化工学报 ›› 2021, Vol. 72 ›› Issue (1): 609-618.DOI: 10.11949/0438-1157.20200836
收稿日期:2020-06-29
修回日期:2020-09-07
出版日期:2021-01-05
发布日期:2021-01-05
通讯作者:
江浩
作者简介:朱华威(1997—),男,博士研究生,基金资助:
ZHU Huawei( ),YU Haifeng,JIANG Qianqian,YANG Zhaofeng,JIANG Hao(
),YU Haifeng,JIANG Qianqian,YANG Zhaofeng,JIANG Hao( ),LI Chunzhong
),LI Chunzhong
Received:2020-06-29
Revised:2020-09-07
Online:2021-01-05
Published:2021-01-05
Contact:
JIANG Hao
摘要:
高键能异质原子的高效掺杂是稳定高电压LiNi0.5Co0.2Mn0.3O2(NCM)三元正极材料并提升其电化学性能的有效策略。借助含硼前体在二次颗粒表面富集及随后高温煅烧强化B3+体相扩散的策略,构建了硼离子高效掺杂NCM正极材料(NCM-B)。引入B—O键(键能:809 kJ·mol-1)抑制了电化学反应过程中晶格氧析出,进而稳定材料的氧离子框架;此外,表面残余的高锂离子导体Li2O-B2O3包覆层可以在一定程度上稳定电极-电解液界面。与改性前NCM相比,改性后的NCM-B正极材料在3.0~4.5 V电压区间的可逆比电容量可以达到193.7 mA·h·g-1,在10 C大功率下,比电容量仍保持120 mA·h·g-1(NCM仅为78.2 mA·h·g-1)。1 C下连续循环100圈后,比电容量保持率从73%提升到90%。表面富集和扩散强化的思想也有望实现其他正极材料的高效掺杂。
中图分类号:
朱华威, 余海峰, 江仟仟, 杨兆峰, 江浩, 李春忠. 硼高效掺杂LiNi0.5Co0.2Mn0.3O2正极材料及其性能提升机制[J]. 化工学报, 2021, 72(1): 609-618.
ZHU Huawei, YU Haifeng, JIANG Qianqian, YANG Zhaofeng, JIANG Hao, LI Chunzhong. Synthesis and performance improvement mechanism of high-efficiency B doped LiNi0.5Co0.2Mn0.3O2 cathode materials for Li-ion batteries[J]. CIESC Journal, 2021, 72(1): 609-618.

图1 NCM-B的制备示意图(a);NCM的SEM图(b)和TEM图[(c),(d)];B-coated NCM的SEM图(e)和TEM图[(f),(g)];NCM-B的SEM图(h)和TEM图[(i),(j)];NCM-B的不同刻蚀深度的XPS总谱图(k),O 1s谱图(l)和B 1s谱图(m)
Fig.1 Schematic illustration for the preparation of NCM-B (a); SEM (b), and TEM [(c),(d)] images of the NCM; SEM (e) and TEM [(f),(g)] images of the B-coated NCM; SEM (h) and TEM [(i),(j)] images of the NCM-B; XPS survey spectra (k), O 1s (l) and B 1s (m) XPS spectra of the NCM-B with different etching depth
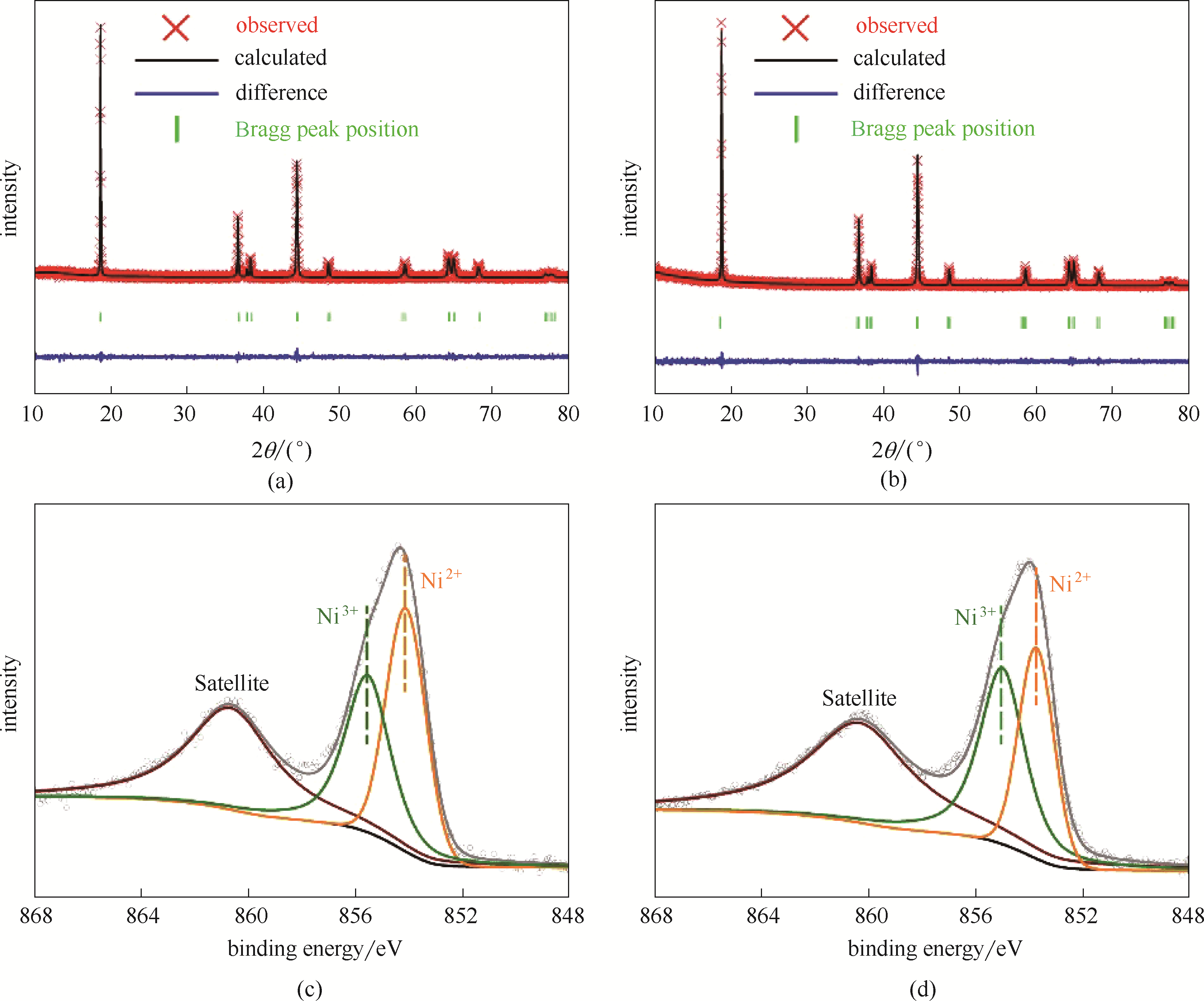
图2 NCM-B(a)和NCM(b)的XRD精修谱图; NCM-B(c)和NCM(d)的XPS Ni 2p3/2谱图
Fig.2 XRD Rietveld refinement patterns of the NCM-B (a) and NCM (b); Ni 2p3/2 XPS patterns of the NCM-B (c) and NCM (d)
| Sample | a/? | c/? | Volume/?3 | c/a | Rwp/% |
|---|---|---|---|---|---|
| NCM-B | 2.8703 | 14.2502 | 101.674 | 4.9620 | 14.68 |
| NCM | 2.8670 | 14.2289 | 101.291 | 4.9630 | 12.56 |
