化工学报 ›› 2019, Vol. 70 ›› Issue (6): 2102-2109.DOI: 10.11949/j.issn.0438-1157.20181517
收稿日期:2018-12-26
修回日期:2019-04-08
出版日期:2019-06-05
发布日期:2019-06-05
通讯作者:
任永胜
基金资助:
Yongsheng REN1,2,3( ),Jing CAO1,2,Bingjie YU1
),Jing CAO1,2,Bingjie YU1
Received:2018-12-26
Revised:2019-04-08
Online:2019-06-05
Published:2019-06-05
Contact:
Yongsheng REN
摘要:
采用等温溶解法研究313.15 K下四元体系Na+//
中图分类号:
任永胜, 曹晶, 于冰洁. 313.15 K四元体系Na+//
Yongsheng REN, Jing CAO, Bingjie YU. Solid-liquid equilibria of quaternary system Na +//
| Ternary system | Initial point | Solid phase | Additive |
|---|---|---|---|
| Na2SO4-NaNO3-H2O | E | NaNO3+NaNO3·Na2SO4·H2O | NaCl |
| F | Na2SO4+NaNO3·Na2SO4·H2O | NaCl | |
| Na2CO3-Na2SO4-H2O | H | Na2SO4 + Na2CO3·2Na2SO4 | NaNO3 |
| L | Na2CO3 + Na2CO3·2Na2SO4 | NaNO3 | |
| Na2CO3-NaNO3-H2O | G | Na2CO3 + NaNO3 | Na2SO4 |
表1 不同单变曲线阶段饱和溶液的配制方案
Table 1 Preparation of solutions corresponding to different sections of isotherm
| Ternary system | Initial point | Solid phase | Additive |
|---|---|---|---|
| Na2SO4-NaNO3-H2O | E | NaNO3+NaNO3·Na2SO4·H2O | NaCl |
| F | Na2SO4+NaNO3·Na2SO4·H2O | NaCl | |
| Na2CO3-Na2SO4-H2O | H | Na2SO4 + Na2CO3·2Na2SO4 | NaNO3 |
| L | Na2CO3 + Na2CO3·2Na2SO4 | NaNO3 | |
| Na2CO3-NaNO3-H2O | G | Na2CO3 + NaNO3 | Na2SO4 |
| No. | Composition of solution, w(b)×100 | J?necke index of dry salt/(g/100 g S) | 平衡固相② | |||||
|---|---|---|---|---|---|---|---|---|
| Na2SO4 | Na2CO3 | NaNO3 | J(Na2SO4) | J(Na2CO3) | J(NaNO3) | J(H2O) | ||
| 1,E | 2.47 | 0.00 | 48.91 | 4.81 | 0.00 | 95.19 | 94.63 | N + NS |
| 2 | 2.49 | 2.44 | 46.48 | 4.83 | 4.74 | 90.42 | 94.52 | N + NS |
| 3 | 2.54 | 4.93 | 42.94 | 5.04 | 9.77 | 85.18 | 98.39 | N + NS |
| 4 | 2.42 | 7.36 | 41.69 | 4.70 | 14.30 | 81.00 | 94.28 | N + NS |
| 5,Q | 2.38 | 8.26 | 40.05 | 4.70 | 16.30 | 79.01 | 97.29 | N + NS + C |
| 6,F | 7.76 | 0.00 | 36.07 | 17.70 | 0.00 | 82.30 | 128.20 | S + NS |
| 7 | 7.09 | 2.79 | 34.93 | 15.83 | 6.22 | 77.95 | 123.14 | S + NS |
| 8 | 5.30 | 5.24 | 33.96 | 11.91 | 11.78 | 76.31 | 124.67 | S + NS + CS |
| 9,G | 0.00 | 9.06 | 40.70 | 0.00 | 18.21 | 81.79 | 100.98 | N + C |
| 10,Q | 2.37 | 8.74 | 39.29 | 4.69 | 17.35 | 77.96 | 98.41 | N + C + CS |
| 11,H | 20.85 | 13.35 | 0.00 | 60.97 | 39.03 | 0.00 | 192.39 | S + CS |
| 12 | 18.79 | 13.17 | 3.29 | 53.31 | 37.36 | 9.33 | 183.68 | S + CS |
