化工学报 ›› 2019, Vol. 70 ›› Issue (4): 1464-1471.DOI: 10.11949/j.issn.0438-1157.20180752
李学玲1,2( ),刘兴元1,2,赵锋1,2,张建强1,2(
),刘兴元1,2,赵锋1,2,张建强1,2( )
)
收稿日期:2018-07-05
修回日期:2019-01-15
出版日期:2019-04-05
发布日期:2019-04-05
通讯作者:
张建强
作者简介:<named-content content-type="corresp-name">李学玲</named-content>(1984—),女,硕士研究生,助教,<email>lixueling_1006@163.com</email>|张建强(1986—),男,博士研究生,讲师,<email>drjqzhang@126.com</email>
基金资助:
Xueling LI1,2( ),Xingyuan LIU1,2,Feng ZHAO1,2,Jianqiang ZHANG1,2(
),Xingyuan LIU1,2,Feng ZHAO1,2,Jianqiang ZHANG1,2( )
)
Received:2018-07-05
Revised:2019-01-15
Online:2019-04-05
Published:2019-04-05
Contact:
Jianqiang ZHANG
摘要:
为探究亚砜类化合物对水中重金属镉的萃取效率和萃取机理,报道了利用二异辛基亚砜(DIOSO)萃取水溶液中镉的情况,实验制备了DIOSO,以其为萃取剂探索其对水溶液中镉的萃取情况,得出最佳萃取条件,在此条件下最高萃取率为99.7%。为达到萃取剂的回收循环利用,实验研究了不同反萃剂对Cd(Ⅱ)的反萃情况,得出利用0.2 mol/L NaOH为反萃剂时能把有机相中的Cd(Ⅱ)全部洗脱出来,反萃率达99.86%。在此基础上,结合光谱和热力学分析,DIOSO对Cd(Ⅱ)的萃取过程可能是离子间发生了缔合作用。DIOSO对水中Cd(Ⅱ)的成功萃取,可以为工业废水污染中Cd(Ⅱ)的处理提供重要理论研究基础。
中图分类号:
李学玲, 刘兴元, 赵锋, 张建强. 用DIOSO从水溶液中萃取镉的研究[J]. 化工学报, 2019, 70(4): 1464-1471.
Xueling LI, Xingyuan LIU, Feng ZHAO, Jianqiang ZHANG. Extraction of cadmium(Ⅱ) from aqueous solutions by DIOSO[J]. CIESC Journal, 2019, 70(4): 1464-1471.
| 稀释剂 | E/% |
|---|---|
| 煤油 | 99.4 |
| 正己烷 | 99.38 |
| 正庚烷 | 99.47 |
| 甲苯 | 90.97 |
| 二甲苯 | 93.13 |
| 二氯甲烷 | 84.93 |
| 三氯甲烷 | 84.97 |
表1 稀释剂对Cd(Ⅱ)萃取率的影响
Table 1 Effect of diluent on Cd(Ⅱ) extraction
| 稀释剂 | E/% |
|---|---|
| 煤油 | 99.4 |
| 正己烷 | 99.38 |
| 正庚烷 | 99.47 |
| 甲苯 | 90.97 |
| 二甲苯 | 93.13 |
| 二氯甲烷 | 84.93 |
| 三氯甲烷 | 84.97 |
| T/K | ΔH/(kJ/mol) | ΔG/(kJ/mol) | ΔS/(J/(mol·K)) |
|---|---|---|---|
| 293 | -98.8 | -14.2 | -288.8 |
| 298 | -98.8 | -12.7 | -289 |
| 303 | -98.8 | -11.1 | -289.3 |
| 313 | -98.8 | -8.4 | -288.7 |
| 323 | -988 | -5.4 | -289 |
表2 不同温度下DIOSO萃取Cd的热力学常数
Table 2 Thermodynamic constants of Cd extraction with DIOSO at different temperatures
| T/K | ΔH/(kJ/mol) | ΔG/(kJ/mol) | ΔS/(J/(mol·K)) |
|---|---|---|---|
| 293 | -98.8 | -14.2 | -288.8 |
| 298 | -98.8 | -12.7 | -289 |
| 303 | -98.8 | -11.1 | -289.3 |
| 313 | -98.8 | -8.4 | -288.7 |
| 323 | -988 | -5.4 | -289 |
| 反萃剂 | S/% |
|---|---|
| HCl | 97 |
| H2SO4 | 89.9 |
| NaOH | 97.9 |
| CH3COONH4 | 94.5 |
| EDTA | 93.4 |
| H2O | 93.8 |
表3 不同反萃剂与反萃率的关系
Table 3 Table 3 Relationship between different stripping agents and stripping rate
| 反萃剂 | S/% |
|---|---|
| HCl | 97 |
| H2SO4 | 89.9 |
| NaOH | 97.9 |
| CH3COONH4 | 94.5 |
| EDTA | 93.4 |
| H2O | 93.8 |
| C NaOH/(mol/L) | 反萃率/% |
|---|---|
| 0.05 | 94.46 |
| 0.1 | 98.41 |