表1 根据XRD精修得到的NCM-B和NCM的晶格常数
Table 1 Lattice constants of the NCM-B and NCM calculated from X-ray Rietveld refinement
| Sample | a/? | c/? | Volume/?3 | c/a | Rwp/% |
|---|---|---|---|---|---|
| NCM-B | 2.8703 | 14.2502 | 101.674 | 4.9620 | 14.68 |
| NCM | 2.8670 | 14.2289 | 101.291 | 4.9630 | 12.56 |

图3 NCM-B和NCM的倍率(a)、循环性能(b)及其不同循环圈数的充放电曲线[(c),(d)]
Fig.3 Rate capability (a), cycling stability (b) and the corresponding charge-discharge curves [(c),(d)] of the NCM-B and the NCM for 100 cycles
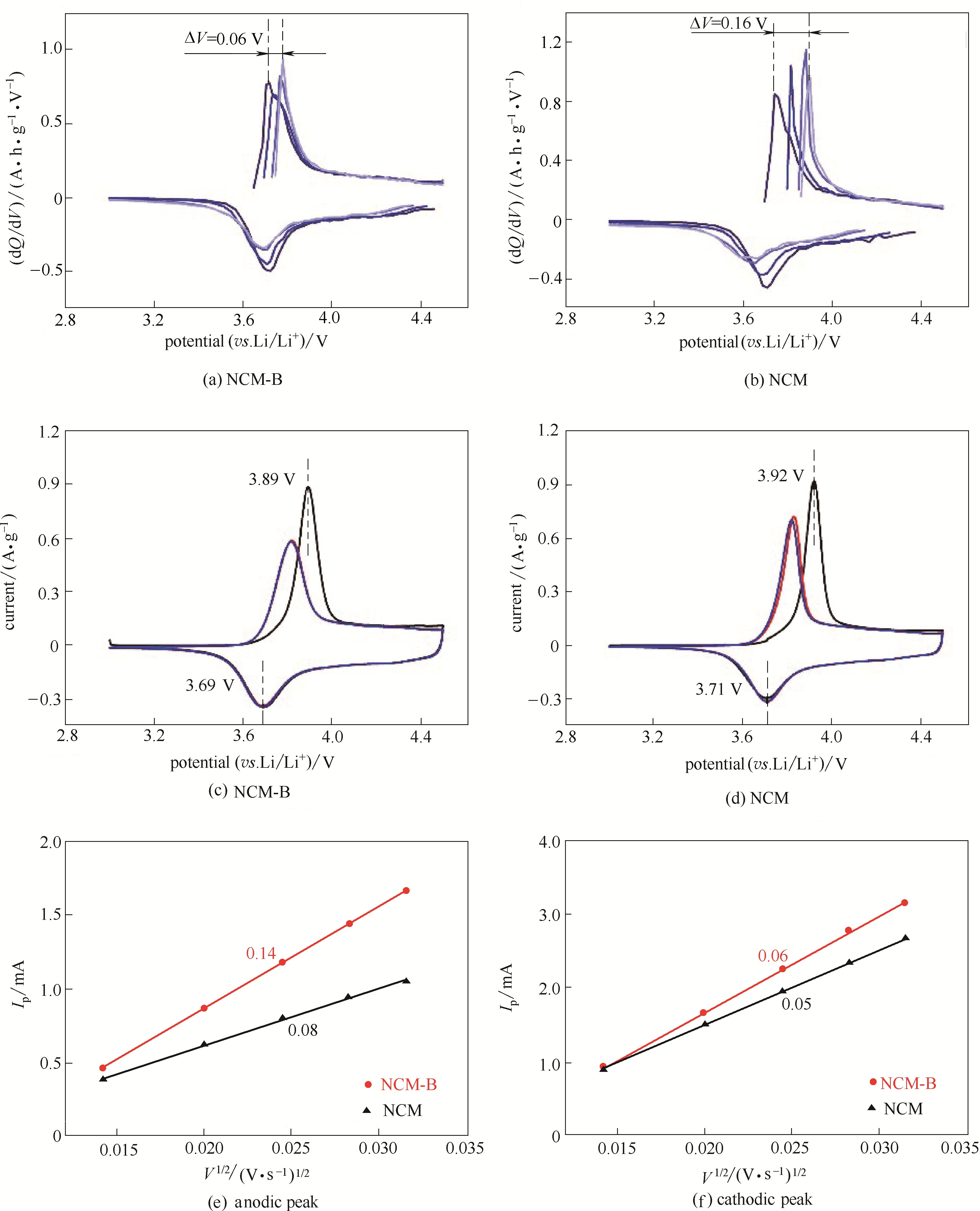
图4 NCM-B和NCM的dQ/dV曲线[(a),(b)]、前三圈循环伏安曲线[(c),(d)];NCM-B和NCM的峰电流和扫速平方根的线性关系图[(e),(f)]
Fig.4 The calculated dQ/dV profiles [(a),(b)], the initial three CV curves at 0.2 mV·s-1 [(c),(d)] of the NCM-B and the NCM;Linear relationship between the anodic/cathodic peak current (ip) and the square root of the scan rate (v1/2) of the NCM-B and the NCM [(e),(f)], respectively

图5 NCM-B和NCM在1 C电流密度下循环100圈后的XRD谱图[(a),(b)]和SEM图[(c),(d)]
Fig.5 XRD patterns [(a),(b)] and SEM images [(c),(d)] of the NCM-B and the NCM after 100 cycles at 1 C

图6 NCM-B和NCM在1 C下循环100圈后的C 1s[(a),(c)]和O 1s XPS[(b),(d)]谱图
Fig.6 XPS spectra of C 1s [(a),(c)] and O 1s [(b),(d)] regions for the NCM-B and the NCM after 100 cycles at 1 C
| 1 | Lu L, Han X, Li J, et al. A review on the key issues for lithium-ion battery management in electric vehicles [J]. J. Power Sources, 2013, 226: 272-288. |
| 2 | 江浩, 李春忠. 表面化学反应控制制备多级结构电极材料及性能[J].化工学报, 2015, 66(8): 2872-2876. |
| Jiang H, Li C Z. Surface reaction controlled preparation of hierarchical structure nanomaterials and their electrochemical performances [J]. CIESC Journal, 2015, 66(8): 2872-2876. | |
| 3 | Huang Y, Jin F M, Chen F J, et al. Improved cycle stability and high-rate capability of Li3VO4-coated Li[Ni0.5Co0.2Mn0.3]O2 cathode material under different voltages [J]. J. Power Sources, 2014, 256: 1-7. |
| 4 | Li L J, Chen Z Y, Zhang Q B, et al. A hydrolysis-hydrothermal route for the synthesis of ultrathin LiAlO2-inlaid LiNi0.5Co0.2Mn0.3O2 as a high-performance cathode material for lithium ion batteries [J]. J. Mater. Chem. A, 2015, 3(2): 894-904. |