| 13 | 16.98 | 12.87 | 6.48 | 46.74 | 35.42 | 17.84 | 175.25 | S + CS |
| 14 | 15.46 | 10.98 | 9.25 | 43.32 | 30.76 | 25.92 | 180.16 | S + CS |
| 15 | 13.74 | 10.28 | 12.40 | 37.72 | 28.24 | 34.04 | 174.62 | S + CS |
| 16 | 12.60 | 9.01 | 15.48 | 33.96 | 24.30 | 41.74 | 169.57 | S + CS |
| 17 | 11.53 | 8.18 | 17.74 | 30.79 | 21.84 | 47.37 | 167.08 | S + CS |
| 18 | 10.19 | 7.76 | 20.90 | 26.23 | 19.98 | 53.79 | 157.32 | S + CS |
| 19 | 9.24 | 7.55 | 22.72 | 23.39 | 19.11 | 57.50 | 153.11 | S + CS |
| 20 | 6.45 | 7.18 | 28.27 | 15.39 | 17.14 | 67.47 | 138.68 | S + CS |
| 21 | 5.64 | 7.10 | 30.29 | 13.11 | 16.51 | 70.39 | 132.36 | S + CS |
| 22,S | 3.81 | 6.64 | 32.60 | 8.85 | 15.41 | 75.74 | 132.29 | S + CS + NS |
| 23,L | 5.12 | 29.71 | 0.00 | 14.69 | 85.31 | 0.00 | 187.18 | C + CS |
| 24 | 5.07 | 27.88 | 3.54 | 14.14 | 76.19 | 9.67 | 173.34 | C + CS |
| 25 | 4.96 | 25.81 | 6.31 | 13.38 | 69.62 | 17.01 | 169.70 | C + CS |
| 26 | 4.58 | 23.61 | 9.52 | 12.15 | 62.60 | 25.25 | 165.09 | C + CS |
| 27 | 4.50 | 21.82 | 12.47 | 11.61 | 56.25 | 32.14 | 157.78 | C + CS |
| 28 | 4.00 | 19.90 | 15.25 | 10.22 | 50.83 | 38.95 | 155.44 | C + CS |
| 29 | 3.50 | 18.58 | 18.35 | 8.66 | 45.96 | 45.38 | 147.31 | C + CS |
| 30 | 3.15 | 17.21 | 20.63 | 7.69 | 41.99 | 50.32 | 143.95 | C + CS |
| 31 | 2.84 | 15.92 | 23.55 | 6.72 | 37.62 | 55.66 | 136.33 | C + CS |
| 32 | 2.61 | 14.60 | 26.20 | 6.01 | 33.64 | 60.35 | 130.34 | C + CS |
| 33 | 2.42 | 13.48 | 28.61 | 5.44 | 30.29 | 64.27 | 124.66 | C + CS |
| 34 | 2.32 | 11.52 | 32.91 | 4.96 | 24.64 | 70.40 | 113.90 | C + CS |
| 35,Q | 2.37 | 8.74 | 39.29 | 4.69 | 17.35 | 77.96 | 98.41 | N + C + CS |
表2 四元体系(Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O)在313.15 K时固液相平衡数据①
Table 2 Solid-liquid equilibrium of quaternary system Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O at 313.15 K
| No. | Composition of solution, w(b)×100 | J?necke index of dry salt/(g/100 g S) | 平衡固相② | |||||
|---|---|---|---|---|---|---|---|---|
| Na2SO4 | Na2CO3 | NaNO3 | J(Na2SO4) | J(Na2CO3) | J(NaNO3) | J(H2O) | ||
| 1,E | 2.47 | 0.00 | 48.91 | 4.81 | 0.00 | 95.19 | 94.63 | N + NS |
| 2 | 2.49 | 2.44 | 46.48 | 4.83 | 4.74 | 90.42 | 94.52 | N + NS |
| 3 | 2.54 | 4.93 | 42.94 | 5.04 | 9.77 | 85.18 | 98.39 | N + NS |
