化工学报 ›› 2025, Vol. 76 ›› Issue (9): 4462-4473.DOI: 10.11949/0438-1157.20250350
徐佳琪1,2( ), 张文君2(
), 张文君2( ), 余燕萍1, 苏宝根1,2, 任其龙1,2, 杨启炜1,2(
), 余燕萍1, 苏宝根1,2, 任其龙1,2, 杨启炜1,2( )
)
收稿日期:2025-04-07
修回日期:2025-06-21
出版日期:2025-09-25
发布日期:2025-10-23
通讯作者:
张文君,杨启炜
作者简介:徐佳琪(2000—),女,硕士研究生,22260345@zju.edu.cn
基金资助:
Jiaqi XU1,2( ), Wenjun ZHANG2(
), Wenjun ZHANG2( ), Yanping YU1, Baogen SU1,2, Qilong REN1,2, Qiwei YANG1,2(
), Yanping YU1, Baogen SU1,2, Qilong REN1,2, Qiwei YANG1,2( )
)
Received:2025-04-07
Revised:2025-06-21
Online:2025-09-25
Published:2025-10-23
Contact:
Wenjun ZHANG, Qiwei YANG
摘要:
热等离子体技术以其高温、高焓、高电子密度的特性,在CO2与富烷烃气体重整领域展现出巨大潜力。本文首先通过数值模拟,从热力学和动力学两个层面揭示了原料配比对热等离子体重整体系粒子时空演化行为的影响机制并预测产物组成,表明热等离子体重整CH4和CO2的反应速度极快,可在毫秒尺度内完成转化;受键能影响,CO2的解离难度大于CH4,导致体系内CO的生成速率低于H2的生成速率。进一步实验研究了CH4以及复杂炼厂气(含CH4、C2H6等组分)与CO2在热等离子体反应器中的重整行为,建立了原料配比、输入功率等关键参数与产物组成的关系,发现炼厂气重整的产物仍以H2和CO为主,最佳条件下,CH4和CO2的转化率分别达到99.6%和93.2%,对应H2和CO的选择性分别为83.7%和98.3%。上述结果为炼厂气与CO2协同转化制备高附加值合成气提供了新的思路。
中图分类号:
徐佳琪, 张文君, 余燕萍, 苏宝根, 任其龙, 杨启炜. 热等离子体重整炼厂气制合成气过程数值模拟与实验研究[J]. 化工学报, 2025, 76(9): 4462-4473.
Jiaqi XU, Wenjun ZHANG, Yanping YU, Baogen SU, Qilong REN, Qiwei YANG. Numerical simulation and experimental study of the conversion of refinery gas to syngas via thermal plasma[J]. CIESC Journal, 2025, 76(9): 4462-4473.
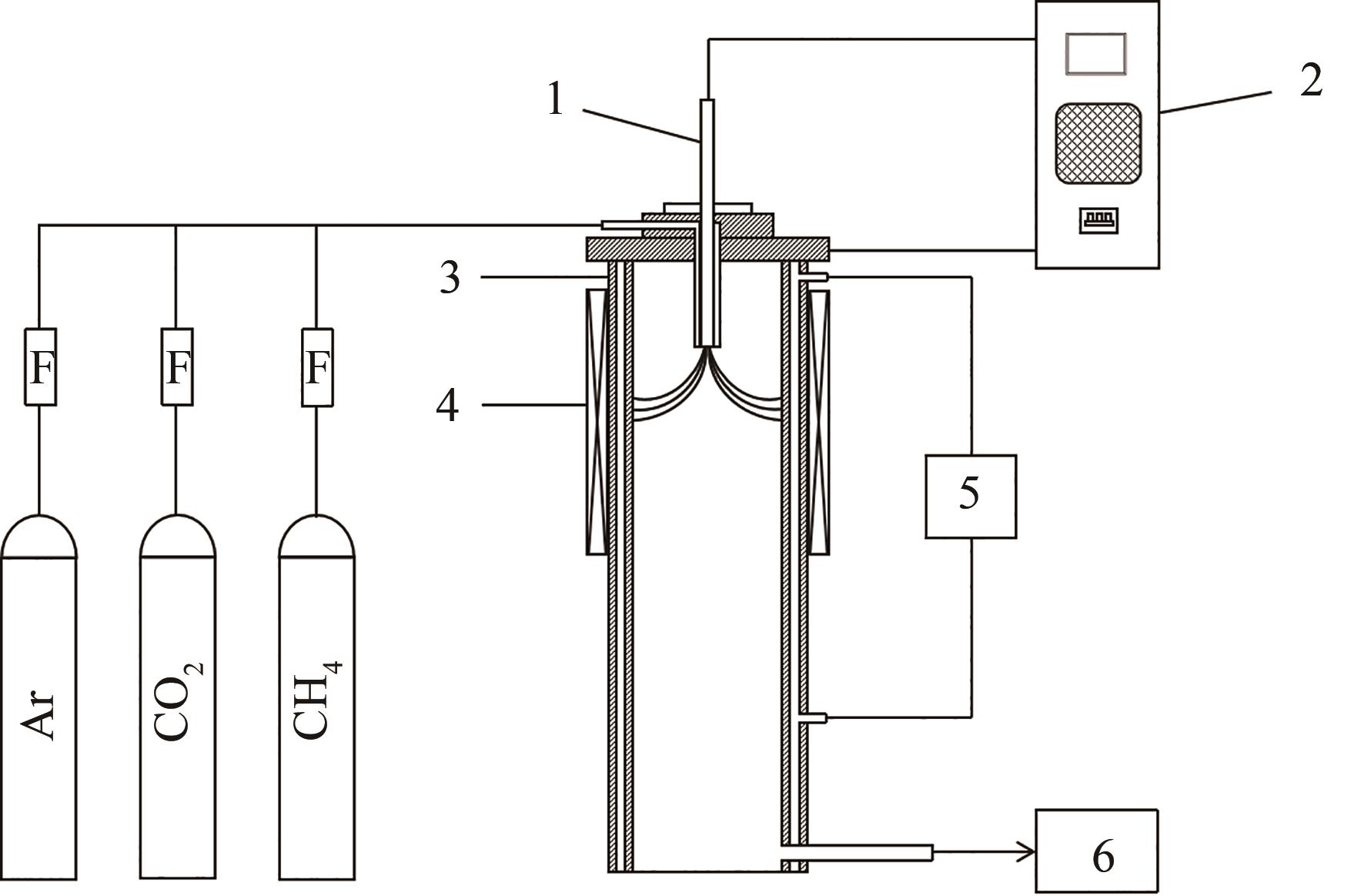
图1 重整实验流程示意图1—阴极;2—直流电源;3—阳极;4—励磁线圈;5—热交换器;6—气相色谱
Fig.1 Schematic diagram of the experimental process of reforming1—cathode; 2—DC power supply; 3—anode; 4—magnetic coils; 5—heat exchanger; 6—gas chromatography
| 气体 | 体积分数/% | 气体 | 体积分数/% |
|---|---|---|---|
| H2 | 18.93 | C3H8 | 1.35 |
| CH4 | 46.49 | C3H6 | 0.32 |
| C2H6 | 15.72 | C4H10 | 0.37 |
| C2H4 | 0.89 | N2 | 14.05 |
| CO2 | 1.20 |
表1 炼厂气的主要组成及含量
Table 1 Main composition and content of refinery gas
| 气体 | 体积分数/% | 气体 | 体积分数/% |
|---|---|---|---|
| H2 | 18.93 | C3H8 | 1.35 |
| CH4 | 46.49 | C3H6 | 0.32 |
| C2H6 | 15.72 | C4H10 | 0.37 |
| C2H4 | 0.89 | N2 | 14.05 |
| CO2 | 1.20 |
| [1] | Tagliapietra S, Zachmann G, Edenhofer O, et al. The European union energy transition: key priorities for the next five years[J]. Energy Policy, 2019, 132: 950-954. |