| 0.2 | 99.86 |
表4 NaOH浓度对反萃率的影响
Table 4 Effect of sodium hydroxide concentration on cadmium stripping
| C NaOH/(mol/L) | 反萃率/% |
|---|---|
| 0.05 | 94.46 |
| 0.1 | 98.41 |
| 0.2 | 99.86 |
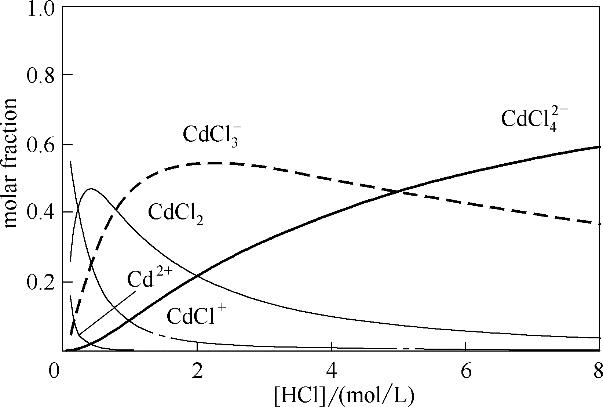
图11 镉络合物在不同浓度HCl溶液中的分布(离子强度I=3 mol/L)
Fig.11 Distribution of cadmium chloride complexes at various hydrochloric acid concentrations (ionic strength I = 3 mol/L)
| 1 | 邱廷省, 成先雄, 郝志伟, 等 . 含镉废水处理技术现状及发展[J]. 四川有色金属, 2002, 4: 38-39. |
| Qiu T S , Cheng X X , Hao Z W , et al . Present situation and development for wastewater containing cadmium treatment technology[J]. Sichuan Nonferrous Metals, 2002, 4: 38-39. | |
| 2 | 张微微, 万素琴, 梁斌 . 含铅含镉废水处理技术[J]. 现代冶金, 2013, 41(5): 64-66. |
| Zhang W W , Wan S Q , Liang B . Lead and cadmium bearing wastewater treatment technology[J]. Modern Metallurgy, 2013, 41(5): 64-66. | |
| 3 | Andrew H P K , Li C , Banerji S K , et al . Extraction, recovery, and biostability of EDTA for remediation of heavy metal-contaminated soil[J]. Journal of Soil Contamination, 1999, 8(1): 81-103. |
| 4 | Mánuel V , Pinto J J , Mendiguchía C , et al . Solvent extraction with organophosphorus extractants in environmental samples: determination of cadmium(Ⅱ) in natural water[J]. Central European Journal of Chemistry, 2014, 12(3): 348-353. |
| 5 | Szilassy I , Sumeghy L , Vadasdi K . Solvent extraction separation and recovery of components from scraps containing nickel and cadmium[J]. Mineral Processing and Extractive Metullargy Review, 1997, 17(4): 227-238. |
| 6 | Billah M , Honjo T . Separation and determination of cadmium and zinc as their thenoyltrifluoroacetone complexes with dibenzo-18-crown-6 by means of synergistic extraction and atomic absorption spectrometry[J]. Fresenius Journal of Analytical Chemistry, 1997, 357(1): 61-64. |
| 7 | Jha M K , Kumar V , Jeong J , et al . Review on solvent extraction of cadmium from various solutions[J]. Hydrometallurgy, 2012, 111(1): 1-9. |
| 8 | Reddy B R , Priya D N , Kumar J R . Solvent extraction of cadmium(Ⅱ) from sulphate solutions using TOPS 99, PC 88A, Cyanex 272 and their mixtures[J]. Hydrometallurgy, 2004, 74(3/4): 277-283. |
| 9 | Alonso A I , Urtiaga A M , Zamacona S , et al . Kinetic modelling of cadmium removal from phosphoric acid by non-dispersive solvent extraction[J]. Journal of Membrane Science, 1997, 130(1/2): 193-203. |
| 10 | Menoyo B , Elizalde M P , Almela A . Extraction of lead by Cyanex 302 from phosphoric acid media[J]. Solvent Extraction and Ion Exchange, 2001, 19(4): 677-698. |