| 4 | 2.42 | 7.36 | 41.69 | 4.70 | 14.30 | 81.00 | 94.28 | N + NS |
| 5,Q | 2.38 | 8.26 | 40.05 | 4.70 | 16.30 | 79.01 | 97.29 | N + NS + C |
| 6,F | 7.76 | 0.00 | 36.07 | 17.70 | 0.00 | 82.30 | 128.20 | S + NS |
| 7 | 7.09 | 2.79 | 34.93 | 15.83 | 6.22 | 77.95 | 123.14 | S + NS |
| 8 | 5.30 | 5.24 | 33.96 | 11.91 | 11.78 | 76.31 | 124.67 | S + NS + CS |
| 9,G | 0.00 | 9.06 | 40.70 | 0.00 | 18.21 | 81.79 | 100.98 | N + C |
| 10,Q | 2.37 | 8.74 | 39.29 | 4.69 | 17.35 | 77.96 | 98.41 | N + C + CS |
| 11,H | 20.85 | 13.35 | 0.00 | 60.97 | 39.03 | 0.00 | 192.39 | S + CS |
| 12 | 18.79 | 13.17 | 3.29 | 53.31 | 37.36 | 9.33 | 183.68 | S + CS |
| 13 | 16.98 | 12.87 | 6.48 | 46.74 | 35.42 | 17.84 | 175.25 | S + CS |
| 14 | 15.46 | 10.98 | 9.25 | 43.32 | 30.76 | 25.92 | 180.16 | S + CS |
| 15 | 13.74 | 10.28 | 12.40 | 37.72 | 28.24 | 34.04 | 174.62 | S + CS |
| 16 | 12.60 | 9.01 | 15.48 | 33.96 | 24.30 | 41.74 | 169.57 | S + CS |
| 17 | 11.53 | 8.18 | 17.74 | 30.79 | 21.84 | 47.37 | 167.08 | S + CS |
| 18 | 10.19 | 7.76 | 20.90 | 26.23 | 19.98 | 53.79 | 157.32 | S + CS |
| 19 | 9.24 | 7.55 | 22.72 | 23.39 | 19.11 | 57.50 | 153.11 | S + CS |
| 20 | 6.45 | 7.18 | 28.27 | 15.39 | 17.14 | 67.47 | 138.68 | S + CS |
| 21 | 5.64 | 7.10 | 30.29 | 13.11 | 16.51 | 70.39 | 132.36 | S + CS |
| 22,S | 3.81 | 6.64 | 32.60 | 8.85 | 15.41 | 75.74 | 132.29 | S + CS + NS |
| 23,L | 5.12 | 29.71 | 0.00 | 14.69 | 85.31 | 0.00 | 187.18 | C + CS |
| 24 | 5.07 | 27.88 | 3.54 | 14.14 | 76.19 | 9.67 | 173.34 | C + CS |
| 25 | 4.96 | 25.81 | 6.31 | 13.38 | 69.62 | 17.01 | 169.70 | C + CS |
| 26 | 4.58 | 23.61 | 9.52 | 12.15 | 62.60 | 25.25 | 165.09 | C + CS |
| 27 | 4.50 | 21.82 | 12.47 | 11.61 | 56.25 | 32.14 | 157.78 | C + CS |
| 28 | 4.00 | 19.90 | 15.25 | 10.22 | 50.83 | 38.95 | 155.44 | C + CS |
| 29 | 3.50 | 18.58 | 18.35 | 8.66 | 45.96 | 45.38 | 147.31 | C + CS |
| 30 | 3.15 | 17.21 | 20.63 | 7.69 | 41.99 | 50.32 | 143.95 | C + CS |
| 31 | 2.84 | 15.92 | 23.55 | 6.72 | 37.62 | 55.66 | 136.33 | C + CS |
| 32 | 2.61 | 14.60 | 26.20 | 6.01 | 33.64 | 60.35 | 130.34 | C + CS |
| 33 | 2.42 | 13.48 | 28.61 | 5.44 | 30.29 | 64.27 | 124.66 | C + CS |
| 34 | 2.32 | 11.52 | 32.91 | 4.96 | 24.64 | 70.40 | 113.90 | C + CS |
| 35,Q | 2.37 | 8.74 | 39.29 | 4.69 | 17.35 | 77.96 | 98.41 | N + C + CS |