| [2] | Shao T M, Pan X Z, Li X, et al. China's industrial decarbonization in the context of carbon neutrality: a sub-sectoral analysis based on integrated modelling[J]. Renewable and Sustainable Energy Reviews, 2022, 170: 112992. |
| [3] | Liu P K, Zhao R Q, Han X. Assessing the efficiency and the justice of energy transformation for the United States of America, China, and the European Union[J]. Sustainable Development, 2023, 31(5): 3387-3407. |
| [4] | Wen L, Diao P X. Simulation study on carbon emission of China's electricity supply and demand under the dual-carbon target[J]. Journal of Cleaner Production, 2022, 379: 134654. |
| [5] | Cai W, Lai K H, Liu C H, et al. Promoting sustainability of manufacturing industry through the lean energy-saving and emission-reduction strategy[J]. Science of The Total Environment, 2019, 665: 23-32. |
| [6] | Zhao S J, Song Q B, Liu L L, et al. Uncovering the lifecycle carbon emissions and its reduction pathways: a case study of petroleum refining enterprise[J]. Energy Conversion and Management, 2024, 301: 118048. |
| [7] | Li Z X, Åhman M, Nilsson L J, et al. Towards carbon neutrality: transition pathways for the Chinese ethylene industry[J]. Renewable and Sustainable Energy Reviews, 2024, 199: 114540. |
| [8] | Pawar V, Ray D, Subrahmanyam C, et al. Study of short-term catalyst deactivation due to carbon deposition during biogas dry reforming on supported Ni catalyst[J]. Energy & Fuels, 2015, 29(12): 8047-8052. |
| [9] | Zambrano D, Soler J, Herguido J, et al. Kinetic study of dry reforming of methane over Ni–Ce/Al2O3 catalyst with deactivation[J]. Topics in Catalysis, 2019, 62(5): 456-466. |
| [10] | Takahashi Y, Yamazaki T. Behavior of high-pressure CH4/CO2 reforming reaction over mesoporous Pt/ZrO2 catalyst[J]. Fuel, 2012, 102: 239-246. |
| [11] | Tu X, Whitehead J C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: understanding the synergistic effect at low temperature[J]. Applied Catalysis B: Environmental, 2012, 125: 439-448. |
| [12] | Fidalgo B, Menéndez J A. Study of energy consumption in a laboratory pilot plant for the microwave-assisted CO2 reforming of CH4 [J]. Fuel Processing Technology, 2012, 95: 55-61. |
