化工学报 ›› 2022, Vol. 73 ›› Issue (12): 5625-5637.DOI: 10.11949/0438-1157.20221116
王朋朋( ), 贾洋刚, 邵霞, 程婕, 冒爱琴(
), 贾洋刚, 邵霞, 程婕, 冒爱琴( ), 檀杰, 方道来
), 檀杰, 方道来
收稿日期:2022-08-08
修回日期:2022-11-01
出版日期:2022-12-05
发布日期:2023-01-17
通讯作者:
冒爱琴
作者简介:王朋朋(1995—),男,硕士研究生,wang_pengpeng2022@163.com
基金资助:
Pengpeng WANG( ), Yanggang JIA, Xia SHAO, Jie CHENG, Aiqin MAO(
), Yanggang JIA, Xia SHAO, Jie CHENG, Aiqin MAO( ), Jie TAN, Daolai FANG
), Jie TAN, Daolai FANG
Received:2022-08-08
Revised:2022-11-01
Online:2022-12-05
Published:2023-01-17
Contact:
Aiqin MAO
摘要:
通过溶液燃烧法成功合成了一系列非活性K+掺杂的尖晶石型 (K x CoCrFeMnNi)3/(5+x)O4(x=0,0.5,1,1.5)高熵氧化物锂离子电池负极材料,系统研究了K+掺杂对结构和储锂性能的影响。结果表明:随着K+掺杂量的增加,均可制备出具有单一尖晶石结构的纳米晶粉体材料,其中等摩尔K+掺杂的 (K1/6Co1/6Cr1/6Fe1/6Mn1/6Ni1/6)3O4高熵氧化物负极材料具有最高的比容量、优异的循环稳定性和倍率性能。(K1/6Co1/6Cr1/6Fe1/6Mn1/6Ni1/6)3O4电极在200 mA·g-1电流密度下,首次放电比容量为1295 mA·h·g-1(首次库仑效率78%);随着循环的进行,可逆比容量先降低后增加,循环150次可逆比容量增加至1505 mA·h·g-1;即使在1000 mA·g-1大电流密度下循环500次后仍具有1402 mA·h·g-1的可逆比容量(均高于理论比容量898 mA·h·g-1)。低价非活性K+的掺杂由于电荷补偿效应使晶格常数降低,但高构型熵稳定的晶体结构提高了循环稳定性;丰富的表面氧空位、较小的晶粒尺寸和介孔结构,增加了赝电容贡献率和电子/离子传输能力,从而显著提升了材料的比容量和倍率性能。
中图分类号:
王朋朋, 贾洋刚, 邵霞, 程婕, 冒爱琴, 檀杰, 方道来. K+掺杂尖晶石型(Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4高熵氧化物负极材料制备与储锂性能研究[J]. 化工学报, 2022, 73(12): 5625-5637.
Pengpeng WANG, Yanggang JIA, Xia SHAO, Jie CHENG, Aiqin MAO, Jie TAN, Daolai FANG. Preparation and lithium storage performance of K+-doped spinel (Co0.2Cr0.2Fe0.2Mn0.2Ni0.2)3O4 high-entropy oxide anode materials[J]. CIESC Journal, 2022, 73(12): 5625-5637.

