化工学报 ›› 2020, Vol. 71 ›› Issue (10): 4826-4835.DOI: 10.11949/0438-1157.20191231
收稿日期:2019-10-23
修回日期:2020-02-12
出版日期:2020-10-05
发布日期:2020-10-05
通讯作者:
李静
作者简介:蔡迪(1994—),男,硕士研究生,基金资助:Received:2019-10-23
Revised:2020-02-12
Online:2020-10-05
Published:2020-10-05
Contact:
Jing LI
摘要:
向正十八烷中加入高导热填充物形成复合相变材料(PCM),可以很好地提升其导热性能,同时,为了保证符合相变材料的高热导率、分散性和再循环可靠性,利用硬脂醇修饰氧化石墨烯(GO),形成改性石墨烯(MG)与正十八烷的复合相变材料。分别制备了改性石墨烯质量分数为0、1%、2%、3%、4%(质量)的改性石墨烯/正十八烷复合相变材料,并经过扫描电镜测试、红外光谱分析、差示扫描量热实验及导热分析等测试对其形貌结构及热物性进行表征和研究。实验表明制备的改性石墨烯/正十八烷复合相变材料具有很好的分散性;当纳米石墨烯片的质量分数达到4%时,复合相变材料的热导率相对于纯正十八烷高出了131.9%。
中图分类号:
蔡迪, 李静. 硬脂醇改性的氧化石墨烯/正十八烷复合相变材料的热物性研究[J]. 化工学报, 2020, 71(10): 4826-4835.
Di CAI, Jing LI. Study on thermal properties of stearyl alcohol modified graphene oxide/ n-octadecane composite phase change materials[J]. CIESC Journal, 2020, 71(10): 4826-4835.
| 样品 | 起始温度Tms /℃ | 峰值Tmp /℃ | 终止温度Tme/℃ | 相变焓Hm/(J/g) |
|---|---|---|---|---|
| 正十八烷 | 24.6 | 28.6 | 34.9 | 232.0 |
| 1%(质量)改性石墨烯/正十八烷 | 22.6 | 29.2 | 34.4 | 229.0 |
| 2%(质量)改性石墨烯/正十八烷 | 24.2 | 28.8 | 34.3 | 228.3 |
| 3%(质量)改性石墨烯/正十八烷 | 24.0 | 28.5 | 34.5 | 223.3 |
| 4%(质量)改性石墨烯/正十八烷 | 23.2 | 28.5 | 33.8 | 220.3 |
表1 正十八烷及其复合相变材料熔化过程的相变温度及相变焓
Table 1 Phase transition temperature and enthalpy of n-octadecane and composite phase change materials during melting process
| 样品 | 起始温度Tms /℃ | 峰值Tmp /℃ | 终止温度Tme/℃ | 相变焓Hm/(J/g) |
|---|---|---|---|---|
| 正十八烷 | 24.6 | 28.6 | 34.9 | 232.0 |
| 1%(质量)改性石墨烯/正十八烷 | 22.6 | 29.2 | 34.4 | 229.0 |
| 2%(质量)改性石墨烯/正十八烷 | 24.2 | 28.8 | 34.3 | 228.3 |
| 3%(质量)改性石墨烯/正十八烷 | 24.0 | 28.5 | 34.5 | 223.3 |
| 4%(质量)改性石墨烯/正十八烷 | 23.2 | 28.5 | 33.8 | 220.3 |
| 样品 | 起始温度Tss/℃ | 峰值Tsp/℃ | 终止温度Tse/℃ | 相变焓Hs/(J/g) |
|---|---|---|---|---|
| 正十八烷 | 27.8 | 26.9 | 23.2 | -225.8 |
| 1%(质量)改性石墨烯/正十八烷 | 27.9 | 27.0 | 23.1 | -222.9 |
| 2%(质量)改性石墨烯/正十八烷 | 28.2 | 27.2 | 23.2 | -220.2 |
| 3%(质量)改性石墨烯/正十八烷 | 28.4 | 26.9 | 23.2 | -218.1 |
| 4%(质量)改性石墨烯/正十八烷 | 28.4 | 27.1 | 22.9 | -214.5 |
表2 正十八烷及其复合相变材料凝固过程的相变温度及相变焓
Table 2 Phase transition temperature and enthalpy of n-octadecane and composite phase change materials during solidification process
| 样品 | 起始温度Tss/℃ | 峰值Tsp/℃ | 终止温度Tse/℃ | 相变焓Hs/(J/g) |
|---|---|---|---|---|
| 正十八烷 | 27.8 | 26.9 | 23.2 | -225.8 |
| 1%(质量)改性石墨烯/正十八烷 | 27.9 | 27.0 | 23.1 | -222.9 |
| 2%(质量)改性石墨烯/正十八烷 | 28.2 | 27.2 | 23.2 | -220.2 |
| 3%(质量)改性石墨烯/正十八烷 | 28.4 | 26.9 | 23.2 | -218.1 |
