化工学报 ›› 2021, Vol. 72 ›› Issue (12): 6399-6406.DOI: 10.11949/0438-1157.20211067
• 材料化学工程与纳米技术 • 上一篇
收稿日期:2021-08-02
修回日期:2021-09-23
出版日期:2021-12-05
发布日期:2021-12-22
通讯作者:
张正国
作者简介:张文波(1994—),男,博士研究生,基金资助:
Wenbo ZHANG1( ),Ziye LING1,2,Xiaoming FANG1,2,Zhengguo ZHANG1,2(
),Ziye LING1,2,Xiaoming FANG1,2,Zhengguo ZHANG1,2( )
)
Received:2021-08-02
Revised:2021-09-23
Online:2021-12-05
Published:2021-12-22
Contact:
Zhengguo ZHANG
摘要:
我国青海盐湖镁资源的利用率低,带来严重的资源浪费和环境污染,若将镁盐开发为相变储热材料,则可扩大其应用领域,从而促进镁盐资源的充分利用。针对中低温保温隔热的应用需求,在前期工作基础上,选用MgCl2·6H2O-Mg(NO3)2·6H2O(MCH-MNH)质量比为41∶59的共晶盐作为相变材料,为降低其过冷度并提高隔热性能,选用多孔介质石墨相氮化碳(g-C3N4,CN)作为支撑材料,制备低热导率的MCH-MNH/CN复合相变材料。先将尿素在550℃高温下煅烧得到多孔的CN,再采用吸附法制备出MCH-MNH/CN复合相变材料,并对复合相变材料的形貌、结构与热性能进行了表征和测量。结果表明,共晶盐相变材料均匀地吸附在CN的微孔结构内,其与CN的复合是一个物理过程,没有发生化学反应;复合相变材料的相变温度为55.2℃,相变焓值为92.7 J/g,几乎没有过冷度,其热导率为0.3 W/(m·K),仅是共晶盐MCH-MNH的一半,提高了隔热性能。此外,复合相变材料还具有良好的热稳定性,在中低温保温隔热领域具有应用前景。
中图分类号:
张文波, 凌子夜, 方晓明, 张正国. 新型六水氯化镁-六水硝酸镁/石墨相氮化碳复合相变材料的制备及其热性能研究[J]. 化工学报, 2021, 72(12): 6399-6406.
Wenbo ZHANG, Ziye LING, Xiaoming FANG, Zhengguo ZHANG. Preparation and thermal properties research of a novel magnesium chloride hexahydrate-magnesium nitrate hexahydrate/graphite phase carbon nitride composite phase change material[J]. CIESC Journal, 2021, 72(12): 6399-6406.
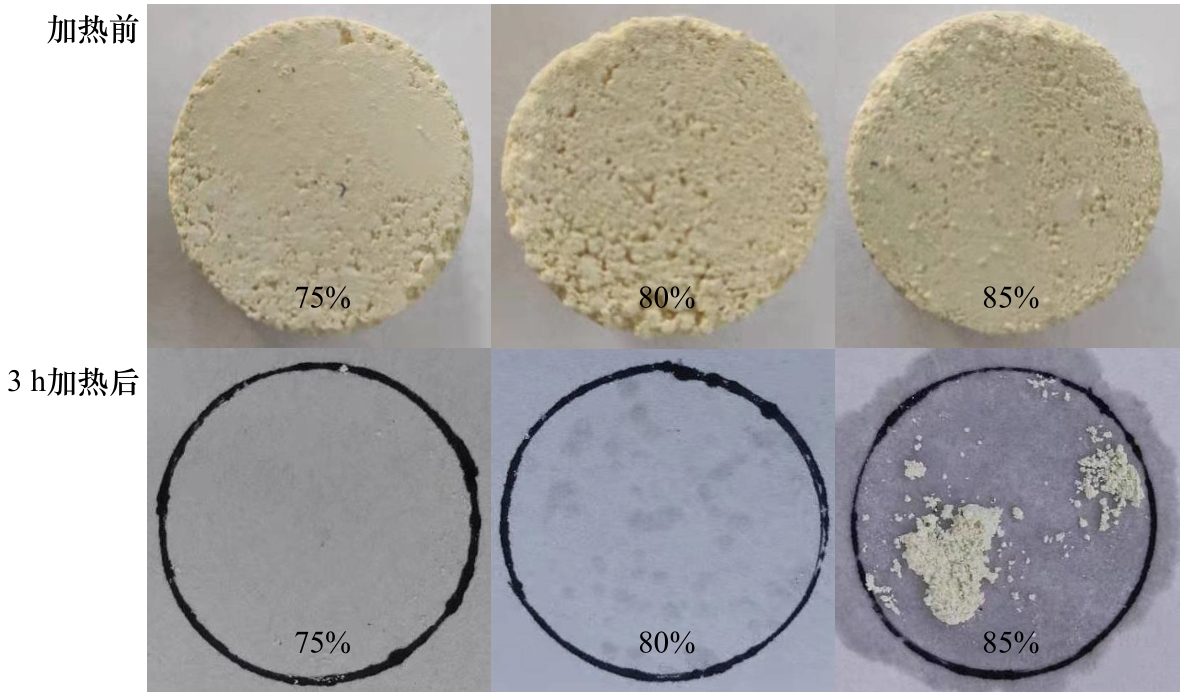
图1 MCH-MNH质量分数分别为75%、80%、85%的MCH-MNH/CN复合相变材料的液漏痕迹照片
Fig.1 Photographs of the liquid leakage traces of MCH-MNH/CN composite phase change materials with 75%, 80%, and 85% mass fractions of MCH-MNH
| MCH-MNH质量分数 | 加热前质量/g | 加热后质量/g | 质量变化/g |
|---|---|---|---|
| 75% | 0.31 | 0.32 | 0.01 |
| 80% | 0.32 | 0.32 | 0 |
| 85% | 0.32 | 0.56 | 0.24 |
表1 MCH-MNH/CN复合相变材料3 h加热后滤纸质量变化
Table 1 Mass variation of the filter paper with MCH-MNH/CNcomposite phase change material before and after heating for 3 h
| MCH-MNH质量分数 | 加热前质量/g | 加热后质量/g | 质量变化/g |
|---|---|---|---|
| 75% | 0.31 | 0.32 | 0.01 |
| 80% | 0.32 | 0.32 | 0 |
| 85% | 0.32 | 0.56 | 0.24 |

图2 CN(a)、MCH-MNH/CN复合相变材料(b)、MCH-MNH/CN复合相变材料循环50次后(c)的SEM图
Fig.2 SEM images of CN (a) and MCH-MNH/CN composite phase change material before (b) and after (c) 50 thermal cycles
