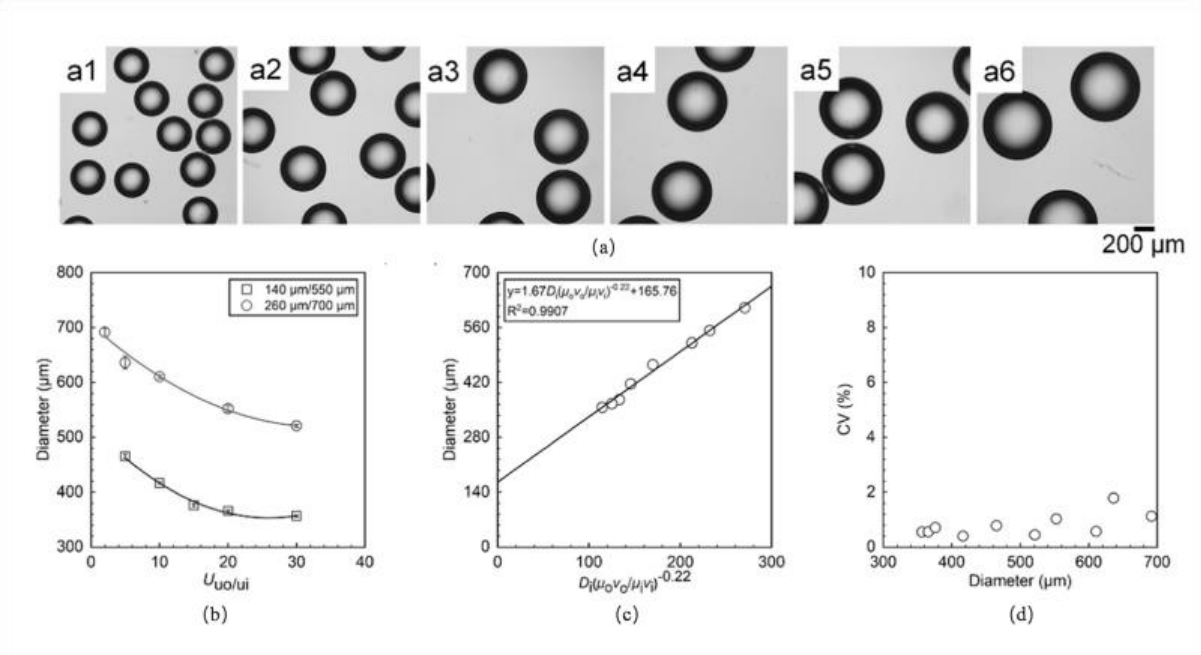
图4 微流控设备的尺寸和内外相流体流速对液滴尺寸的影响:(a) 平均直径为356.59μm(a1)、465.43μm(a2)、552.38μm(a3)、610.54μm(a4)、636.16μm(a5)和691.54μm(a6)的单分散液滴的光学显微照片;(b) 外相与内相的流速比(Uu0/ui)和微流控设备的尺寸对液滴尺寸的影响;(c) 不同平均尺寸液滴的变异系数(CV)值;(d) 液滴尺寸与微流控设备尺寸、连续相和分散相液体的粘度及流速之间的关系[61]
Fig.4 Effects of microfluidic device dimensions and fluid flow rates of both continuous and dispersed phases on droplet size:(a) Optical micrographs of monodisperse droplets with average diameters of 356.59 μm (a1), 465.43 μm (a2), 552.38 μm (a3), 610.54 μm (a4), 636.16 μm (a5), and 691.54 μm (a6).;(b) Influence of flow rate ratio (Uu0/ui) between continuous and dispersed phases and microfluidic device dimensions on droplet size;(c) Coefficient of variation (CV) values of droplets with different average sizes;(d) Relationship between droplet size and microfluidic device dimensions, viscosities and flow rates of continuous and dispersed phases[61]
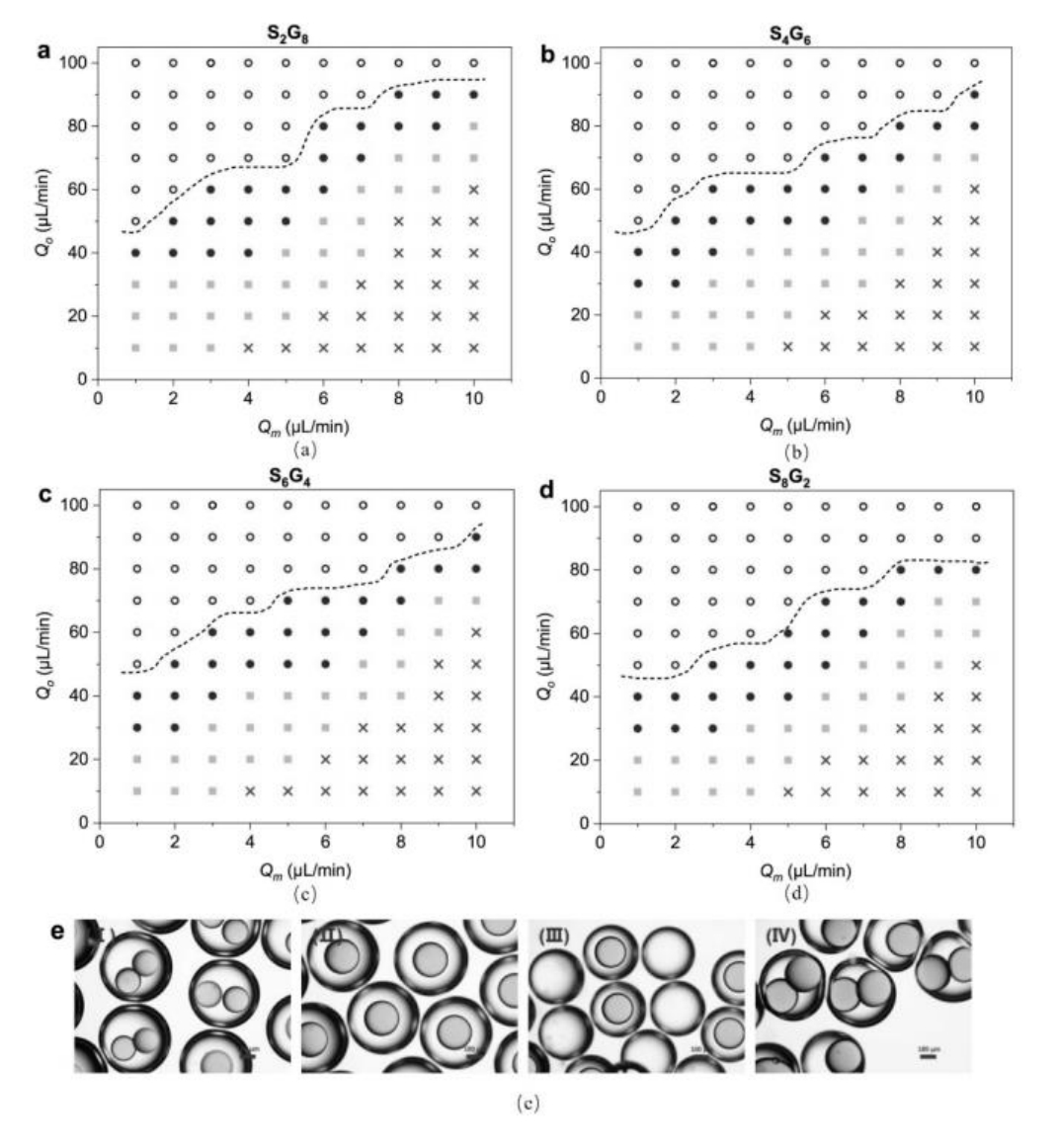
图6 不同外部流量下乳液类型的相图:(a)S2G8(b)S4G6(c)S6G4以及(d)S8G2,其中×-不稳定乳液,■-多核乳液,●-单核乳液和⚪-非核乳液;(e)不同类型乳液的图像:I - 多核乳液,II - 单核乳液,III - 非核乳液,IV - 不稳定乳液[63]
Fig.6 Phase diagram of emulsion types under different external flow rates: (a) S2G8, (b) S4G6, (c) S6G4, and (d) S8G2, where ×- unstable emulsion, ■- multi-core emulsion, ●- single-core emulsion, and ⚪- non-nucleated emulsion; (e) Images of different emulsion types: I - multi-core emulsion, II - single-core emulsion, III - non-nucleated emulsion, IV - unstable emulsion[63]
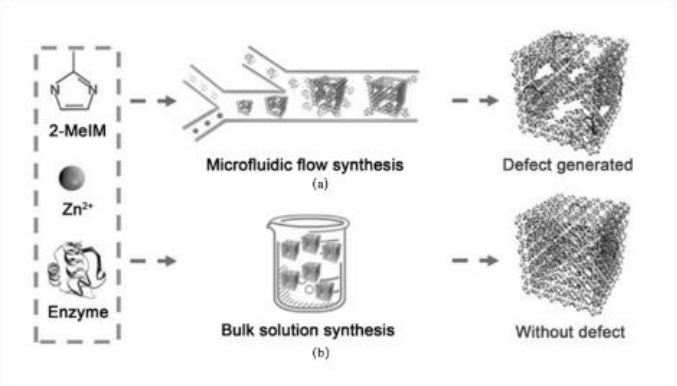
图8 酶-MOF复合材料的微流控层流合成和本体溶液合成示意图[75]
Fig.8 Schematic illustration of microfluidic laminar flow synthesis versus bulk solution synthesis for enzyme-MOF composites[75]
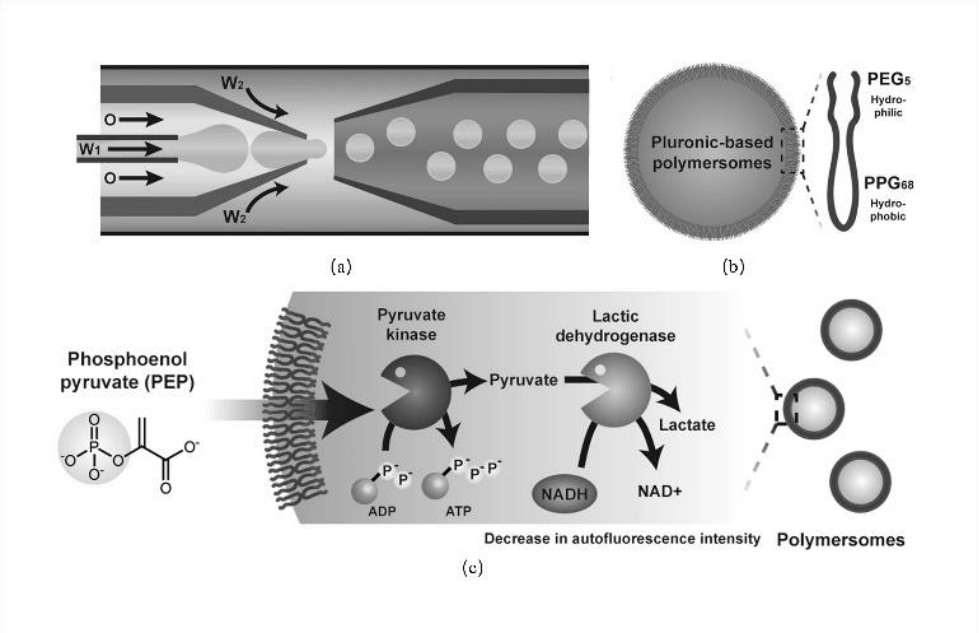
图10 囊泡制备及信号传递:(a)通过微流控方法制备水包油包水(W1/O/W2)液滴;(b)由Pluronic L121组成的产生的聚合物体的示意图。PEG:聚乙二醇,PPG:聚丙二醇;(c)通过引入丙酮酸激酶(PyK)和乳酸脱氢酶(LDH)在聚合物体中驱动的PEP级联酶促反应的示意图[60]
Fig.10 Capsule Preparation and Signal Transduction: (a) Preparation of water-in-oil-in-water (W1/O/W2) double emulsion droplets via microfluidics; (b) Schematic diagram of the generated polymersomes composed of Pluronic L121. PEG: Polyethylene glycol, PPG: Polypropylene glycol; (c) Schematic illustration of the PEP-driven cascade enzymatic reaction in polymersomes through the introduction of pyruvate kinase (PyK) and lactate dehydrogenase (LDH) [60]
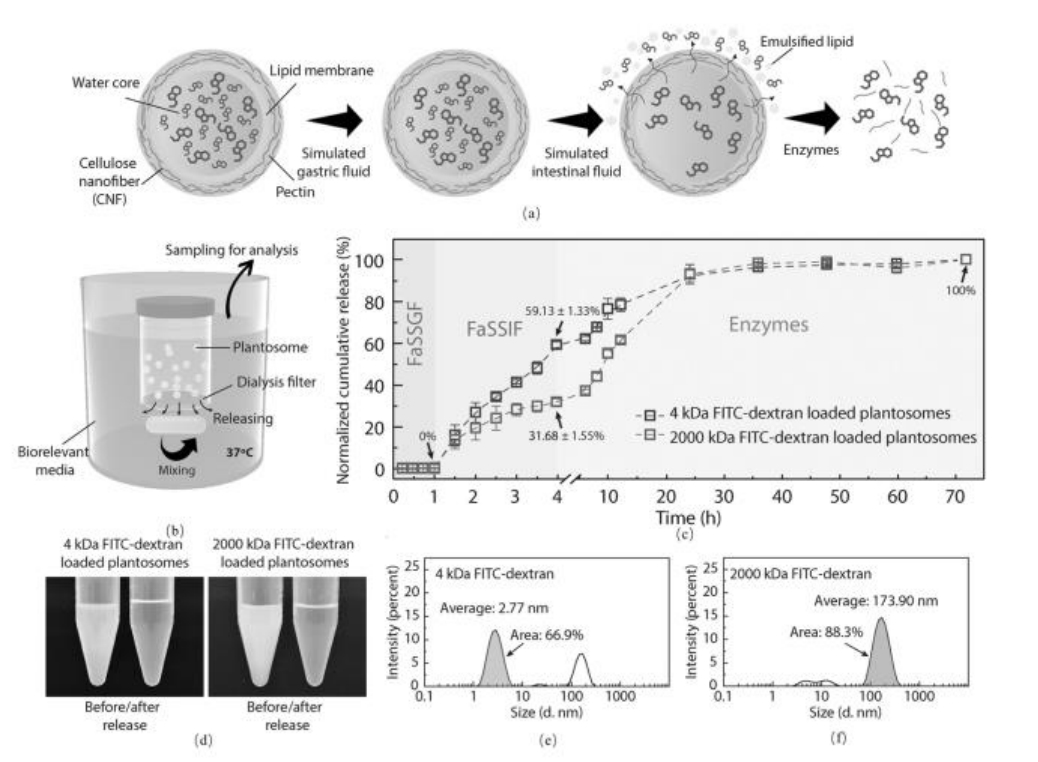
图11 模拟植物体中的双触发释放机制及具体性能 (a) 载药尺寸响应型两步释放机制示意图;(b) 体外释放研究装置的示意图;(c) 装载有4-或2000-kDa FITC-右旋糖酐的植物体的顺序释放曲线;(d) FITC-右旋糖酐负载的植物体在释放研究前后的照片;(e) 和(f) 4-kDa与2000-kDa FITC-葡聚糖尺寸分布的动态光散射(DLS)表征结果[92]
Fig.11 Simulation of Dual-Triggered Release Mechanism and Specific Performance in Phytomimetic Systems:(a) Schematic diagram of the cargo size-dependent two-step release mechanism; (b) Illustration of the in vitro release study setup.;(c) Sequential release profiles of 4-kDa or 2000-kDa FITC-dextran loaded phytosomes.;(d) Photographs of FITC-dextran loaded phytosomes before and after the release study; (e, f) DLS measurements showing the size distribution of 4-kDa and 2000-kDa FITC-dextran[92]