| 11 | Almela A , Elizalde M , Ocio A . Cadmium(Ⅱ) extraction from phosphoric media by bis(2, 4, 4-trimethylpentyl)dithiophosphinic acid (CYANEX 301)[J]. Fluid Phase Equilibria, 2004, 22(6): 961-977. |
| 12 | Gupta B , Deep A , Malik P . Extraction and recovery of cadmium using Cyanex 923[J]. Hydrometallurgy, 2001, 61(1): 65-71. |
| 13 | Wang W Y , Schaumberg D A , Park S K . Cadmium and lead exposure and risk of cataract surgery in U.S. adults[J]. International Journal of Hygiene and Environmental Health, 2016, 219(8): 850-856. |
| 14 | Yuan C Y , Long H Y , Ma E X . Synthesis of sulfoxides and their structure-reactivity studies on the lanthanide extraction[J]. Science China Chemistry, 1984, 27(9): 887-897. |
| 15 | 中华人民共和国国家技术监督局 . 污水综合排放标准: GB/T 8978—1988[S]. 北京: 中国标准出版社, 1998. |
| State Bureau of Technical Supervision of the People s Republic of China . Integrated sastewater discharge standard: GB /T 8978—1988[S]. Beijing: Standards Press of China, 1988. | |
| 16 | Parhi P K , Das N N , Sarangi K . Extraction of cadmium from dilute solution using supported liquid membrane[J]. Journal of Hazardous Materials, 2009, 172: 773-779. |
| 17 | Alamdari E K , Moradkhani D , Darvishi D , et al . Synergistic effect of MEHPA on co-extraction of zinc and cadmium with DEHPA[J]. Minerals Engineering, 2004, 17: 89-92. |
| 18 | Norman L N . Separating hydrocarbons with liquid membranes: US3410794[P]. 1968-11-12. |
| 19 | 杨项军, 陈景, 谢琦莹 . 表面活性剂从碱性氰化液中萃取金[J]. 化学进展, 2009, 21(7/8): 1583 -1591. |
| Yang X J , Chen J , Xie Q Y . Surfactant extracts gold from alkaline cyanide liquid [J]. Progress in Chemistry, 2009, 21(7/8): 1583-1591. | |
| 20 | Jiang J Z , Wang X Y , Zhou W J , et al . Extraction of gold from alkaline cyanide solution by the tetradecyldimethylbenzyl- ammonium chloride/tri-n-butyl phosphate/n-heptane system based on a microemulsion mechanism[J]. Physical Chemistry Chemical Physics, 2002, 4: 4489 - 4494. |
| 21 | Gupta B , Malik P , Deep A . Solvent extraction and separation of tervalent lanthanides and yttrium using Cyanex 923[J]. Solvent Extraction and Ion Exchange, 2003, 21(2): 239-258. |
| 22 | Kumar V , Kumar M , Jha M K , et al . Solvent extraction of cadmium from sulfate solution with di-(2-ethylhexyl) phosphoric acid diluted in kerosene[J]. Hydrometallurgy, 2009, 96: 230-234. |