| 5 | Kong J Z, Ren C, Tai G A, et al. Ultrathin ZnO coating for improved electrochemical performance of LiNi0.5Co0.2Mn0.3O2 cathode material [J]. J. Power Sources, 2014, 266: 433-439. |
| 6 | Shi J L, Xiao D D, Ge M, et al. High-capacity cathode material with high voltage for Li-ion batteries [J]. Adv. Mater., 2018, 30(9): 1705575. |
| 7 | Liu B, Jia Y, Yuan C, et al. Safety issues and mechanisms of lithium-ion battery cell upon mechanical abusive loading: a review [J]. Energy Storage Materials, 2020, 24: 85-112. |
| 8 | Mao Y W, Wang X L, Xia S H, et al. High-voltage charging-induced strain, heterogeneity, and micro-cracks in secondary particles of a nickel-rich layered cathode material [J]. Adv. Funct. Mater., 2019, 29(18): 1900247. |
| 9 | Li Y G, Yu H F, Jiang H, et al. Surface-engineering of layered LiNi0.815Co0.15Al0.035O2 cathode material for high-energy and stable Li-ion batteries [J]. J. Energy Chem., 2018, 27(2): 559-564. |
| 10 | Zhao Z K, Chen S, Mu D B, et al. Understanding the surface decoration on primary particles of nickel-rich layered LiNi0.6Co0.2Mn0.2O2 cathode material with lithium phosphate [J]. J. Power Sources, 2019, 431: 84-92. |
| 11 | Chen Z, Kim G T, Guang Y, et al. Manganese phosphate coated Li[Ni0.6Co0.2Mn0.2]O2 cathode material: towards superior cycling stability at elevated temperature and high voltage [J]. J. Power Sources, 2018, 402: 263-271. |
| 12 | Li X L, Jin L B, Song D W, et al. LiNbO3-coated LiNi0.8Co0.1Mn0.1O2 cathode with high discharge capacity and rate performance for all-solid-state lithium battery [J]. J. Energy Chem., 2020, 40: 39-45. |
| 13 | Liang J N, Lu Y, Wang J, et al. Well-ordered layered LiNi0.8Co0.1Mn0.1O2 submicron sphere with fast electrochemical kinetics for cathodic lithium storage [J]. J. Energy Chem., 2020, 47: 188-195. |
| 14 | 宋刘斌, 蒋鹏, 肖忠良, 等. 核壳结构正极材料界面设计与性能研究[J]. 化工学报, 2019, 70(7): 2426-2438. |
| Song L B, Jiang P, Xiao Z L, et al. Interface design and properties of core-shell structure cathode materials[J]. CIESC Journal, 2019, 70(7): 2426-2438. | |