| [1] | Rothschild L J, Averesch N J H, Strychalski E A, et al. Building synthetic Cells─From the technology infrastructure to cellular entities[J]. ACS Synthetic Biology, 2024, 13(4): 974-997. |
| [2] | Ribatti D. An historical note on the cell theory[J]. Experimental Cell Research, 2018, 364(1): 1-4. |
| [3] | Chang T M. Pharmaceutical and therapeutic applications of artificial cells including microencapsulation[J]. European Journal of Pharmaceutics and Biopharmaceutics, 1998, 45(1): 3-8. |
| [4] | Zhang B Y, Montgomery M, Chamberlain M D, et al. Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis[J]. Nature Materials, 2016, 15(6): 669-678. |
| [5] | Garrett W S. From cell biology to the microbiome: an intentional infinite loop[J]. The Journal of Cell Biology, 2015, 210(1): 7-8. |
| [6] | Spoelstra W K, Deshpande S, Dekker C. Tailoring the appearance: what will synthetic cells look like [J]. Current Opinion in Biotechnology, 2018, 51: 47-56. |
| [7] | Gaitzsch J, Huang X, Voit B. Engineering functional polymer capsules toward smart nanoreactors[J]. Chemical Reviews, 2016, 116(3): 1053-1093. |
| [8] | Elani Y, Trantidou T, Wylie D, et al. Constructing vesicle-based artificial cells with embedded living cells as organelle-like modules[J]. Scientific Reports, 2018, 8: 4564. |
| [9] | Buddingh' B C, Van Hest J C M. Artificial cells: synthetic compartments with life-like functionality and adaptivity[J]. Accounts of Chemical Research, 2017, 50(4): 769-777. |
| [10] | Jeong S, Nguyen H T, Kim C H, et al. Toward artificial cells: novel advances in energy conversion and cellular motility[J]. Advanced Functional Materials, 2020, 30(11): 1907182. |
| [11] | Pohorille A, Deamer D. Artificial cells: prospects for biotechnology[J]. Trends in Biotechnology, 2002, 20(3): 123-128. |
| [12] | Szostak J W, Bartel D P, Luisi P L. Synthesizing life[J]. Nature, 2001, 409(6818): 387-390. |
| [13] | Saraniti M. Designing biomimetic nanomachines[J]. Nature Nanotechnology, 2008, 3(11): 647-648. |
| [14] | Zhang S, Zhang R F, Yan X Y, et al. Nanozyme-based artificial organelles: an emerging direction for artificial organelles[J]. Small, 2022, 18(33): e2202294. |
| [15] | Peng H S, Zhao M, Liu X Y, et al. Biomimetic materials to fabricate artificial cells[J]. Chemical Reviews, 2024, 124(23): 13178-13215. |
| [16] | Göpfrich K, Platten M, Frischknecht F, et al. Bottom-up synthetic immunology[J]. Nature Nanotechnology, 2024, 19(11): 1587-1596. |
| [17] | Mutschler H, Robinson T, Dora Tang T Y, et al. Special issue on bottom-up synthetic biology[J]. ChemBioChem, 2019, 20(20): 2533-2534. |
| [18] | Ugrinic M, DeMello A, Dora Tang T Y. Microfluidic tools for bottom-up synthetic cellularity[J]. Chem, 2019, 5(7): 1727-1742. |