| 4%(质量)改性石墨烯/正十八烷 | 28.4 | 27.1 | 22.9 | -214.5 |
| 样品 | 热扩散系数/(mm2/s) | 密度/(g/cm3) | 比热容/(J/(g·K)) | 热导率/(W/(m·K)) |
|---|---|---|---|---|
| 正十八烷 | 0.137 | 0.777 | 1.658 | 0.177 |
| 1%(质量)改性石墨烯/正十八烷 | 0.184 | 0.771 | 2.007 | 0.285 |
| 2%(质量)改性石墨烯/正十八烷 | 0.230 | 0.766 | 1.973 | 0.338 |
| 3%(质量)改性石墨烯/正十八烷 | 0.266 | 0.760 | 1.934 | 0.391 |
| 4%(质量)改性石墨烯/正十八烷 | 0.278 | 0.755 | 1.950 | 0.409 |
表3 正十八烷及其复合相变材料的热导率及相关数据
Table 3 Thermal conductivity and relevant data of n-octadecane and composite phase change materials
| 样品 | 热扩散系数/(mm2/s) | 密度/(g/cm3) | 比热容/(J/(g·K)) | 热导率/(W/(m·K)) |
|---|---|---|---|---|
| 正十八烷 | 0.137 | 0.777 | 1.658 | 0.177 |
| 1%(质量)改性石墨烯/正十八烷 | 0.184 | 0.771 | 2.007 | 0.285 |
| 2%(质量)改性石墨烯/正十八烷 | 0.230 | 0.766 | 1.973 | 0.338 |
| 3%(质量)改性石墨烯/正十八烷 | 0.266 | 0.760 | 1.934 | 0.391 |
| 4%(质量)改性石墨烯/正十八烷 | 0.278 | 0.755 | 1.950 | 0.409 |
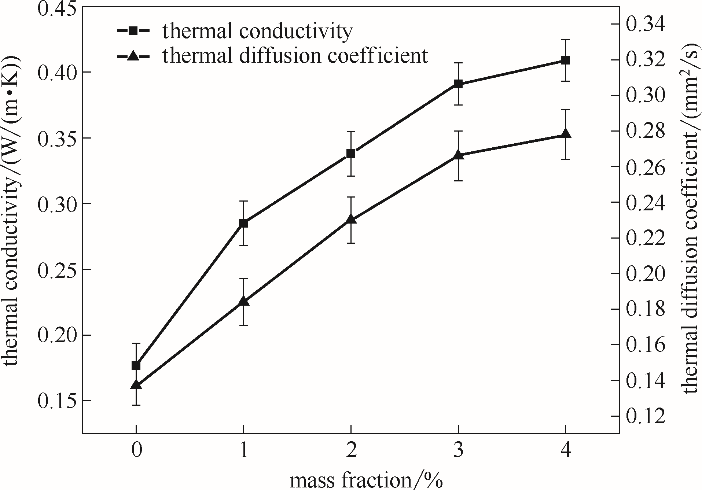
图8 不同质量分数的改性石墨烯/正十八烷复合相变材料在20℃时的热导率及热扩散系数
Fig.8 Thermal conductivity and thermal diffusion coefficient of modified graphene/n-octadecane composite phase change materials with different mass fractions at 20℃
| 1 | Zhang P, Ma Z W, Wang R Z. An overview of phase change material slurries: MPCS and CHS[J]. Renewable and Sustainable Energy Reviews, 2010, 14(2): 598-614. |
| 2 | Zhang Q, Wang H, Ling Z, et al. RT100/expand graphite composite phase change material with excellent structure stability, photo-thermal performance and good thermal reliability[J]. Solar Energy Materials and Solar Cells, 2015, 140: 158-166. |
| 3 | Zhang Z, Zhang N, Peng J, et al. Preparation and thermal energy storage properties of paraffin/expanded graphite composite phase change material[J]. Applied Energy, 2012, 91(1): 426-431. |
| 4 | Lin Y, Jia Y, Alva G, et al. Review on thermal conductivity enhancement, thermal properties and applications of phase change materials in thermal energy storage[J]. Renewable & Sustainable Energy Reviews, 2018, 82: 2730-2742. |
| 5 | Liu L K, Su D, Tang Y J, et al. Thermal conductivity enhancement of phase change materials for thermal energy storage: a review[J]. Renewable & Sustainable Energy Reviews, 2016, 62: 305-317. |
| 6 | Karaman S, Karaipekli A, Sari A, et al. Polyethylene glycol (PEG)/diatomite composite as a novel form-stable phase change material for thermal energy storage[J]. Solar Energy Materials & Solar Cells, 2011, 95(7): 1647-1653. |