| [13] | Zeng Y X, Zhu X B, Mei D H, et al. Plasma-catalytic dry reforming of methane over γ-Al2O3 supported metal catalysts[J]. Catalysis Today, 2015, 256: 80-87. |
| [14] | Chung W C, Chang M B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects[J]. Renewable and Sustainable Energy Reviews, 2016, 62: 13-31. |
| [15] | Delikonstantis E, Scapinello M, Stefanidis G D. Investigating the plasma-assisted and thermal catalytic dry methane reforming for syngas production: process design, simulation and evaluation[J]. Energies, 2017, 10(9): 1429. |
| [16] | Andersen J A, Christensen J M, Østberg M, et al. Plasma-catalytic dry reforming of methane: screening of catalytic materials in a coaxial packed-bed DBD reactor[J]. Chemical Engineering Journal, 2020, 397: 125519. |
| [17] | Sun J T, Chen Q, Qin W Y, et al. Plasma-catalytic dry reforming of CH4: effects of plasma-generated species on the surface chemistry[J]. Chemical Engineering Journal, 2024, 498: 155847. |
| [18] | Bhuiyan S I, Kraus J, Hil Baky M A, et al. Greenhouse gas emission reduction and energy impact of electrifying upgraders in refineries using plasma processing technology[J]. Sustainable Energy & Fuels, 2023, 7(9): 2178-2199. |
| [19] | Sanlisoy A, Ozdinc Carpinlioglu M. Microwave plasma gasification of a variety of fuel for syngas production[J]. Plasma Chemistry and Plasma Processing, 2019, 39(5): 1211-1225. |
| [20] | Hrabovsky M, Hlina M, Kopecky V, et al. Steam plasma treatment of organic substances for hydrogen and syngas production[J]. Plasma Chemistry and Plasma Processing, 2017, 37(3): 739-762. |
| [21] | Yan B H, Wang Q, Jin Y, et al. Dry reforming of methane with carbon dioxide using pulsed DC arc plasma at atmospheric pressure[J]. Plasma Chemistry and Plasma Processing, 2010, 30(2): 257-266. |
| [22] | Lu T F, Law C K. A criterion based on computational singular perturbation for the identification of quasi steady state species: a reduced mechanism for methane oxidation with NO chemistry[J]. Combustion and Flame, 2008, 154(4): 761-774. |
| [23] | Wang H, Frenklach M. A detailed kinetic modeling study of aromatics formation in laminar premixed acetylene and ethylene flames[J]. Combustion and Flame, 1997, 110(1/2): 173-221. |
| [24] | 王佳杰, 毛震波, 李军, 等. 等离子体CO2-CH4干重整反应技术进展[J]. 低碳化学与化工, 2023, 48(3): 78-88. |