| [19] | Xu C, Hu S, Chen X Y. Artificial cells: from basic science to applications[J]. Materials Today, 2016, 19(9): 516-532. |
| [20] | Palivan C G, Goers R, Najer A, et al. Bioinspired polymer vesicles and membranes for biological and medical applications[J]. Chemical Society Reviews, 2016, 45(2): 377-411. |
| [21] | Chen L, Yang C, Xiao Y, et al. Millifluidics, microfluidics, and nanofluidics: manipulating fluids at varying length scales[J]. Materials Today Nano, 2021, 16: 100136. |
| [22] | 吉笑盈, 郑园, 李晓鹏, 等. 微流控可控制备液滴、颗粒和胶囊及其应用[J]. 化工学报, 2024, 75(4): 1455-1468. |
| Ji X.Y, Zheng Y, Li X.P,et al. Controlled preparation of droplets, particles and capsules by microfluidics and their applications[J]. CIESC Journal, 2024, 75(4): 1455-1468. | |
| [23] | Hirschi S, Ward T R, Meier W P, et al. Synthetic biology: bottom-up assembly of molecular systems[J]. Chemical Reviews, 2022, 122(21): 16294-16328. |
| [24] | Kaneko K. Origin of a cell with recursive growth[M]//Life: An Introduction to Complex Systems Biology, 2006: 111-133. |
| [25] | Liu W L, Zou M Z, Qin S Y, et al. Recent advances of cell membrane-coated nanomaterials for biomedical applications[J]. Advanced Functional Materials, 2020, 30(39): 2003559. |
| [26] | Fernandez-de-Cossio-Diaz J, Vazquez A. A physical model of cell metabolism[J]. Scientific Reports, 2018, 8(1): 8349. |
| [27] | Vance J A, Devaraj N K. Membrane mimetic chemistry in artificial cells[J]. Journal of the American Chemical Society, 2021, 143(22): 8223-8231. |
| [28] | Lv C, Gu X Y, Li H W, et al. Molecular transport through a biomimetic DNA channel on live cell membranes[J]. ACS Nano, 2020, 14(11): 14616-14626. |
| [29] | de Weerd S, Ruiter E A, Calicchia E, et al. Optimization of cell membrane purification for the preparation and characterization of cell membrane liposomes[J]. Small Methods, 2024, 8(12): e2400498. |
| [30] | Burns J R, Seifert A, Fertig N, et al. A biomimetic DNA-based channel for the ligand-controlled transport of charged molecular cargo across a biological membrane[J]. Nature Nanotechnology, 2016, 11(2): 152-156. |
| [31] | Geng J, Kim K, Zhang J F, et al. Stochastic transport through carbon nanotubes in lipid bilayers and live cell membranes[J]. Nature, 2014, 514(7524): 612-615. |
| [32] | Banghart M, Borges K, Isacoff E, et al. Light-activated ion channels for remote control of neuronal firing[J]. Nature Neuroscience, 2004, 7(12): 1381-1386. |
| [33] | Volgraf M, Gorostiza P, Numano R, et al. Allosteric control of an ionotropic glutamate receptor with an optical switch[J]. Nature Chemical Biology, 2006, 2(1): 47-52. |
| [34] | Zhang Z, Kong X Y, Xiao K, et al. A bioinspired multifunctional heterogeneous membrane with ultrahigh ionic rectification and highly efficient selective ionic gating[J]. Advanced Materials, 2016, 28(1): 144-150. |
| [35] | Messager L, Burns J R, Kim J, et al. Biomimetic hybrid nanocontainers with selective permeability[J]. Angewandte Chemie (International Ed. in English), 2016, 55(37): 11106-11109. |