| 7 | Dao T D, Jeong H M. Novel stearic acid/graphene core–shell composite microcapsule as a phase change material exhibiting high shape stability and performance[J]. Solar Energy Materials and Solar Cells, 2015, 137: 227-234. |
| 8 | Iwata H, Oodate M, Uyama Y, et al. Preparation of temperature-sensitive membranes by grafting polymerization onto a porous membrane[J]. J. Membrane Sci., 1991, 55: 119-124. |
| 9 | Okahata Y, Noguchi H, Seki T. Thermoselective permeation from a polymer-grafted capsule membrane[J]. Macromolecules, 1986, 19: 493-499. |
| 10 | Chu L Y, Park S H, Yamaguchi T, et al. Preparation of thermo-responsive core-shell microcapsule with a porous membrane and poly(N-isopropylacrylamide) gates[J]. J. Membrane Sci., 2001, 192: 27-37. |
| 11 | Peng T, Cheng Y L. Temperature-responsive permeability of porous PNIPAAm-g-PE membranes[J]. J. Appl. Polym. Sci., 1998, 70: 2133-2140. |
| 12 | Ito Y, Ochiai Y, Park Y S, et al. pH-sensitive gating by conformational change of a polypeptide brush grafted onto a porous polymer membrane[J]. J. Am. Chem. Soc., 1997, 119: 1619-1631. |
| 13 | Ito Y, Park Y S, Imanishi Y. Visualization of critical pH-controlled gating of a porous membrane grafted with polyelectrolyte brushes[J]. J. Am. Chem. Soc., 1997, 119: 2739-2755. |
| 14 | Chung D J, Ito Y, Imanishi Y. Preparation of porous membranes grafted with poly(spiropyran-containing methacrylate) and photo control of permeability[J]. J. Appl. Polym. Sci., 1994, 51: 2027-2040. |
| 15 | Tan S H, Mohamedali A, Kapur A, et al. A novel, cost-effective and efficient chicken egg IgY purification procedure[J]. Journal of Immunological Methods, 2012, 380(1/2): 73-76. |
| 16 | Marcet I, Laca A, Paredes B, et al. IgY isolation from a watery by-product obtained from an egg yolk fractionation process[J]. Food and Bioproducts Processing, 2011, (2): 87-91. |
| 17 | Dao T D, Jeong H M. A Pickering emulsion route to a stearic acid/graphene core-shell composite phase change material[J]. Carbon, 2016, 99: 49-57. |
| 18 | Mehrali M, Latibari S T, Mehrali M, et al. Preparation of nitrogen-doped graphene/palmitic acid shape stabilized composite phase change material with remarkable thermal properties for thermal energy storage[J]. Appl. Energy, 2014, 135: 339-349. |
| 19 | 蔡迪, 李静, 焦乃勋. 纳米石墨烯片-正十八烷复合相变材料制备及热物性研究[J]. 物理学报, 2019, 68(10): 100502. |