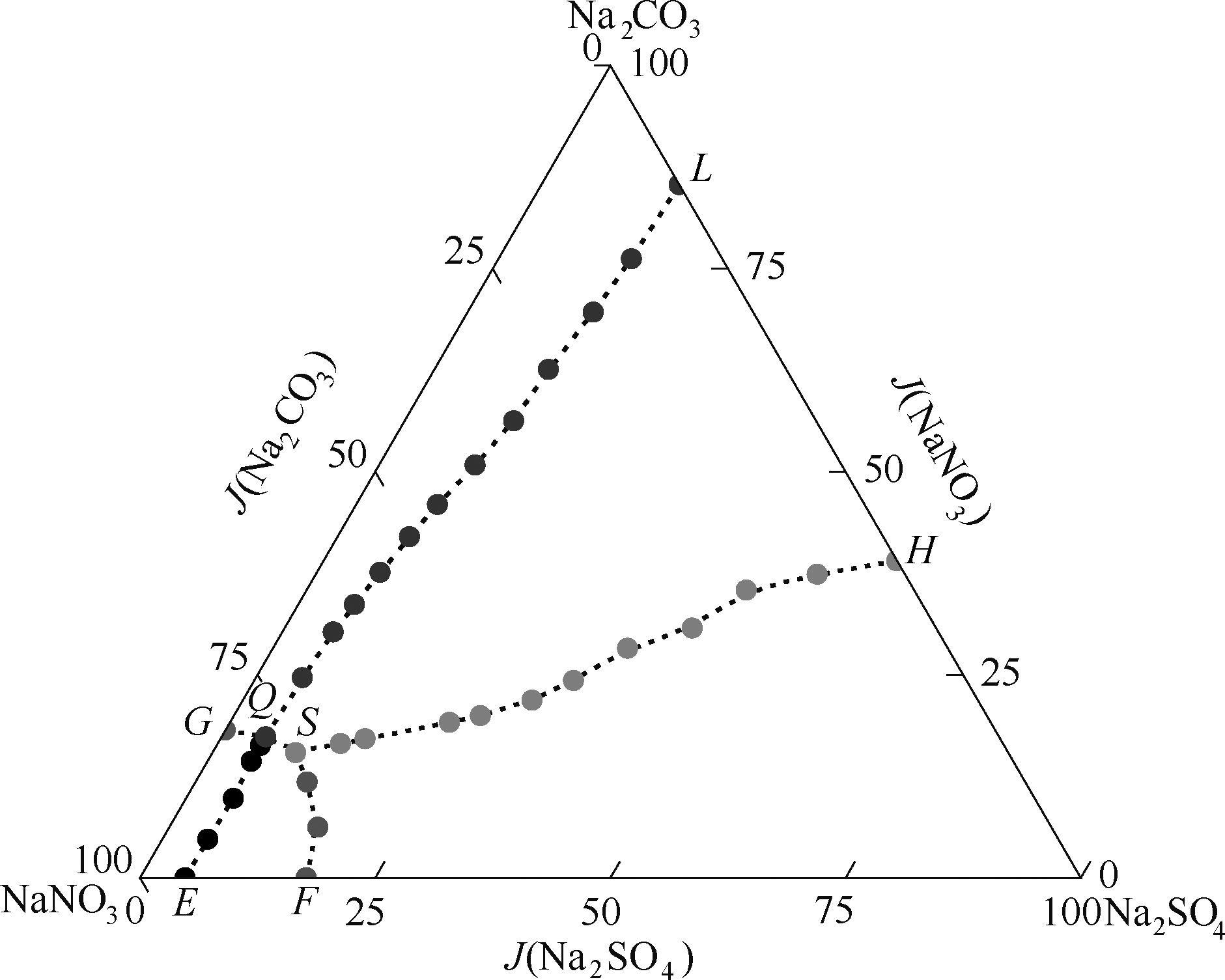
图1 四元体系(Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O)在313.15 K时干盐图
Fig.1 Dry-salt phase diagram of system Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O at 313.15 K
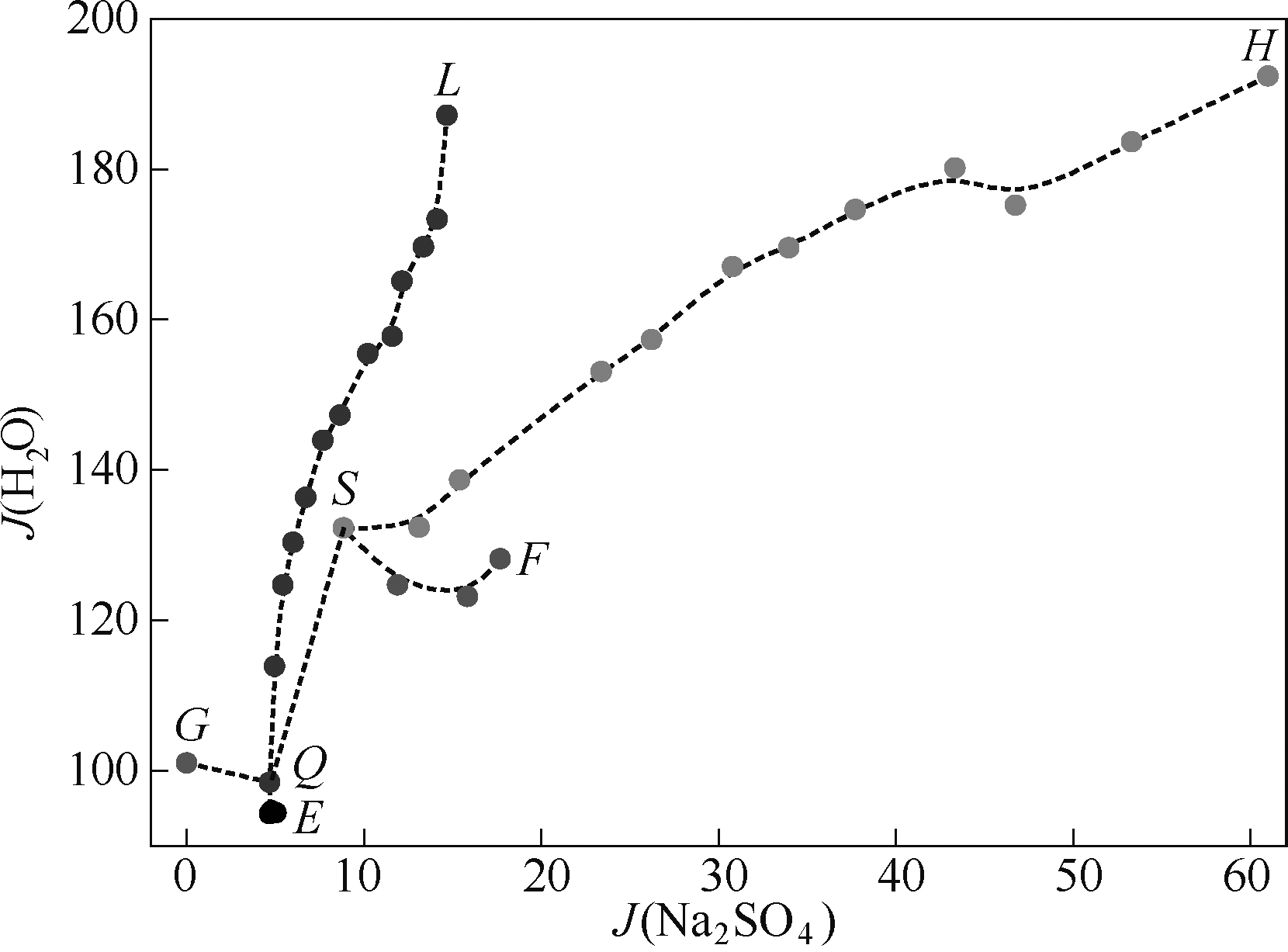
图3 四元体系(Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O)在313.15 K时水图
Fig.3 Water-content diagram of system Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O at 313.15 K
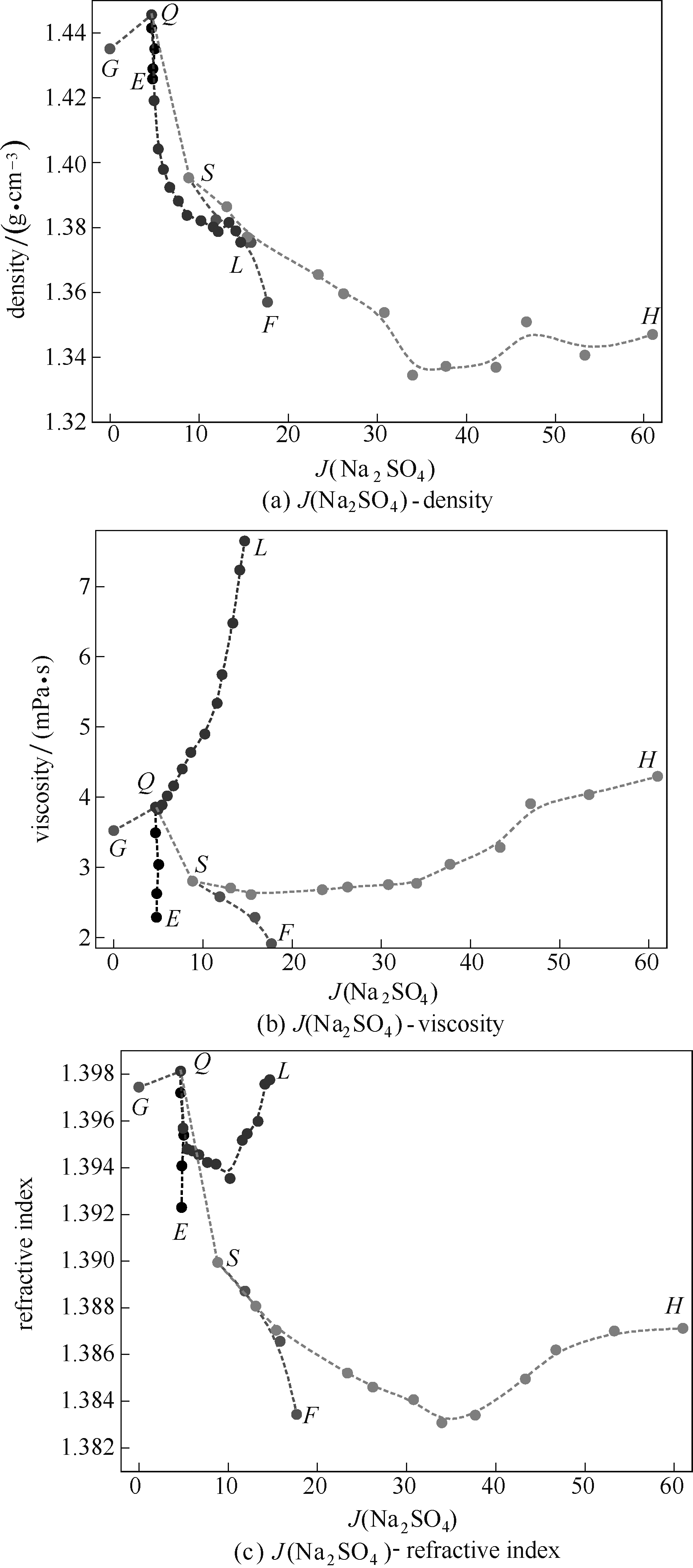
图4 四元体系(Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O) 在313.15 K时物理性质-组成图
Fig.4 Physical properties as function of J (Na2SO4) composition in quaternary system Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O at 313.15 K
| No.① | J(Na2SO4) | Viscosity | Refractive index | Density/(g·cm?3) |
|---|---|---|---|---|
| 1,E | 4.81 | 2.2861 | 1.39229 | 1.4258 |
| 2 | 4.83 | 2.6218 | 1.39407 | 1.4290 |
| 3 | 5.04 | 3.0358 | 1.39539 | 1.4351 |
| 4 | 4.70 | 3.4904 | 1.39720 | 1.4415 |
| 5,Q | 4.70 | 3.6338 | 1.39801 | 1.4414 |
| 6,F | 17.70 | 1.9106 | 1.38343 | 1.3569 |
| 7 | 15.83 | 2.2850 | 1.38656 | 1.3754 |
| 8 | 11.91 | 2.5782 | 1.38871 | 1.3824 |
| 9,G | 0.00 | 3.5211 | 1.39744 | 1.4351 |
| 10,Q | 4.69 | 3.8538 | 1.39813 | 1.4456 |
| 11,H | 60.97 | 4.2930 | 1.38712 | 1.3470 |
| 12 | 53.31 | 4.0326 | 1.38700 | 1.3406 |
| 13 | 46.74 | 3.9056 | 1.38719 | 1.3508 |
| 14 | 43.32 | 3.2815 | 1.38495 | 1.3369 |
| 15 | 37.72 | 3.0421 | 1.38340 | 1.3372 |
| 16 | 33.96 | 2.7706 | 1.38308 | 1.3345 |
| 17 | 30.79 | 2.7511 | 1.38407 | 1.3538 |
| 18 | 26.23 | 2.7164 | 1.38460 | 1.3596 |
| 19 | 23.39 | 2.6785 | 1.38520 | 1.3655 |
| 20 | 15.39 | 2.6085 | 1.38704 | 1.3771 |
| 21 | 13.11 | 2.7023 | 1.38807 | 1.3864 |
| 22,S | 8.85 | 2.8018 | 1.38994 | 1.3953 |
| 23,L | 14.69 | 7.6458 | 1.39776 | 1.3754 |
| 24 | 14.14 | 7.2336 | 1.39757 | 1.3790 |
| 25 | 13.38 | 6.4775 | 1.39598 | 1.3815 |
| 26 | 12.15 | 5.7420 | 1.39545 | 1.3787 |
| 27 | 11.61 | 5.3353 | 1.39517 | 1.3802 |
| 28 | 10.22 | 4.8954 | 1.39353 | 1.3821 |
| 29 | 8.66 | 4.6353 | 1.39414 | 1.3837 |
| 30 | 7.69 | 4.3998 | 1.39422 | 1.3881 |
| 31 | 6.72 | 4.1579 | 1.39454 | 1.3923 |
| 32 | 6.01 | 4.0156 | 1.39471 | 1.3979 |
| 33 | 5.44 | 3.8859 | 1.39479 | 1.4042 |
| 34 | 4.96 | 3.7780 | 1.39516 | 1.4123 |