| [36] | Shen W T, Zhou Z D, Yu Y Y, et al. Continuous separation of cell membranes via affinity chromatography[J]. Separation and Purification Technology, 2025, 376: 133948. |
| [37] | Wang Q P, Chen K, Jiang H, et al. Cell-inspired design of cascade catalysis system by 3D spatially separated active sites[J]. Nature Communications, 2023, 14: 5338. |
| [38] | Li A T, Ilie A, Sun Z T, et al. Whole-cell-catalyzed multiple regio- and stereoselective functionalizations in cascade reactions enabled by directed evolution[J]. Angewandte Chemie International Edition, 2016, 55(39): 12026-12029. |
| [39] | Vriezema D M, Garcia P M L, Sancho Oltra N, et al. Positional assembly of enzymes in polymersome nanoreactors for cascade reactions[J]. Angewandte Chemie (International Ed. in English), 2007, 46(39): 7378-7382. |
| [40] | Tan H L, Guo S, Dinh N D, et al. Heterogeneous multi-compartmental hydrogel particles as synthetic cells for incompatible tandem reactions[J]. Nature Communications, 2017, 8: 663. |
| [41] | Guo M Y, Gu F J, Meng L D, et al. Synthesis of formaldehyde from CO2 catalyzed by the coupled photo-enzyme system[J]. Separation and Purification Technology, 2022, 286: 120480. |
| [42] | Lomora M, Itel F, Dinu I A, et al. Selective ion-permeable membranes by insertion of biopores into polymersomes[J]. Physical Chemistry Chemical Physics, 2015, 17(24): 15538-15546. |
| [43] | Sheng Y K, Guo F, Guo B C, et al. Light-driven CO2 reduction with a surface-displayed enzyme cascade-C(3)N(4) hybrid[J]. ACS Synthetic Biology, 2023, 12(9): 2715-2724. |
| [44] | Wang Y M, Wang C, Zheng Y L, et al. Tuning covalent organic frameworks for nucleic acid loading: toward creating a hierarchical artificial cell[J]. Journal of the American Chemical Society, 2025, 147(25): 21926-21939. |
| [45] | Sümbelli Y, Mason A F, van Hest J C M. Toward artificial cell-mediated tissue engineering: a new perspective[J]. Advanced Biology, 2023, 7(12): 2300149. |
| [46] | 陈凤凤, 陶胜男, 龚穗菁, 等. 化妆品乳液及乳化新技术(Ⅰ):皮克林乳液的基本原理及其在化妆品中的应用[J]. 日用化学工业, 2021, 51(2): 89-97, 114. |
| Chen F.F, Tao S.N, Gong S.J,et al. Cosmetic emulsions and new technologies of emulsification(Ⅰ) Fundamental principles of Pickering emulsions and their applications in cosmetics [J]. China Surfactant Detergent & Cosmetics, 2021, 51(2): 89-97, 114. | |
| [47] | 程芳琴, 焦玉花, 李恩泽, 等. 智能响应型Pickering乳液的制备及在物质分离中的应用进展[J]. 化工进展, 2021, 40(4): 2206-2214. |
| Cheng F Q, Jiao Y H, Li E Z, et al. Progress on the preparation of intelligent responsive Pickering emulsions and their applications in matter separation[J]. Chemical Industry and Engineering Progress, 2021, 40(4): 2206-2214. | |
| [48] | 齐亚兵, 吴子波, 杨清翠. Pickering乳液制备及稳定性研究进展[J]. 化工进展, 2024, 43(4): 2017-2030. |
| Qi Y B, Wu Z B, Yang Q C. Research advances of preparation of Pickering emulsions and their stability[J]. Chemical Industry and Engineering Progress, 2024, 43(4): 2017-2030. | |