| Cai D, Li J, Jiao N X. Preparation and thermophysical properties of graphene nanoplatelets-octadecane phase change composite materials[J]. Acta Physica Sinica, 2019, 68(10): 100502. | |
| 20 | 陈轩. 含纳米颗粒相变复合储能材料的强化传热机理与工艺研究[D]. 哈尔滨: 哈尔滨工程大学, 2016. |
| Chen X. Research on heat transfer mechanism and optimization of experiment process of nanoparticles containing phase change composite[D]. Harbin: Harbin Engineering University, 2016. | |
| 21 | 张金辉. 石蜡及其复合相变材料的热物性研究[D]. 青岛: 青岛科技大学, 2014. |
| Zhang J H. Thermophysical properties of paraffin wax and its composite phase change materials[D]. Qingdao: Qingdao University of Science and Technology, 2014. | |
| 22 | Wu S, Li T X, Yan T, et al. High performance form-stable expanded graphite/stearic acid composite phase change material for modular thermal energy storage[J]. International Journal of Heat and Mass Transfer, 2016, 102: 733-744. |
| 23 | Tahani M, et al. Experimental evaluation and ANN modeling of thermal conductivity of graphene oxide nanoplatelets/deionized water nanofluid[J]. International Communications in Heat and Mass Transfer, 2016, 76: 358-365. |
| 24 | Alazmi A, Rasul S, Patole S P, et al. Comparative study of synthesis and reduction methods for graphene oxide[J]. Polyhedron, 2016, 116: 153-161. |
| 25 | Yuan K, Wang H, Liu J, et al. Novel slurry containing graphene oxide-grafted microencapsulated phase change material with enhanced thermo-physical properties and photo-thermal performance[J]. Solar Energy Materials & Solar Cells, 2015, 143(143): 29-37. |
| 26 | Stengl V, Bakardjieva S, Bakardjiev M, et al. Carborane functionalized graphene oxide, a precursor for conductive self-assembled monolayers[J]. Carbon, 2014, 67: 336-343. |
| 27 | 周建伟, 程玉良, 王储备, 等. 硬脂酸/氧化石墨烯复合相变储热材料研究[J]. 化工新型材料, 2013, 41(6): 47-49. |
| Zhou J W, Cheng Y L, Wang C B, et al. Study on stearic acid/ graphene oxide composite phase-change for thermal storage[J]. New Chemical Materials, 2013, 41(6): 47-49. | |
| 28 | 王赫, 王建平, 王艳, 等. 加入改性石墨烯的聚甲基丙烯酸甲酯/正十八烷相变材料微胶囊的制备与表征[J]. 化工新型材料, 2014, 42(1): 118-121. |
| Wang H, Wang J P, Wang Y, et al. Preparation and characterization of microcapsules of graphite modified poly(methyl methacrylate)/n-octadecane phase change material[J]. New Chemical Materials, 2014, 42(1): 118-121. | |
| 29 | 陈素清, 黄国波, 鲍建设, 等. 石墨烯导热增强相变储能材料的制备及性能[J]. 新型炭材料, 2018, 33(3): 262-267. |