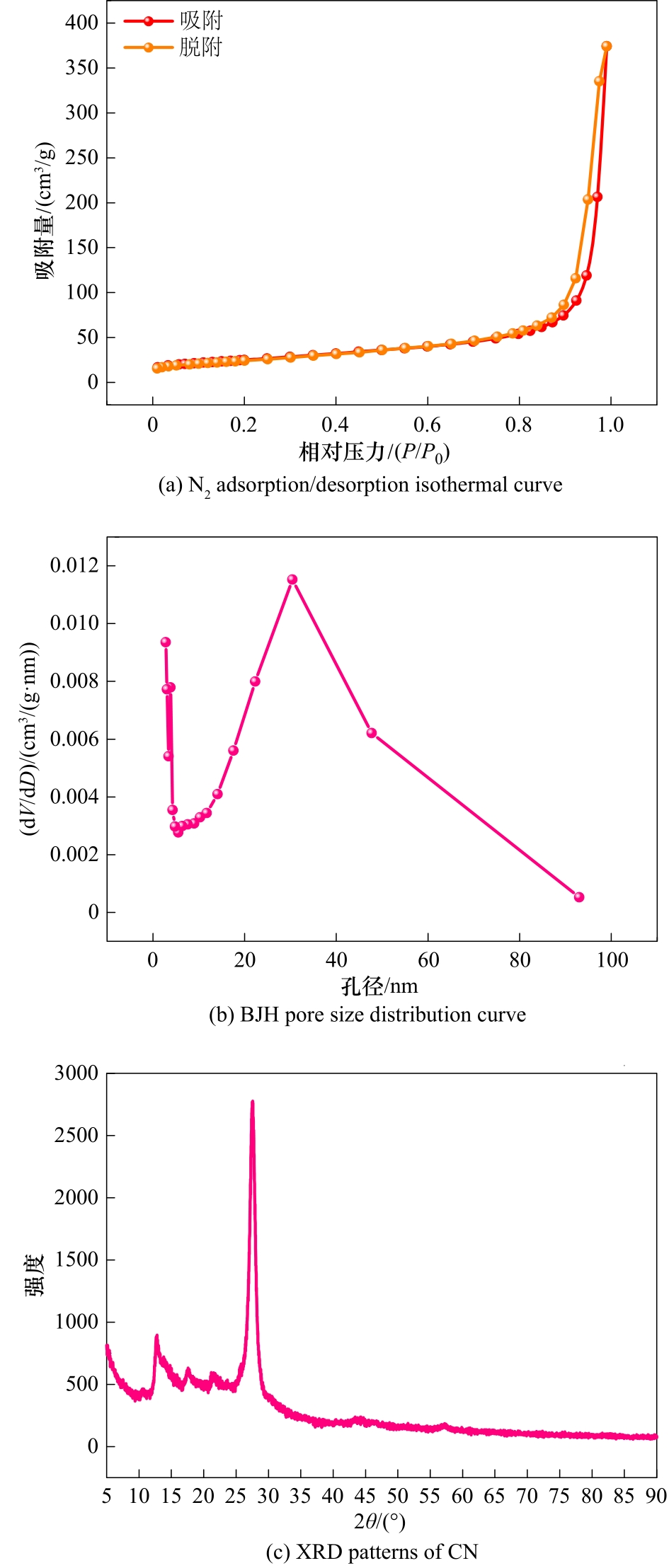
图3 CN的N2吸附/脱附等温曲线(a),BJH孔径分布曲线(b)和XRD谱图(c)
Fig.3 N2 adsorption/desorption isothermal curve (a) , BJH pore size distribution curve (b) and XRD patterns (c) of CN
| 样品 | 熔化温度/ ℃ | 凝固温度/ ℃ | 熔化焓值/ (J/g) | 凝固焓值/ (J/g) |
|---|---|---|---|---|
| MCH-MNH | 57.7±0.8 | 38.6 | 119.8 | 110.6 |
| MCH-MNH/CN | 55.2±0.7 | 42.8 | 92.7 | 88.1 |
表2 MCH-MNH、MCH-MNH/CN复合相变材料的相变特征
Table 2 Phase transformation characteristics of MCH-MNH and MCH-MNH/CNcomposite phase change material
| 样品 | 熔化温度/ ℃ | 凝固温度/ ℃ | 熔化焓值/ (J/g) | 凝固焓值/ (J/g) |
|---|---|---|---|---|
| MCH-MNH | 57.7±0.8 | 38.6 | 119.8 | 110.6 |
| MCH-MNH/CN | 55.2±0.7 | 42.8 | 92.7 | 88.1 |
| 样品 | 热导率/(W/(m·K)) |
|---|---|
| MCH-MNH | 0.62 |
| MCH-MNH/CN | 0.30 |
表3 MCH-MNH、MCH-MNH/CN复合相变材料的热导率
Table 3 Thermal conductivity of MCH-MNH, MCH-MNH/CN composite phase change material
| 样品 | 热导率/(W/(m·K)) |
|---|---|
| MCH-MNH | 0.62 |
| MCH-MNH/CN | 0.30 |

图8 MCH-MNH、MCH-MNH/CN复合相变材料冷热循环50次的DSC曲线
Fig.8 DSC curves of MCH-MNH and MCH-MNH/CN composite phase change material before and after 50 thermal cycles
| 样品 | 循环次数 | 熔化温度/℃ | 熔化焓值/(J/g) |
|---|---|---|---|
| MCH-MNH | 0 | 57.7 | 119.8 |
| 50 | 31.2 | 59.0 | |
| MCH-MNH/CN | 0 | 55.2 | 92.7 |
| 50 | 56.7 | 90.9 |
表4 MCH-MNH、MCH-MNH/CN复合相变材料冷热循环50次前后的相变特征
Table 4 Phase transformation characteristics of MCH-MNH and MCH-MNH/CNcomposite phase change material before and after 50 thermal cycles
| 样品 | 循环次数 | 熔化温度/℃ | 熔化焓值/(J/g) |
|---|---|---|---|
| MCH-MNH | 0 | 57.7 | 119.8 |
| 50 | 31.2 | 59.0 | |
| MCH-MNH/CN | 0 | 55.2 | 92.7 |
| 50 | 56.7 | 90.9 |
| 1 | 史巍, 王传涛. 相变材料研究综述[J]. 硅酸盐通报, 2015, 34(12): 3517-3522. |
| Shi W, Wang C T. Study review on phase change materials[J]. Bulletin of the Chinese Ceramic Society, 2015, 34(12): 3517-3522. | |
| 2 | Hirmiz R, Teamah H M, Lightstone M F, et al. Performance of heat pump integrated phase change material thermal storage for electric load shifting in building demand side management[J]. Energy and Buildings, 2019, 190: 103-118. |