| [49] | 张兆宇, 姜秋艳, 孙宁, 等. 刺激响应型Pickering乳液的制备及应用进展[J]. 精细化工 2021, 38(2): 259-268. |
| Zhang Z Y, Jiang Q Y, Sun N, et al. Progress in preparation and application of stimulus-responsive Pickering emulsion [J]. Fine Chemicals, 2021, 38(2): 259-268. | |
| [50] | Gonzalez Ortiz D, Pochat-Bohatier C, Cambedouzou J, et al. Current trends in Pickering emulsions: Particle morphology and applications[J]. Engineering, 2020, 6(4): 468-482. |
| [51] | Feng M C, Niu Z R, Xing C Y, et al. Covalent organic framework based crosslinked porous microcapsules for enzymatic catalysis[J]. Angewandte Chemie (International Ed. in English), 2023, 62(33): e202306621. |
| [52] | Hao X T, Li J, Zhang B, et al. Transforming crowded coacervates into multi-compartmental hybrid microreactors for practical enzymatic catalysis[J]. Angewandte Chemie (International Ed. in English), 2025, 64(43): e202502479. |
| [53] | Liu L D, Wei J J, Ho K M, et al. Capsules templated from water-in-oil Pickering emulsions for enzyme encapsulation[J]. Journal of Colloid and Interface Science, 2023, 629: 559-568. |
| [54] | Tian D P, Zhang X M, Shi H, et al. Pickering-droplet-derived MOF microreactors for continuous-flow biocatalysis with size selectivity[J]. Journal of the American Chemical Society, 2021, 143(40): 16641-16652. |
| [55] | Xie M Z, Zhan Z H, Li Y F, et al. Functional microfluidics: theory, microfabrication, and applications[J]. International Journal of Extreme Manufacturing, 2024, 6(3): 032005. |
| [56] | Renganathan K, Jain R, Chheda D, et al. Development of a continuous-flow microfluidic process for microsphere production[J]. Materials Letters, 2026, 404: 139599. |
| [57] | Ashikhmin A, Piskunov M, Kochkin D, et al. Droplet microfluidic method for estimating the dynamic interfacial tension of ion-crosslinked sodium alginate microspheres[J]. Langmuir, 2024, 40(30): 15906-15917. |
| [58] | 金聪, 赵旭晟, 郭嘉灏, 等. 基于3D微流控芯片的水凝胶微胶囊可控生成系统[J]. 华东理工大学学报(自然科学版), 2025, 51(6): 1-10. |
| Jin C, Zhao X S, Guo J H, et al. A 3D Microfluidic-Based Hydrogel Microcapsule Fabrication System[J]. Journal of East China University of Science and Technology, 2025, 51(6): 783-792. | |
| [59] | Chen S, Shahar T, Cohen S. Thermo-controlled microfluidic generation of monodisperse alginate microspheres based on external gelation[J]. RSC Advances, 2024, 14(44): 32021-32028. |
| [60] | Seo H, Lee H. Spatiotemporal control of signal-driven enzymatic reaction in artificial cell-like polymersomes[J]. Nature Communications, 2022, 13: 5179. |
| [61] | Long Y H, Ju X J, Yang S H, et al. Microfluidic fabrication of monodisperse hyaluronic acid microspheres with excellent biocompatibility and tunable physicochemical properties[J]. Industrial & Engineering Chemistry Research, 2024, 63(15): 6632-6643. |
| [62] | Nie C H, Ye Q Y, Chen J B, et al. Influences of polysaccharide stabilizer and polyglycerol polyricinoleate on the stability of Pickering double emulsions revealed via microfluidic technology[J]. Food Hydrocolloids, 2025, 163: 111046. |