| 35,Q | 4.69 | 3.7191 | 1.39568 | 1.4191 |
表3 四元体系(Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O)在313.15 K时平衡液相物理性质
Table 3 Physical properties of quaternary system Na+// S O 4 2 - , C O 3 2 - , N O 3 - -H2O at 313.15 K
| No.① | J(Na2SO4) | Viscosity | Refractive index | Density/(g·cm?3) |
|---|---|---|---|---|
| 1,E | 4.81 | 2.2861 | 1.39229 | 1.4258 |
| 2 | 4.83 | 2.6218 | 1.39407 | 1.4290 |
| 3 | 5.04 | 3.0358 | 1.39539 | 1.4351 |
| 4 | 4.70 | 3.4904 | 1.39720 | 1.4415 |
| 5,Q | 4.70 | 3.6338 | 1.39801 | 1.4414 |
| 6,F | 17.70 | 1.9106 | 1.38343 | 1.3569 |
| 7 | 15.83 | 2.2850 | 1.38656 | 1.3754 |
| 8 | 11.91 | 2.5782 | 1.38871 | 1.3824 |
| 9,G | 0.00 | 3.5211 | 1.39744 | 1.4351 |
| 10,Q | 4.69 | 3.8538 | 1.39813 | 1.4456 |
| 11,H | 60.97 | 4.2930 | 1.38712 | 1.3470 |
| 12 | 53.31 | 4.0326 | 1.38700 | 1.3406 |
| 13 | 46.74 | 3.9056 | 1.38719 | 1.3508 |
| 14 | 43.32 | 3.2815 | 1.38495 | 1.3369 |
| 15 | 37.72 | 3.0421 | 1.38340 | 1.3372 |
| 16 | 33.96 | 2.7706 | 1.38308 | 1.3345 |
| 17 | 30.79 | 2.7511 | 1.38407 | 1.3538 |
| 18 | 26.23 | 2.7164 | 1.38460 | 1.3596 |
| 19 | 23.39 | 2.6785 | 1.38520 | 1.3655 |
| 20 | 15.39 | 2.6085 | 1.38704 | 1.3771 |
| 21 | 13.11 | 2.7023 | 1.38807 | 1.3864 |
| 22,S | 8.85 | 2.8018 | 1.38994 | 1.3953 |
| 23,L | 14.69 | 7.6458 | 1.39776 | 1.3754 |
| 24 | 14.14 | 7.2336 | 1.39757 | 1.3790 |
| 25 | 13.38 | 6.4775 | 1.39598 | 1.3815 |
| 26 | 12.15 | 5.7420 | 1.39545 | 1.3787 |
| 27 | 11.61 | 5.3353 | 1.39517 | 1.3802 |
| 28 | 10.22 | 4.8954 | 1.39353 | 1.3821 |
| 29 | 8.66 | 4.6353 | 1.39414 | 1.3837 |
| 30 | 7.69 | 4.3998 | 1.39422 | 1.3881 |
| 31 | 6.72 | 4.1579 | 1.39454 | 1.3923 |
| 32 | 6.01 | 4.0156 | 1.39471 | 1.3979 |
| 33 | 5.44 | 3.8859 | 1.39479 | 1.4042 |
| 34 | 4.96 | 3.7780 | 1.39516 | 1.4123 |
| 35,Q | 4.69 | 3.7191 | 1.39568 | 1.4191 |
| 1 | 黄开东, 李强, 汪炎 . 煤化工废水“零排放”技术及工程应用现状分析[J]. 工业用水与废水, 2012, 43(5): 1-6. |
| Huang K D , Li Q , Wang Y . Techniques for wastewater zero discharge in coal chemical industry and their application status[J]. Industrial Water & Wastewater, 2012, 43(5): 1-6. | |
| 2 | 曲风臣 . 煤化工废水“零排放”技术要点及存在问题[J]. 化学工业, 2013, 31(2): 18-24. |
| Qu F C . The key technologies and problems of wastewater zero discharge in coal chemical industry[J]. Chemical Industry, 2013, 31(2): 18-24. | |