| 23 | Alguacil F J , Tayibi H . Carrier-facilitated transport of Cd(Ⅱ) from a high-salinity chloride medium across a supported liquid membrane containing Cyanex 923 in Solvesso 100[J]. Desalination, 2005, 180(1): 181-187. |
| 24 | Leopolda A A , Colla M T , Fortunya A , et al . Mathematical modeling of cadmium(Ⅱ) solvent extraction from neutral and acidic chloride media using Cyanex 923 extractant as a metal carrier[J]. Journal of Hazardous Materials, 2010, 182(1/2/3): 903-911. |
| 25 | Regel M , Sastre A M , Szymanowski J . Recovery of zinc(Ⅱ) from HCl spent pickling solutions by solvent extraction[J]. Environmental Science & Technology, 2001, 35(3): 630-635. |
| 26 | Cierpiszewski R , Miesiac I , Regelrosocka M , et al . Removal of zinc(Ⅱ) from spent hydrochloric acid solutions from zinc hot galvanizing plants[J]. Industrial & Engineering Chemistry Research, 2002, 41: 598-603. |
| 27 | Alguacil F J , Lopez F A . The extraction of mineral acids by the phosphine oxide Cyanex 923[J]. Hydrometallurgy, 1996, 42: 245-255. |
| 28 | Martfnez S , Sastre A , Alguacil F J . Gold extraction equilibrium in the system Cyanex 921-HC1-Au(III) [J]. Hydrometallurgy, 1997, 46: 205-214. |
| 29 | Barroso M A , Lopez F A , Sastre A . Study of the extraction of gold(Ⅲ) in aqueous hydrochloric acid media by the phosphine oxide Cyanex 925[J]. Hydrometallurgy, 1997, 45(1): 199-209. |
| [1] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [2] | 韩晨, 司徒友珉, 朱斌, 许建良, 郭晓镭, 刘海峰. 协同处理废液的多喷嘴粉煤气化炉内反应流动研究[J]. 化工学报, 2023, 74(8): 3266-3278. |
| [3] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [4] | 张佳怡, 何佳莉, 谢江鹏, 王健, 赵鹬, 张栋强. 渗透汽化技术用于锂电池生产中N-甲基吡咯烷酮回收的研究进展[J]. 化工学报, 2023, 74(8): 3203-3215. |
| [5] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [6] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [7] | 陈朝光, 贾玉香, 汪锰. 以低浓度废酸驱动中和渗析脱盐的模拟与验证[J]. 化工学报, 2023, 74(6): 2486-2494. |
| [8] | 肖忠良, 尹碧露, 宋刘斌, 匡尹杰, 赵亭亭, 刘成, 袁荣耀. 废旧锂离子电池回收工艺研究进展及其安全风险分析[J]. 化工学报, 2023, 74(4): 1446-1456. |
| [9] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [10] | 刘定平, 陈爱桦, 张向阳, 何文浩, 王海. 铝灰半干法水解脱氮研究[J]. 化工学报, 2023, 74(3): 1294-1302. |
| [11] | 许万, 陈振斌, 张慧娟, 牛昉昉, 火婷, 刘兴盛. 线性温敏性聚合物嵌段调控的 |
| [12] | 罗欣宜, 冯超, 刘晶, 乔瑜. 污泥不同热处理工艺产物磷的浸出回收实验研究[J]. 化工学报, 2022, 73(9): 4034-4044. |
| [13] | 马语峻, 刘向军. 多孔陶瓷膜烟气水分回收理论与模型研究[J]. 化工学报, 2022, 73(9): 4103-4112. |
| [14] | 戚新刚, 路利波, 陈渝楠, 葛志伟, 郭烈锦. 造纸黑液超临界水气化制氢与高附加值化学品回收研究进展[J]. 化工学报, 2022, 73(8): 3338-3354. |
| [15] | 杨双桥, 韦宝杰, 徐大伟, 李莉, 王琪. 铝塑复合包装的应用及废弃物回收利用新技术[J]. 化工学报, 2022, 73(8): 3326-3337. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号