| [63] | Tu W Y, Hu W T, Chen J, et al. Controllable fabrication of core-shell microcapsules using sodium alginate/gellan gum as shell material by microfluidics[J]. Food Hydrocolloids, 2025, 168: 111514. |
| [64] | Deng N N, Yelleswarapu M, Zheng L F, et al. Microfluidic assembly of monodisperse vesosomes as artificial cell models[J]. Journal of the American Chemical Society, 2017, 139(2): 587-590. |
| [65] | Utech S, Prodanovic R, Mao A S, et al. Microfluidic generation of monodisperse, structurally homogeneous alginate microgels for cell encapsulation and 3D cell culture[J]. Advanced Healthcare Materials, 2015, 4(11): 1628-1633. |
| [66] | Zheng Y, Luo C H, Chai Z S, et al. High-throughput preparation of monodisperse biocompatible core-shell capsules by 3D-Printed Microfluidics[J]. Chemical Engineering Science, 2025, 304: 121104. |
| [67] | Dinu M V, Dinu I A, Saxer S S, et al. Stabilizing enzymes within polymersomes by coencapsulation of trehalose[J]. Biomacromolecules, 2021, 22(1): 134-145. |
| [68] | Fan Y, Wang D H, Yang J L, et al. Top-down approach for fabrication of polymer microspheres by interfacial engineering[J]. Chinese Journal of Polymer Science, 2020, 38(12): 1286-1293. |
| [69] | Pelletier J F, Sun L J, Wise K S, et al. Genetic requirements for cell division in a genomically minimal cell[J]. Cell, 2021, 184(9): 2430-2440.e16. |
| [70] | Lim B, Yin Y T, Ye H, et al. Reprogramming synthetic cells for targeted cancer therapy[J]. ACS Synthetic Biology, 2022, 11(3): 1349-1360. |
| [71] | Ji Y, Lin Y Y, Qiao Y. Interfacial assembly of biomimetic MOF-based porous membranes on coacervates to build complex protocells and prototissues[J]. Nature Chemistry, 2025, 17(7): 986-996. |
| [72] | Li D Y, Zhang H P, Xie S Z, et al. Lattice distortion in a confined structured ZnS/ZnO heterojunction for efficient photocatalytic CO2 reduction[J]. ACS Applied Materials & Interfaces, 2023, 15(30): 36324-36333. |
| [73] | Ly Q V, Cui L L, Asif M B, et al. Membrane-based nanoconfined heterogeneous catalysis for water purification: a critical review✰[J]. Water Research, 2023, 230: 119577. |
| [74] | Meng C C, Ding B F, Zhang S Z, et al. Angstrom-confined catalytic water purification within Co-TiOx laminar membrane nanochannels[J]. Nature Communications, 2022, 13(1): 4010. |
| [75] | Hu C, Bai Y X, Hou M, et al. Defect-induced activity enhancement of enzyme-encapsulated metal-organic frameworks revealed in microfluidic gradient mixing synthesis[J]. Science Advances, 2020, 6(5): eaax5785. |
| [76] | Zhang W, Ye W B, Wang Y J, et al. Microfluidic fabrication of tunable alginate-based microfibers for the stable immobilization of enzymes[J]. Biotechnology Journal, 2022, 17(9): 2200098. |
| [77] | Guo X M, Xue N, Zhang M, et al. A supraparticle-based biomimetic cascade catalyst for continuous flow reaction[J]. Nature Communications, 2022, 13: 5935. |