图2 样品K1的HRTEM图(a),选区电子衍射图(b),不同放大倍数SEM图[(c),(d)],元素EDS图(e)
Fig.2 High-resolution TEM image (a), selected area electron diffraction (SAED) pattern (b), SEM images at different magnifications [(c),(d)] and EDS mapping images (e) of K1 sample
| 样品 | 比表面积/ (m2·g-1) | 孔体积/ (cm3·g-1) | 平均孔径/nm | 最可几孔径/nm |
|---|---|---|---|---|
| K0 | 26.31 | 0.14 | 21.45 | 2.77 |
| K0.5 | 38.69 | 0.18 | 18.83 | 2.78 |
| K1 | 22.28 | 0.08 | 14.17 | 3.06 |
| K1.5 | 42.35 | 0.14 | 13.39 | 2.58 |
表1 样品的BET比表面积、孔体积、平均孔径和最可几孔径
Table 1 BET surface area, pore volume, average pore size and the most probable pore size of the samples
| 样品 | 比表面积/ (m2·g-1) | 孔体积/ (cm3·g-1) | 平均孔径/nm | 最可几孔径/nm |
|---|---|---|---|---|
| K0 | 26.31 | 0.14 | 21.45 | 2.77 |
| K0.5 | 38.69 | 0.18 | 18.83 | 2.78 |
| K1 | 22.28 | 0.08 | 14.17 | 3.06 |
| K1.5 | 42.35 | 0.14 | 13.39 | 2.58 |
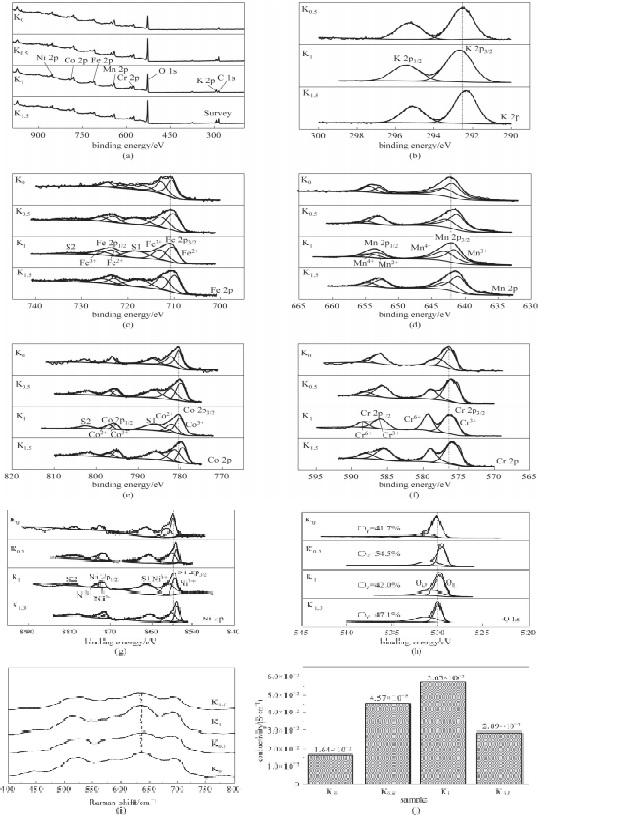
图5 样品的XPS全谱图(a),各元素精细谱图[(b)~(h)],拉曼光谱(i),四探针电导率(j)
Fig.5 XPS survey spectra (a), high-resolution XPS spectrum of all elements [(b)—(h)] and Raman spectra (i) and four-probes conductivity (j) of the samples
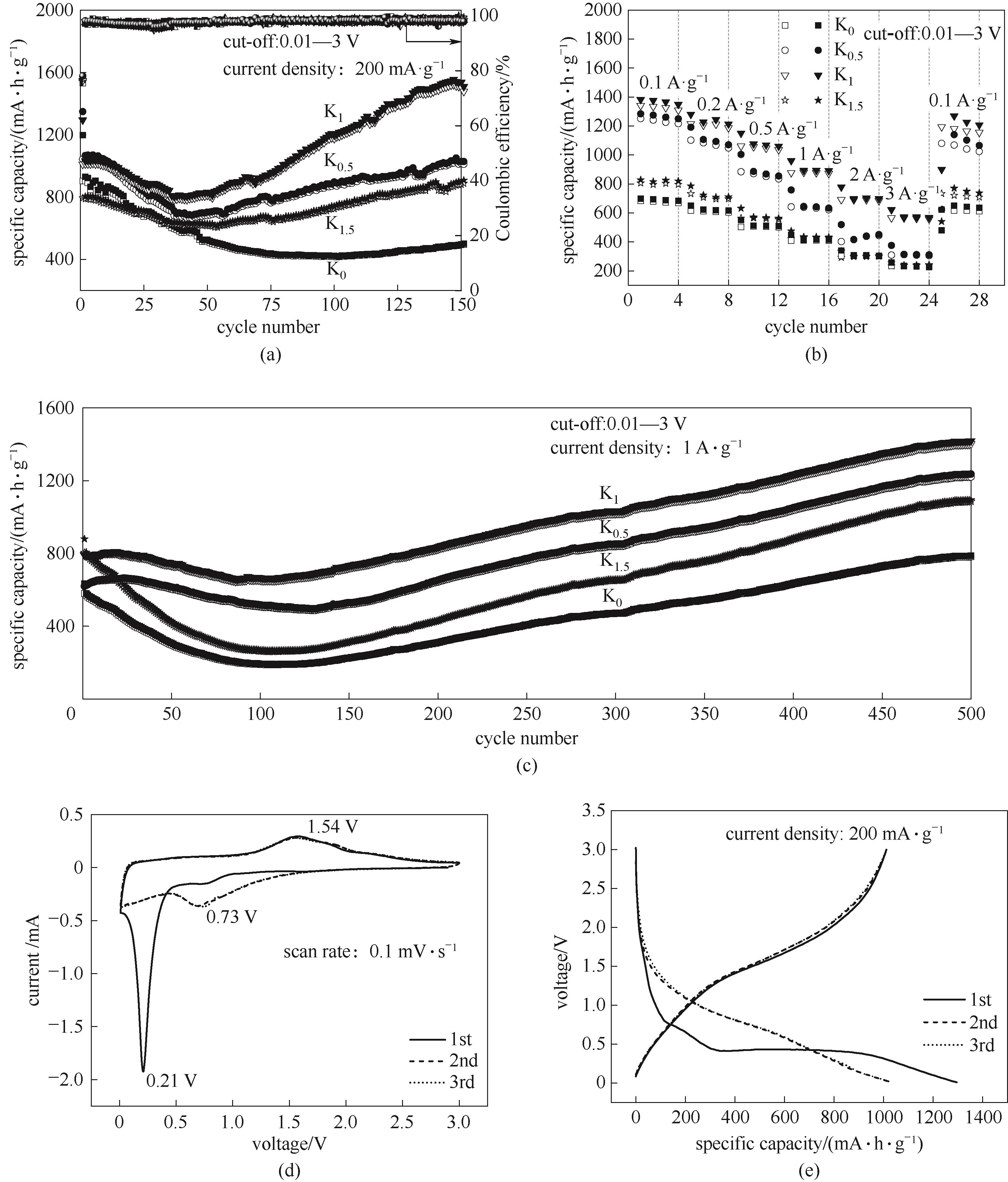
图6 各电极在不同电流密度下的循环性能[(a),(c)]和阶梯倍率性能(b);K1电极的循环伏安图(d)和容量电压曲线(e)
Fig.6 Cycling performance at different current density [(a), (c)] and stepped rate capability (b) of the electrodes; CV curves (d) and charge-discharge profiles (e) of K1 electrode

图7 K1电极循环前(a)和150次后(b)SEM图,循环150次后HRTEM图(c),循环150次前后XRD谱图(d)