| 3 | Pandey A K, Hossain M S, Tyagi V V, et al. Novel approaches and recent developments on potential applications of phase change materials in solar energy[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 281-323. |
| 4 | Khadiran T, Hussein M Z, Zainal Z, et al. Advanced energy storage materials for building applications and their thermal performance characterization: a review[J]. Renewable and Sustainable Energy Reviews, 2016, 57: 916-928. |
| 5 | Maleki M, Karimian H, Shokouhimehr M, et al. Development of graphitic domains in carbon foams for high efficient electro/photo-to-thermal energy conversion phase change composites[J]. Chemical Engineering Journal, 2019, 362: 469-481. |
| 6 | Fu L L, Wang Q H, Ye R D, et al. A calcium chloride hexahydrate/expanded perlite composite with good heat storage and insulation properties for building energy conservation[J]. Renewable Energy, 2017, 114: 733-743. |
| 7 | Huang R, Feng J X, Ling Z Y, et al. A sodium acetate trihydrate-formamide/expanded perlite composite with high latent heat and suitable phase change temperatures for use in building roof[J]. Construction and Building Materials, 2019, 226: 859-867. |
| 8 | Zalba B, Marı́n J M, Cabeza L F, et al. Review on thermal energy storage with phase change: materials, heat transfer analysis and applications[J]. Applied Thermal Engineering, 2003, 23(3): 251-283. |
| 9 | 王会春, 凌子夜, 方晓明, 等. 六水氯化镁相变储热材料的研究进展[J]. 储能科学与技术, 2017, 6(2): 204-212. |
| Wang H C, Ling Z Y, Fang X M, et al. Recent progress in the use of magnesium chloride hexahydrate used as a phase change material[J]. Energy Storage Science and Technology, 2017, 6(2): 204-212. | |
| 10 | Pilar R, Svoboda L, Honcova P, et al. Study of magnesium chloride hexahydrate as heat storage material[J]. Thermochimica Acta, 2012, 546: 81-86. |
| 11 | Choi J C, Kim S D. Heat transfer in a latent heat-storage system using MgCl2·6H2O at the melting point[J]. Energy, 1995, 20(1): 13-25. |
| 12 | Gutierrez A, Ushak S, Galleguillos H, et al. Use of polyethylene glycol for the improvement of the cycling stability of bischofite as thermal energy storage material[J]. Applied Energy, 2015, 154: 616-621. |