| 15 | Wu F, Li Q, Chen L, et al. Use of Ce to reinforce the interface of Ni-rich LiNi0.8Co0.1Mn0.1O2 cathode materials for lithium-ion batteries under high operating voltage [J]. ChemSusChem, 2019, 12(4): 935-943. |
| 16 | Zhu H W, Yu H F, Jiang H, et al. High-efficiency Mo doping stabilized LiNi0.9Co0.1O2 cathode materials for rapid charging and long-life Li-ion batteries[J]. Chem. Eng. Sci., 2020, 217, 115518. |
| 17 | Yu H F, Li Y G, Jiang H, et al. 110th anniversary: concurrently coating and doping high-valence vanadium in nickel-rich lithiated oxides for high-rate and stable lithium-ion batteries[J]. Ind. Eng. Chem. Res., 2019, 58(10): 4108-4115. |
| 18 | Kong D, Hu J, Chen Z, et al. Ti-gradient doping to stabilize layered surface structure for high performance high-Ni oxide cathode of Li-ion battery[J]. Adv. Energy Mater., 2019, 9(41): 1901756. |
| 19 | Steiner J D, Cheng H, Walsh J, et al. Targeted surface doping with reversible local environment improves oxygen stability at the electrochemical interfaces of nickel-rich cathode materials [J]. ACS Appl. Mater. Interfaces, 2019, 11(41): 37885-37891. |
| 20 | Zhang C X, Xu S, Han B, et al. Towards rational design of high-performance Ni-rich layered oxide cathodes: the interplay of borate-doping and excess lithium [J]. J. Power Sources, 2019, 431: 40-47. |
| 21 | Jiang Y, Liu Z, Zhang Y, et al. Full-gradient structured LiNi0.8Co0.1Mn0.1O2 cathode material with improved rate and cycle performance for lithium ion batteries [J]. Electrochem. Acta, 2019, 309: 74-85. |
| 22 | Xie H L, Li C L, Kan W H, et al. Consolidating the grain boundary of garnet electrolyte LLZTO with Li3BO3 for high performance LiNi0.8Co0.1Mn0.1O2/LiFePO4 hybrid solid batteries [J]. J. Mater. Chem. A, 2019, 7(36): 20965-20965. |
| 23 | Kang S, Kim, J, Stoll M, et al. Layered Li(Ni0.5-xMn0.5-xM′2x)O2, (M′=Co, Al, Ti, x = 0, 0.025) cathode materials for Li-ion rechargeable batteries [J]. J. Power Sources, 2002, 112: 41-48. |