| Chen S Q, Huang G B, Bao J S, et al. Preparation and thermal properties of phase change material modified by a functionalized reduced graphene oxide[J]. New Carbon Materials, 2018, 33(3): 262-267. | |
| 30 | 刘焕炳. 聚乙二醇正十六烷基醚接枝氧化石墨烯相变材料的制备及性能研究[D].天津: 天津工业大学, 2018. |
| Liu H B. Preparation and properties of polyethylene glycol N-cetyl ether grafted go phase change materials[D]. Tianjin: Tianjin University of Technology, 2018. | |
| 31 | 杨志涛, 张军强, 宗冬冬, 等. SiO2改性石墨烯-石蜡复合相变乳液的制备及热性能[J]. 新能源进展, 2017, 5(2): 110-116. |
| Yang Z T, Zhang J Q, Zong D D, et al. Preparation and thermal properties of SiO2 modified graphene-paraffin composite phase change emulsion[J]. Advances in New and Renewable Energy, 2017, 5(2): 110-116. | |
| 32 | Wang Y, Liu Z, Zhang T, et al. Preparation and characterization of graphene oxide-grafted hexadecanol composite phase-change material for thermal energy storage[J]. Energy Technology, 2017, 5(11): 2005-2014. |
| [1] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [2] | 代佳琳, 毕唯东, 雍玉梅, 陈文强, 莫晗旸, 孙兵, 杨超. 热物性对混合型CPCMs固液相变特性影响模拟研究[J]. 化工学报, 2023, 74(5): 1914-1927. |
| [3] | 杜江龙, 杨雯棋, 黄凯, 练成, 刘洪来. 复合相变材料/空冷复合式锂离子电池模块散热性能[J]. 化工学报, 2023, 74(2): 674-689. |
| [4] | 余后川, 任腾, 张宁, 姜晓滨, 代岩, 张晓鹏, 鲍军江, 贺高红. 二维氧化石墨烯膜离子选择性传递调控的研究进展[J]. 化工学报, 2023, 74(1): 303-312. |
| [5] | 李智超, 郑瑜, 张润楠, 姜忠义. 高通量抗污染氧化石墨烯膜研究进展[J]. 化工学报, 2022, 73(6): 2370-2380. |
| [6] | 张苗, 杨洪海, 尹勇, 徐悦, 沈俊杰, 卢心诚, 施伟刚, 王军. 氧化石墨烯/水脉动热管的启动及传热特性[J]. 化工学报, 2022, 73(3): 1136-1146. |
| [7] | 徐欢, 柯律, 张生辉, 张子林, 韩广东, 崔金声, 唐道远, 黄东辉, 高杰峰, 何新建. GO表面原位生长CNTs改善聚丙烯导热复合材料分散与界面形态[J]. 化工学报, 2022, 73(11): 5150-5157. |
| [8] | 林肯, 许肖永, 李强, 胡定华. 石蜡-膨胀石墨复合相变材料热导率研究[J]. 化工学报, 2021, 72(8): 4425-4432. |
| [9] | 夏东, 黄朋, 李恒. 水热法制备三维导电石墨烯气凝胶及其焦耳热性能研究[J]. 化工学报, 2021, 72(7): 3839-3848. |
| [10] | 张文波, 凌子夜, 方晓明, 张正国. 新型六水氯化镁-六水硝酸镁/石墨相氮化碳复合相变材料的制备及其热性能研究[J]. 化工学报, 2021, 72(12): 6399-6406. |
| [11] | 奥德, 张皓冰, 吕美婵, 王海涛, 常娜. MOF-199@GO改性PVDF荷电纳滤膜的制备及其性能[J]. 化工学报, 2020, 71(S2): 297-305. |
| [12] | 田隆, 刘婷, 孙克宁. 用于水质净化的氧化石墨烯膜研究进展[J]. 化工学报, 2020, 71(9): 4112-4130. |
| [13] | 冯雪廷, 矫庆泽, 李群, 冯彩虹, 赵芸, 黎汉生, 李海军, 蔡惠群. NiCo2S4/N,S-rGO纳米复合材料的制备和电化学储钠性能[J]. 化工学报, 2020, 71(9): 4314-4324. |
| [14] | 汪菊, 牛淑锋, 费莹, 漆虹. GO/Al2O3复合纳滤膜的制备及其稳定性能研究[J]. 化工学报, 2020, 71(6): 2795-2803. |
| [15] | 毛东阳, 杨丹, 范杰平. 氧化石墨烯杂化分子印迹复合膜制备及性能研究[J]. 化工学报, 2020, 71(6): 2900-2911. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号