| Wang J J, Mao Z B, Li J, et al. Progress in plasma-driven dry reforming of CO2 and CH4 [J]. Low-Carbon Chemistry and Chemical Engineering, 2023, 48(3): 78-88. | |
| [25] | Gleizes A, Gonzalez J J, Freton P. Thermal plasma modelling[J]. Journal of Physics D: Applied Physics, 2005, 38(9): R153-R183. |
| [26] | Fincke J R, Anderson R P, Hyde T, et al. Plasma thermal conversion of methane to acetylene[J]. Plasma Chemistry and Plasma Processing, 2002, 22(1): 105-136. |
| [27] | 余徽, 印永祥, 戴晓雁. 等离子体射流裂解甲烷制乙炔的数值模拟[J]. 化工学报, 2006, 57(10): 2319-2326. |
| Yu H, Yin Y X, Dai X Y. Numerical simulation of methane conversion to acetylene in plasma jet reactor[J]. Journal of Chemical Industry and Engineering (China), 2006, 57(10): 2319-2326. | |
| [28] | Hori M. Radical-controlled plasma processes[J]. Reviews of Modern Plasma Physics, 2022, 6(1): 36. |
| [29] | 孙艳朋, 聂勇, 吴昂山, 等. 热等离子体重整甲烷和二氧化碳制合成气的热力学研究[J]. 天然气化工(C1化学与化工), 2010, 35(4): 22-26. |
| Sun Y P, Nie Y, Wu A S, et al. Thermodynamic study on carbon dioxide reforming of methane to syngas by thermal plasma[J]. Natural Gas Chemical Industry, 2010, 35(4): 22-26. | |
| [30] | Giammaria G, van Rooij G, Lefferts L. Plasma catalysis: distinguishing between thermal and chemical effects[J]. Catalysts, 2019, 9(2): 185. |
| [31] | Trelles J P, Chazelas C, Vardelle A, et al. Arc plasma torch modeling[J]. Journal of Thermal Spray Technology, 2009, 18(5): 728-752. |
| [32] | Živný O, Hlína M, Serov A, et al. Abatement of tetrafluormethane using thermal steam plasma[J]. Plasma Chemistry and Plasma Processing, 2020, 40(1): 309-323. |
| [33] | Zhong H T, Shneider M N, Mao X Q, et al. Dynamics and chemical mode analysis of plasma thermal-chemical instability[J]. Plasma Sources Science and Technology, 2021, 30(3): 035002. |
| [34] | Feng J Y, Sun X, Li Z, et al. Plasma-assisted reforming of methane[J]. Advanced Science, 2022, 9(34): 2203221. |
| [35] | Kok D H K, Ibrahim R K R, Toemen S, et al. The catalytic efficiency of Ru/Mn/Ce-Al2O3 in the reduction of HCN in dry methane reforming with CO2 assisted by non-thermal plasma[J]. Journal of Physics: Conference Series, 2023, 2432(1): 012011. |