| 24 | Xie H, Du K, Hu G, et al. Synthesis of LiNi0.8Co0.15Al0.05O2 with 5-sulfosalicylic acid as a chelating agent and its electrochemical properties [J]. J. Mater. Chem. A, 2015, 3(40): 20236-20243. |
| 25 | 耿淑君, 黄青山, 朱全红, 等. 共沉淀法制备LiNi1-x-yCoxMnyO2正极材料工艺条件探究[J]. 化工学报, 2018, 69(1): 175-187. |
| Geng S J, Huang Q S, Zhu Q H, et al. Investigation on synthesis conditions of LiNi1-x-yCoxMnyO2 cathode material via co-precipitation[J]. CIESC Journal, 2018, 69(1): 175-187. | |
| 26 | Li J Y, Li W D, Wang S Y, et al. Facilitating the operation of lithium-ion cells with high-nickel layered oxide cathodes with a small dose of aluminum [J]. Chem. Mater., 2018, 30(9): 3101-3109. |
| 27 | Ryu H H, Park K J, Yoon D R, et al. Li[Ni0.9Co0.09W0.01]O2: a new type of layered oxide cathode with high cycling stability [J]. Adv. Energy Mater., 2019, 9(44): 1902698. |
| 28 | Hou P, Li F, Sun Y, et al. Multishell precursors facilitated synthesis of concentration-gradient nickel-rich cathodes for long-life and high-rate lithium-ion batteries [J]. ACS Appl. Mater. Interfaces, 2018, 10(29): 24508-24515. |
| 29 | Deng Z N, Jiang H, Hu Y J, et al. 3D ordered macroporous MoS2@C nanostructure for flexible Li-ion batteries [J]. Adv. Mater., 2017, 29(10): 1603020. |
| 30 | Jiang Q Q, Yu H F, Hu Y J, et al. Exposed surface engineering of high-voltage LiNi0.5Co0.2Mn0.3O2 cathode materials enables high-rate and durable Li-ion batteries [J]. Ind. Eng. Chem. Res., 2019, 58(51): 23099-23105. |
| 31 | Park K J, Jung H G, Kuo L Y, et al. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries [J]. Adv. Energy Mater., 2018, 8(25): 1801202. |
| 32 | Fu J, Mu D, Wu B, et al. Enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode at high cutoff voltage by modifying electrode/electrolyte interface with lithium metasilicate [J]. Electrochim. Acta, 2017, 246: 27-34. |
| 33 | Li Y, Wan S, Veith G M, et al. A novel electrolyte salt additive for lithium-ion batteries with voltages greater than 4.7 V [J]. Adv. Energy Mater., 2017, 7(4): 1601397. |