| 3 | 童莉, 郭森, 周学双 .煤化工废水零排放的制约性问题[J].化工环保, 2010, 30(5): 371−375. |
| Tong L , Guo S , Zhou X S . Control problem of zero discharge of coal chemical wastewater[J]. Chemical Industry Environmental Protection, 2010, 30(5): 371-375. | |
| 4 | Dellantonio A , Fitz W J , Custovic H , et al . Environmental risks of farmed and barren alkaline coal ash landfills in Tuzla, Bosnia and Herzegovina[J]. Environmental Pollution, 2008, 153: 677-686. |
| 5 | Zuberi M J S , Ali S F . Greenhouse effect reduction by recovering energy from waste landfills in Pakistan[J]. Renewable & Sustainable Energy Reviews, 2015, 44: 117-131. |
| 6 | Bosmans A , Vanderreydt I , Geysen D , et al . The crucial role of waste-to-energy technologies in enhanced landfill mining: a technology review[J]. Journal of Cleaner Production, 2013, 55: 10-23. |
| 7 | Ericsson B , Hallmans B . Treatment of saline wastewater for zero discharge at the Debiensko coal mines in poland[J]. Desalination, 1996, 105(1/2): 115-123. |
| 8 | Turek M , Dydo P , Surma A . Zero discharge utilization of saline waters from “Wesola” coal-mine[J]. Desalination, 2005, 185(1): 275-280. |
| 9 | Du C H , Zheng S L , Li H Q , et al . Solid-liquid equilibria of K2CO3 + K2CrO4 + H2O System[J]. Journal of Chemical & Engineering Data, 2006, 1(51): 104-106. |
| 10 | Lu H , Wang J , Yu J , et al . Phase equilibria for the pseudo-ternary system (NaCl + Na2SO4 + H2O) of coal gasification wastewater at T = (268.15 to 373.15) K[J]. Chinese Journal of Chemical Engineering, 2017, 25: 955-962. |
| 11 | Zhang X R , Ren Y S , Li P , et al . Solid-liquid equilibrium for the ternary systems (Na2SO4 + NaH2PO4 + H2O) and (Na2SO4 + NaCl + H2O) at 313.15 K and atmospheric pressure[J]. Journal of Chemical & Engineering Data, 2014, 59(12): 3969-3974. |
| 12 | Zhou H , Bao Y , Bai X , et al . Salt-forming regions of seawater type solution in the evaporation and fractional crystallization process[J]. Fluid Phase Equilibr., 2014, 362: 281-287. |
| 13 | Cao J , Ren Y S , Yu B J , et al . Solid-liquid equilibrium for the ternary systems (Na2CO3 + NaCl + H2O) and (Na2CO3 + Na2SO4 + H2O) at 313.15 K and atmospheric pressure [J]. J. Chem. Thermodynamics, 2019, 133: 181-193. |