| [78] | Ivanov I, Castellanos S L, Balasbas S, et al. Bottom-up synthesis of artificial cells: recent highlights and future challenges[J]. Annual Review of Chemical and Biomolecular Engineering, 2021, 12: 287-308. |
| [79] | Kim J, Kim K T. Polymersome-based modular nanoreactors with size-selective transmembrane permeability[J]. ACS Applied Materials & Interfaces, 2020, 12(20): 23502-23513. |
| [80] | Kurihara K, Tamura M, Shohda K I, et al. Self-reproduction of supramolecular giant vesicles combined with the amplification of encapsulated DNA[J]. Nature Chemistry, 2011, 3(10): 775-781. |
| [81] | Lee K Y, Park S J, Lee K A, et al. Photosynthetic artificial organelles sustain and control ATP-dependent reactions in a protocellular system[J]. Nature Biotechnology, 2018, 36(6): 530-535. |
| [82] | Hindley J W, Zheleva D G, Elani Y, et al. Building a synthetic mechanosensitive signaling pathway in compartmentalized artificial cells[J]. PNAS 2019, 116(34): 16711-16716. |
| [83] | Joesaar A, Yang S, Bögels B, et al. DNA-based communication in populations of synthetic protocells[J]. Nature Nanotechnology, 2019, 14(4): 369-378. |
| [84] | Buddingh' B C, Elzinga J, Van Hest J C M. Intercellular communication between artificial cells by allosteric amplification of a molecular signal[J]. Nature Communications, 2020, 11: 1652. |
| [85] | Love C, Steinkühler J, Gonzales D T, et al. Reversible pH-responsive coacervate formation in lipid vesicles activates dormant enzymatic reactions[J]. Angewandte Chemie (International Ed. in English), 2020, 59(15): 5950-5957. |
| [86] | Lin S W, Tsai J C, Shyong Y J. Drug delivery of extracellular vesicles: Preparation, delivery strategies and applications[J]. International Journal of Pharmaceutics, 2023, 642: 123185. |
| [87] | Ullah M, Kodam S P, Mu Q, et al. Microbubbles versus extracellular vesicles as therapeutic cargo for targeting drug delivery[J]. ACS Nano, 2021, 15(3): 3612-3620. |
| [88] | Dad H A, Gu T W, Zhu A Q, et al. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms[J]. Molecular Therapy, 2021, 29(1): 13-31. |
| [89] | Fan R R, Wu J, Duan S W, et al. Droplet-based microfluidics for drug delivery applications[J]. International Journal of Pharmaceutics, 2024, 663: 124551. |
| [90] | Tu S C, Mai S T, Shu D, et al. Microfluidic-based preparation of PLGA microspheres facilitating peptide sustained-release[J]. Materials Letters, 2024, 368: 136675. |
| [91] | Hu J, Chen X Y, Lin J Y, et al. Double-layer coated particles formed by one-step method based on microfluidic technology[J]. Journal of Micromechanics and Microengineering, 2024, 34(4): 045011. |
| [92] | Mao A R, Gebhard A C, Ezazi N Z, et al. Plant cell-inspired colon-targeted cargo delivery systems with dual-triggered release mechanisms[J]. Science Advances, 2025, 11(20): eadt2653. |