| 13 | Zahir M H, Mohamed S A, Saidur R, et al. Supercooling of phase-change materials and the techniques used to mitigate the phenomenon[J]. Applied Energy, 2019, 240: 793-817. |
| 14 | 李玉婷, 周永全, 葛飞, 等. 无机水合盐相变储能材料的过冷及相分离研究进展[J]. 盐湖研究, 2018, 26(1): 81-86. |
| Li Y T, Zhou Y Q, Ge F, et al. Supercooling and phase separation of inorganic salt hydrates as phase change materials[J]. Journal of Salt Lake Research, 2018, 26(1): 81-86. | |
| 15 | El-Sebaii A A, Al-Heniti S, Al-Agel F, et al. One thousand thermal cycles of magnesium chloride hexahydrate as a promising PCM for indoor solar cooking[J]. Energy Conversion and Management, 2011, 52(4): 1771-1777. |
| 16 | Nagano K, Ogawa K, Mochida T, et al. Thermal characteristics of magnesium nitrate hexahydrate and magnesium chloride hexahydrate mixture as a phase change material for effective utilization of urban waste heat[J]. Applied Thermal Engineering, 2004, 24(2/3): 221-232. |
| 17 | Nagano K, Ogawa K, Mochida T, et al. Performance of heat charge/discharge of magnesium nitrate hexahydrate and magnesium chloride hexahydrate mixture to a single vertical tube for a latent heat storage system[J]. Applied Thermal Engineering, 2004, 24(2/3): 209-220. |
| 18 | Galazutdinova Y, Vega M, Grágeda M, et al. Preparation and characterization of an inorganic magnesium chloride/nitrate/graphite composite for low temperature energy storage[J]. Solar Energy Materials and Solar Cells, 2018, 175: 60-70. |
| 19 | Zhou Y, Sun W C, Ling Z Y, et al. Hydrophilic modification of expanded graphite to prepare a high-performance composite phase change block containing a hydrate salt[J]. Industrial & Engineering Chemistry Research, 2017, 56(50): 14799-14806. |
| 20 | Zhou S Y, Zhou Y, Ling Z Y, et al. Modification of expanded graphite and its adsorption for hydrated salt to prepare composite PCMs[J]. Applied Thermal Engineering, 2018, 133: 446-451. |
| 21 | Ling Z Y, Liu J W, Wang Q H, et al. MgCl2·6H2O-Mg(NO3)2·6H2O eutectic/SiO2 composite phase change material with improved thermal reliability and enhanced thermal conductivity[J]. Solar Energy Materials and Solar Cells, 2017, 172: 195-201. |