| 14 | Cao J , Ding M X , Ren Y S . Solid-liquid equilibria for (NaNO3 + NaCl + H2O) and (NaNO3 + Na2CO3 + H2O) systems at 313.15 K [J]. Journal of Chemical & Engineering Data, 2019, 64: 884-894. |
| 15 | Zhang X R , Ren Y S , Li P , et al . Solid-liquid equilibrium for the ternary systems (Na2SO4 + NaH2PO4 + H2O) and (Na2SO4 + NaCl + H2O) at 313.15 K and atmospheric pressure[J]. Journal of Chemical & Engineering Data, 2014, 59: 3969-3974. |
| 16 | Cao J , Ren Y S , Zhu Q N , et al . Investigation of solid-liquid equilibria on the Na+// Cl-, NO3 -, SO4 2−-H2O system and the Na+// NO3 -, SO4 2−-H2O system at 313.15K [J]. Journal of Chemical & Engineering Data, 2019, 64: 1209-1221. |
| 17 | Eysseltová J . IUPAC-NIST solubility data series(89): Alkali metal nitrates (Part 1): Sodium nitrate[J]. Journal of Physical & Chemical Reference Data, 2017, 46(1): 54-56. |
| 18 | 黎玲 . 30℃ Na2CO3-Na2SO4 -NaCl-H2O体系溶解度的研究[J]. 内蒙古工学院学报, 1989, (1): 71-82. |
| Li L . Study on solubility of 30°C Na2CO3-Na2SO4-NaCl-H2O system[J]. Journal of Inner Mongolia Institute of Technology, 1989, (1): 71-82. | |
| 19 | Teeple J E . The Industrial Development of Searles Lake Brine with Equilibrium Data[M]. New York: ACS, 1929. |
| 20 | Bian C , Chen H , Song X F , et al . Stable phase equilibria of the quaternary system Na+// Cl-, NO3 -, SO4 2−-H2O at 353.15 K[J]. J. Chem. Eng. Data, 2018, 63: 3305-3314 |
| 21 | 张杰, 史学伟, 赵双良, 等 . 水盐体系相平衡研究进展[J]. 化工学报, 2016, 67(2): 379-389. |
| Zhang J , Shi X W , Zhao S L , et al . Progress in study on phase equilibria of salt-water systems[J]. CIESC Journal, 2016, 67(2): 379-389. | |
| 22 | 任永胜, 何婷婷, 谢娟, 等 . 333.15 K K+, NH4 +// C1-, SO4 2−-H2O 和K+, NH4 +// C1-, SO4 2−- (CH2OH)2-H2O体系固液相平衡 [J]. 化工学报, 2018, 69(7): 2838-2850. |
| Ren Y S , He T T , Xie J , et al . Phase equilibria in systems K+, NH4 +// C1-, SO4 2−-H2O and K+, NH4 +// C1-, SO4 2−- (CH2OH)2 -H2O at 313.15 K [J]. CIESC Journal, 2018, 69(7): 2838-2850. | |
| 23 | 沈薇, 谢娟, 何婷婷, 等 . 298.15 K下四元体系Na+, K+// SO4 2−, H2PO4 --H2O固液相平衡研究 [J]. 高校化学工程学报, 2017, 31(2): 276-283. |
| Shen W , Xie J , He T T , et al . Solid-liquid equilibria of a quaternary system Na+, K+// SO4 2−, H2PO4 --H2O at 298.15 K [J]. Journal of Chemical Engineering of Chinese Universities, 2017, 31(2): 276-283. | |