| [93] | Ma W J, Yang Y T, Yang B B, et al. Engineered biomimetic nanovesicles derived from bone marrow stromal cells with innate homing capability for targeted delivery[J]. Advanced Materials, 2025, 37(45): e05714. |
| [94] | Zhao X F, Chen W, Wu J, et al. Application of biomimetic cell membrane-coated nanocarriers in cardiovascular diseases[J]. International Journal of Nanomedicine, 2025, 20: 8249-8289. |
| [95] | Xiao W, Fu H L, Rahaman M N, et al. Hollow hydroxyapatite microspheres: a novel bioactive and osteoconductive carrier for controlled release of bone morphogenetic protein-2 in bone regeneration[J]. Acta Biomaterialia, 2013, 9(9): 8374-8383. |
| [96] | Xi G H, Liu W, Chen M, et al. Polysaccharide-based lotus seedpod surface-like porous microsphere with precise and controllable micromorphology for ultrarapid hemostasis[J]. ACS Applied Materials & Interfaces, 2019, 11(50): 46558-46571. |
| [97] | Lei L J, Wang X G, Zhu Y L, et al. Antimicrobial hydrogel microspheres for protein capture and wound healing[J]. Materials & Design, 2022, 215: 110478. |
| [98] | Wang L Y, Zhu X Y, Xu C Y, et al. Artificial breakthrough of cell membrane barrier for transmembrane substance exchange: a review of recent progress[J]. Advanced Functional Materials, 2024, 34(13): 2311920. |
| [99] | Lu Y, Allegri G, Huskens J. Vesicle-based artificial cells: materials, construction methods and applications[J]. Materials Horizons, 2022, 9(3): 892-907. |
| [100] | Chen X F, Elemans J A A W. Artificial Processive Catalytic Systems: Bridging Synthetic Polymers and Biological Precision[J]. Polymer Science & Technology, 2025, 1(3): 233-236. |
| [1] | 孙浩然, 吴成云, 王艳蒙, 孙静楠, 胡仞与, 段钟弟. 热对流影响下液滴蒸发特性模型与实验研究[J]. 化工学报, 2025, 76(S1): 123-132. |
| [2] | 吴馨, 龚建英, 李祥宇, 王宇涛, 杨小龙, 蒋震. 超声波激励疏水表面液滴运动的实验研究[J]. 化工学报, 2025, 76(S1): 133-139. |
| [3] | 王宇涛, 龚建英, 李祥宇, 吴馨, 刘秀芳. 基于压电-声流效应的液滴定向驱动技术研究[J]. 化工学报, 2025, 76(S1): 181-186. |
| [4] | 黄灏, 王文, 贺隆坤. LNG船薄膜型液货舱预冷过程模拟与分析[J]. 化工学报, 2025, 76(S1): 187-194. |
| [5] | 黄博, 黄灏, 王文, 贺隆坤. 薄膜型LNG船液货舱温度场计算分析[J]. 化工学报, 2025, 76(S1): 195-204. |
| [6] | 王三一, 黄文来. 电化学合成氨流程建模与优化[J]. 化工学报, 2025, 76(9): 4474-4486. |
| [7] | 王钰, 冯英楠, 王涛, 赵之平. 原位生长构筑纳米复合纳滤膜:膜制备与应用[J]. 化工学报, 2025, 76(9): 4723-4736. |
| [8] | 张建民, 何美贵, 贾万鑫, 赵静, 金万勤. 聚氧化乙烯/冠醚共混膜及其二氧化碳分离性能[J]. 化工学报, 2025, 76(9): 4862-4871. |
| [9] | 王杰, 林渠成, 张先明. 基于分解算法的混合气体多级膜分离系统全局优化[J]. 化工学报, 2025, 76(9): 4670-4682. |
| [10] | 段炼, 周星睿, 袁文君, 陈飞. 连续相速度脉动对微通道内聚合物液滴生成和形貌的影响规律[J]. 化工学报, 2025, 76(9): 4578-4585. |
| [11] | 徐佳琪, 张文君, 余燕萍, 苏宝根, 任其龙, 杨启炜. 热等离子体重整炼厂气制合成气过程数值模拟与实验研究[J]. 化工学报, 2025, 76(9): 4462-4473. |
| [12] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [13] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [14] | 张荟钦, 赵泓竣, 付正军, 庄力, 董凯, 贾添智, 曹雪丽, 孙世鹏. 纳滤膜在离子型稀土浸出液提浓中的应用研究[J]. 化工学报, 2025, 76(8): 4095-4107. |
| [15] | 刘璐, 杨莹, 杨浩文, 王太, 王腾, 董新宇, 闫润. 星形亲水区组合表面冷凝液滴脱落特性实验研究[J]. 化工学报, 2025, 76(8): 3905-3914. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号