| 34 | Zhang C C, Liu S Y, Su J M, et al. Revealing the role of NH4VO3 treatment in Ni-rich cathode materials with improved electrochemical performance for rechargeable lithium-ion batteries [J]. Nanoscale, 2018, 10(18): 8820-8831. |
| 35 | Wang L F, Liu G Y, Ding X, et al. Simultaneous coating and doping of a nickel-rich cathode by an oxygen ion conductor for enhanced stability and power of lithium-ion batteries [J]. ACS Appl. Mater. Interfaces, 2019, 11(37): 33901-33912. |
| 36 | Li Y C, Veith G M, Browning K L, et al. Lithium malonatoborate additives enabled stable cycling of 5 V lithium metal and lithium ion batteries [J]. Nano Energy, 2017, 40: 9-19. |
| [1] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [2] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [3] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [4] | 李靖, 沈聪浩, 郭大亮, 李静, 沙力争, 童欣. 木质素基碳纤维复合材料在储能元件中的应用研究进展[J]. 化工学报, 2023, 74(6): 2322-2334. |
| [5] | 肖忠良, 尹碧露, 宋刘斌, 匡尹杰, 赵亭亭, 刘成, 袁荣耀. 废旧锂离子电池回收工艺研究进展及其安全风险分析[J]. 化工学报, 2023, 74(4): 1446-1456. |
| [6] | 程伟江, 汪何琦, 高翔, 李娜, 马赛男. 锂离子电池硅基负极电解液成膜添加剂的研究进展[J]. 化工学报, 2023, 74(2): 571-584. |
| [7] | 杜江龙, 杨雯棋, 黄凯, 练成, 刘洪来. 复合相变材料/空冷复合式锂离子电池模块散热性能[J]. 化工学报, 2023, 74(2): 674-689. |
| [8] | 钟磊, 邱学青, 张文礼. 木质素衍生炭在碱金属离子电池负极中的研究进展[J]. 化工学报, 2022, 73(8): 3369-3380. |
| [9] | 胡华坤, 薛文东, 霍思达, 李勇, 蒋朋. 锂离子电池电解液SEI成膜添加剂的研究进展[J]. 化工学报, 2022, 73(4): 1436-1454. |
| [10] | 杨伟, 王昱杰, 方凯斌, 邹汉波, 陈胜洲, 刘自力. Co-Mn比例调控对LiNi0.8Co0.10-y Mn0.05+y Al0.05O2材料性能影响探究[J]. 化工学报, 2022, 73(12): 5615-5624. |
| [11] | 贾理男, 杜一博, 郭邦军, 张希. 基于硫化物电解质的全固态锂离子电池负极研究进展[J]. 化工学报, 2022, 73(12): 5289-5304. |
| [12] | 王朋朋, 贾洋刚, 邵霞, 程婕, 冒爱琴, 檀杰, 方道来. K+掺杂尖晶石型(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4高熵氧化物负极材料制备与储锂性能研究[J]. 化工学报, 2022, 73(12): 5625-5637. |
| [13] | 周弋惟, 陈卓, 徐建鸿. 湿法冶金回收废旧锂电池正极材料的研究进展[J]. 化工学报, 2022, 73(1): 85-96. |
| [14] | 王慧艳, 陈怡沁, 周静红, 曹约强, 周兴贵. 锂离子电池正极涂层孔隙结构优化的数值模拟[J]. 化工学报, 2022, 73(1): 376-383. |
| [15] | 梁坤峰, 王莫然, 高美洁, 吕振伟, 徐红玉, 董彬, 高凤玲. 纯电动车集成热管理系统性能的热力学分析[J]. 化工学报, 2021, 72(S1): 494-502. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号