| 24 | He T T , Sun J J , Shen W , et al . Solid-liquid phase equilibria of quaternary system NH4 + //C1-, SO 4 2 - , H2PO 4 - –H2O and its subsystems NH4 +//C1-, SO 4 2 - -H2O, NH4 +//C1-, H2PO 4 - -H2O at 313.15 K[J]. Journal of Chemical Thermodynamics, 2017, 112: 31-42. |
| 25 | 中国科学院青海盐湖所 . 卤水和盐的分析方法[M]. 北京: 科学出版社, 1988: 75-80 |
| Institute of Qinghai Salt-lake of Chinese Academy of Sciences . Analytical Methods of Brines and Salts[M]. Beijing: Science Press, 1988, 75-80. | |
| 26 | Sang S , Yin H , Tang A , et al . (Liquid + solid) phase equilibria in the quaternary system Na2CO3 + K2B4O7 + K2CO3 + Na2B4O7 + H2O at 288 K[J]. J. Chem. Eng. Data, 2004, 49 (6): 1775-1777. |
| 27 | Yang B , Li J , Jin Y , et al . Solid-liquid equilibria in the quaternary system Na+, NH4 +//Cl-, H2PO 4 - -H2O at 298.15 K and 323.15 K [J]. Fluid Phase Equilibria, 2015, 404: 55-60. |
| 28 | Ren Y S , Zhang X R , Sun Y , et al . Solid-liquid equilibrium of quaternary system Na+//H2PO 4 - ,C1-, SO 4 2 - -H2O at 298.15 K[J]. Fluid Phase Equilibria, 2015, 393: 1-6. |
| 29 | Shen W , Wang Y L , He T T , et al . Study on the equilibrium of the quaternary systems Na+(K+)// H2PO4 - , Cl-, SO 4 2 - –H2O at 313.15 K[J]. Fluid Phase Equilibria, 2015, 403: 85-94. |
| [1] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [2] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [3] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [4] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [5] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [6] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [7] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [8] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [9] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| [10] | 吴子睿, 孙瑞, 石凌峰, 田华, 王轩, 舒歌群. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| [11] | 孙裕坤, 杨焘, 吴江涛. R32+R1234yf+R1234ze(E)混合制冷剂气液相平衡实验研究[J]. 化工学报, 2022, 73(3): 1063-1071. |
| [12] | 许昊, 陈伟, 李邹路. 以[Li(TX-7)]SCN/H2O为工质对的第二类热泵特性研究[J]. 化工学报, 2022, 73(2): 577-586. |
| [13] | 高腾飞, 李国选, 雷志刚. 从催化裂化柴油中分离联苯的溶剂筛选:实验和计算热力学[J]. 化工学报, 2022, 73(12): 5314-5323. |
| [14] | 刘潜, 张香兰, 李志平, 栗卓琦, 喻红. 油酚分离过程离子液体萃取溶剂的多尺度筛选[J]. 化工学报, 2022, 73(11): 5011-5024. |
| [15] | 彭昌炜, 桑世华, 崔瑞芝, 任红保. 五元体系NaBr-KBr-MgBr2-CaBr2-H2O在298.15 K下的空间立体相图研究[J]. 化工学报, 2022, 73(11): 4850-4858. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号