| 22 | Zhang C, Zhang Z Y, Ye R D, et al. Characterization of MgCl2·6H2O-based eutectic/expanded perlite composite phase change material with low thermal conductivity[J]. Materials, 2018, 11(12): 2369. |
| 23 | Ling Z Y, Li S M, Zhang Z G, et al. A shape-stabilized MgCl2·6H2O-Mg(NO3)2·6H2O/expanded graphite composite phase change material with high thermal conductivity and stability[J]. Journal of Applied Electrochemistry, 2018, 48(10): 1131-1138. |
| 24 | Liu J H, Zhang T K, Wang Z C, et al. Simple pyrolysis of urea into graphitic carbon nitride with recyclable adsorption and photocatalytic activity[J]. Journal of Materials Chemistry, 2011, 21(38): 14398. |
| 25 | Yuan Y P, Xu W T, Yin L S, et al. Large impact of heating time on physical properties and photocatalytic H2 production of g-C3N4 nanosheets synthesized through urea polymerization in Ar atmosphere[J]. International Journal of Hydrogen Energy, 2013, 38(30): 13159-13163. |
| 26 | Zhang Y W, Liu J H, Wu G, et al. Porous graphitic carbon nitride synthesized via direct polymerization of urea for efficient sunlight-driven photocatalytic hydrogen production[J]. Nanoscale, 2012, 4(17): 5300-5303. |
| 27 | Mo Z, She X J, Li Y P, et al. Synthesis of g-C3N4 at different temperatures for superior visible/UV photocatalytic performance and photoelectrochemical sensing of MB solution[J]. RSC Advances, 2015, 5(123): 101552-101562. |
| 28 | Gu Q, Gao Z W, Zhao H, et al. Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light[J]. RSC Advances, 2015, 5(61): 49317-49325. |
| 29 | Martin D J, Qiu K P, Shevlin S A, et al. Highly efficient photocatalytic H2 evolution from water using visible light and structure-controlled graphitic carbon nitride[J]. Angewandte Chemie, 2014, 126(35): 9394-9399. |
| 30 | Niu P, Yin L C, Yang Y Q, et al. Increasing the visible light absorption of graphitic carbon nitride (melon) photocatalysts by homogeneous self-modification with nitrogen vacancies[J]. Advanced Materials, 2014, 26(47): 8046-8052. |