| [36] | Wang W Z, Snoeckx R, Zhang X M, et al. Modeling plasma-based CO2 and CH4 conversion in mixtures with N2, O2, and H2O: the bigger plasma chemistry picture[J]. The Journal of Physical Chemistry C, 2018, 122(16): 8704-8723. |
| [37] | Snoeckx R, Setareh M, Aerts R, et al. Influence of N2 concentration in a CH4/N2 dielectric barrier discharge used for CH4 conversion into H2 [J]. International Journal of Hydrogen Energy, 2013, 38(36): 16098-16120. |
| [38] | Zhang H, Wang W Z, Li X D, et al. Plasma activation of methane for hydrogen production in a N2 rotating gliding arc warm plasma: a chemical kinetics study[J]. Chemical Engineering Journal, 2018, 345: 67-78. |
| [39] | McKean D C. Individual CH bond strengths in simple organic compounds: effects of conformation and substitution[J]. Chemical Society Reviews, 1978, 7(3): 399-422. |
| [1] | 周轶磊, 李智, 彭鑫. 基于代理模型的连续重整反应过程自优化控制结构设计[J]. 化工学报, 2025, 76(9): 4499-4511. |
| [2] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| [3] | 吴天灏, 叶霆威, 林延, 黄振. 生物质化学链气化原位补氢制H2/CO可控合成气[J]. 化工学报, 2025, 76(7): 3498-3508. |
| [4] | 麦棹铭, 武颖韬, 王维, 穆海宝, 黄佐华, 汤成龙. 正十二烷-甲烷双燃料非线性着火特性及稀释气体效应研究[J]. 化工学报, 2025, 76(6): 3115-3124. |
| [5] | 赵清萍, 张敏, 赵辉, 王刚, 邱永福. 乙烯氢甲酯化合成丙酸甲酯的氢键作用机制及反应动力学研究[J]. 化工学报, 2025, 76(6): 2701-2713. |
| [6] | 万俊, 宋佳芮, 范春煌, 魏乐乐, 聂依娜, 刘琳. 高效空穴转移助力光催化碱性甲醇-水溶液制氢[J]. 化工学报, 2025, 76(3): 1064-1075. |
| [7] | 杨猛, 丁晓倩, 余涛, 刘畅, 汤成龙, 黄佐华. 甲烷/氧化亚氮绿色推进剂自着火特性实验及动力学[J]. 化工学报, 2025, 76(3): 1221-1229. |
| [8] | 郭珊, 田雨, 徐永滨, 王朋, 刘治明. 废旧电池再资源化制备高性能中熵合金催化剂及其性能研究[J]. 化工学报, 2025, 76(1): 231-240. |
| [9] | 石美琳, 赵连达, 邓行健, 王静松, 左海滨, 薛庆国. 催化甲烷重整工艺的研究进展[J]. 化工学报, 2024, 75(S1): 25-39. |
| [10] | 赵焕娟, 包颖昕, 于康, 刘婧, 钱新明. 多元组分爆轰不稳定性定量实验研究[J]. 化工学报, 2024, 75(S1): 339-348. |
| [11] | 王寅, 初鹏飞, 刘虎, 吕静, 黄守莹, 王胜平, 马新宾. 不同pH铝溶胶对二甲醚羰基化成型丝光沸石催化剂性能的影响[J]. 化工学报, 2024, 75(7): 2533-2543. |
| [12] | 张劲, 郭志斌, 罗来明, 卢善富, 相艳. 5 kW重整甲醇高温质子交换膜燃料电池系统设计与性能[J]. 化工学报, 2024, 75(4): 1697-1704. |
| [13] | 张兆想, 蔡茂坤, 任志英, 贾晓红, 郭飞. 温度及其波动对橡胶密封硫化过程影响的仿真分析[J]. 化工学报, 2024, 75(2): 715-726. |
| [14] | 王学云, 郁肖兵, 彭万旺, 沈岩松. 熔渣气化炉喷嘴燃烧区行为的数值模拟研究[J]. 化工学报, 2024, 75(2): 659-674. |
| [15] | 卓红英, 赵忠正, 沈铮, 杨小峰, 黄延强. 正-仲氢催化转化研究进展[J]. 化工学报, 2024, 75(11): 3883-3895. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号