Fig.7 SEM images before cycling (a) and after 150 cycles (b), HRTEM image after 150 cycles (c) and XRD patterns before cycling and after 150 cycles (d) of K1 electrode
| Samples | Rs/Ω | Rct/Ω | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| K0 | 6.7 | 4.2 | 284 | 156 | 32.1 | 29.0 |
| K0.5 | 4.1 | 5.4 | 246 | 125 | 13.2 | 86.3 |
| K1 | 5.3 | 4.8 | 228 | 102 | 38.5 | 257.3 |
| K1.5 | 7.4 | 5.5 | 265 | 134 | 17.0 | 45.4 |
表2 电极循环前和循环150次后的等效电路图参数
Table 2 Parameters of equivalent circuit diagrams of the electrodes before and after 150 cycles
| Samples | Rs/Ω | Rct/Ω | ||||
|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |
| K0 | 6.7 | 4.2 | 284 | 156 | 32.1 | 29.0 |
| K0.5 | 4.1 | 5.4 | 246 | 125 | 13.2 | 86.3 |
| K1 | 5.3 | 4.8 | 228 | 102 | 38.5 | 257.3 |
| K1.5 | 7.4 | 5.5 | 265 | 134 | 17.0 | 45.4 |
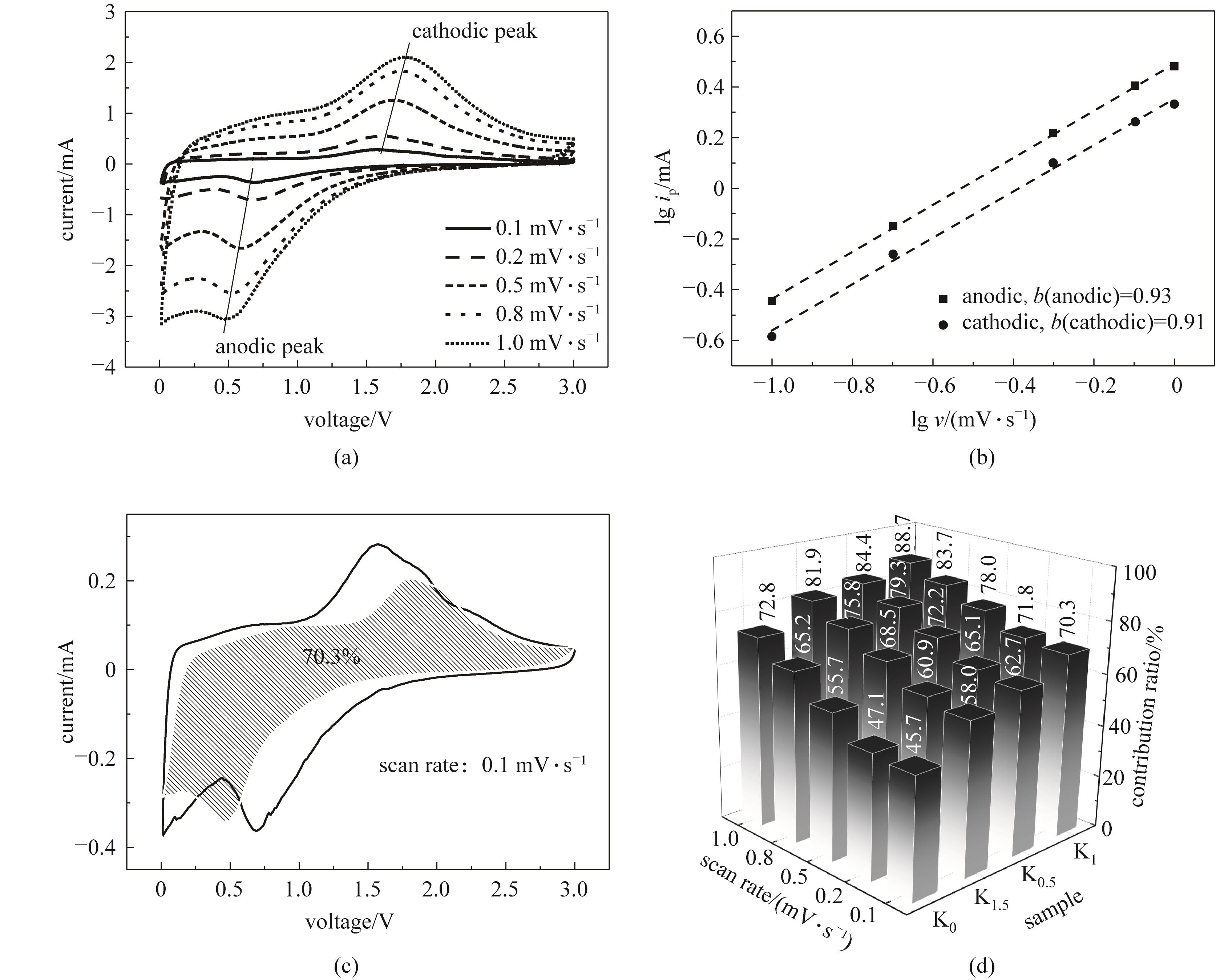
图9 K1电极在不同扫速下的CV曲线(a),lgip-lgv关系(b),0.1 mV·s-1扫速下的赝电容(阴影区域)贡献(c);各电极在不同扫速下的赝电容贡献(d)
Fig.9 CV curves at different scan rates (a), lgip-lgv relation (b) and pseudocapacitive (shaded region) contribution at 0.1 mV·s-1 scan rate (c) of K1 electrode; pseudocapacitive contribution at different scan rates of the electrodes (d)
| 1 | 刘祥哲. 锂离子电池层状复合正极材料的制备与改性[D]. 济南: 济南大学, 2011. |
| Liu X Z. Synthesis and modification of layered compound cathode material for lithium-ion batteries[D]. Jinan: University of Jinan, 2011. | |
| 2 | 何欢. 钴、铁基金属氧化物结构调控及其储锂性能研究[D]. 武汉: 华中科技大学, 2016. |
| He H. Structure controlling of cobalt/iron-based oxides and lithium storage performance research[D]. Wuhan: Huazhong University of Science and Technology, 2016. | |