| 31 | Hwang S, Lee S, Yu J S. Template-directed synthesis of highly ordered nanoporous graphitic carbon nitride through polymerization of cyanamide[J]. Applied Surface Science, 2007, 253(13): 5656-5659. |
| 32 | Lu X, Huang H W, Zhang X Y, et al. Novel light-driven and electro-driven polyethylene glycol/two-dimensional MXene form-stable phase change material with enhanced thermal conductivity and electrical conductivity for thermal energy storage[J]. Composites Part B: Engineering, 2019, 177: 107372. |
| 33 | Ling Z Y, Li S M, Cai C Y, et al. Battery thermal management based on multiscale encapsulated inorganic phase change material of high stability[J]. Applied Thermal Engineering, 2021, 193: 117002. |
| [1] | 郑玉圆, 葛志伟, 韩翔宇, 王亮, 陈海生. 中高温钙基材料热化学储热的研究进展与展望[J]. 化工学报, 2023, 74(8): 3171-3192. |
| [2] | 刘润竹, 储甜甜, 张孝阿, 王成忠, 张军营. α,ω-端羟基亚苯基氟硅聚合物的合成及性能[J]. 化工学报, 2023, 74(3): 1360-1369. |
| [3] | 杜江龙, 杨雯棋, 黄凯, 练成, 刘洪来. 复合相变材料/空冷复合式锂离子电池模块散热性能[J]. 化工学报, 2023, 74(2): 674-689. |
| [4] | 宋超宇, 熊亚选, 张金花, 金宇贺, 药晨华, 王辉祥, 丁玉龙. 污泥焚烧炉渣基定型复合相变储热材料的制备和性能[J]. 化工学报, 2022, 73(5): 2279-2287. |
| [5] | 陈子禾, 赵呈志, 冒文莉, 盛楠, 朱春宇. 定向生物质多孔碳复合相变材料的制备及其热性能研究[J]. 化工学报, 2022, 73(4): 1817-1825. |
| [6] | 孔昕山, 黄仁星, 康丽霞, 刘永忠. 甲醇模块化生产中分时储热系统的优化设计[J]. 化工学报, 2022, 73(2): 770-781. |
| [7] | 沈永亮, 张朋威, 刘淑丽. 肋片和多孔介质强化梯级相变储热系统性能的对比研究[J]. 化工学报, 2022, 73(10): 4366-4376. |
| [8] | 张欣宇, 杨晓宏, 张燕楠, 徐佳锟, 郭枭, 田瑞. 基于二维梯度树状肋相变储热系统强化传热机理[J]. 化工学报, 2022, 73(10): 4399-4409. |
| [9] | 罗伟莉, 王雯雯, 潘权稳, 葛天舒, 王如竹. 基于活性碳纤维毡复合吸附剂的储热性能[J]. 化工学报, 2021, 72(S1): 554-559. |
| [10] | 林肯, 许肖永, 李强, 胡定华. 石蜡-膨胀石墨复合相变材料热导率研究[J]. 化工学报, 2021, 72(8): 4425-4432. |
| [11] | 魏小兰, 谢佩, 王维龙, 陆建峰, 丁静. 含钙三元氯化物体系相图计算与熔盐热稳定性[J]. 化工学报, 2021, 72(6): 3074-3083. |
| [12] | 高剑晨, 赵炳晨, 何峰, 李廷贤. 六水硝酸镁相变储热复合材料改性制备及储/放热性能研究[J]. 化工学报, 2021, 72(6): 3328-3337. |
| [13] | 熊亚选, 钱向瑶, 李烁, 孙明远, 王振宇, 吴玉庭, 徐鹏, 丁玉龙, 马重芳. 制备方法对纳米熔盐储热性能及形成机理的影响[J]. 化工学报, 2021, 72(5): 2857-2868. |
| [14] | 忻睦迪, 邢恩会. 三甲基膦和金属氧化物复合改性ZSM-5分子筛及其裂解性能研究[J]. 化工学报, 2021, 72(5): 2657-2668. |
| [15] | 李威, 王秋旺, 曾敏. 水合盐基中低温热化学储热材料性能测试及数值研究[J]. 化工学报, 2021, 72(5): 2763-2772. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号