| 3 | Chen H, Qiu N, Wu B Z, et al. A new spinel high-entropy oxide (Mg0.2Ti0.2Zn0.2Cu0.2Fe0.2)3O4 with fast reaction kinetics and excellent stability as an anode material for lithium ion batteries[J]. RSC Advances, 2020, 10(16): 9736-9744. |
| 4 | Duan C Q, Tian K H, Li X L, et al. New spinel high-entropy oxides (FeCoNiCrMnXLi)3O4 (X = Cu, Mg, Zn) as the anode material for lithium-ion batteries[J]. Ceramics International, 2021, 47(22): 32025-32032. |
| 5 | 麦永津. 过渡金属氧化物锂离子电池负极材料纳米复合化改性研究[D]. 杭州: 浙江大学, 2012. |
| Mai Y J. Improved electrochemical performance of transition metal oxide nanocomposites as anode materials for lithium ion batteries[D]. Hangzhou: Zhejiang University, 2012. | |
| 6 | 姚煜. 纳米结构氧化物锂离子电池负极材料研究[D]. 上海: 复旦大学, 2012. |
| Yao Y. Nanostructured oxide lithium-ion battery anode materials research[D]. Shanghai: Fudan University, 2012. | |
| 7 | Song J, Lu X M, Tian Q H, et al. Dual-stable engineering enables high-performance Zn2SnO4-based lithium-ion battery anode[J]. Journal of Alloys and Compounds, 2022, 910: 164924. |
| 8 | Yuan D, Adekoya D, Dou Y H, et al. Cation-vacancy induced Li+ intercalation pseudocapacitance at atomically thin heterointerface for high capacity and high power lithium-ion batteries[J]. Journal of Energy Chemistry, 2021, 62: 281-288. |
| 9 | Cao Z Z, Liu C H, Huang Y X, et al. Oxygen-vacancy-rich NiCo2O4 nanoneedles electrode with poor crystallinity for high energy density all-solid-state symmetric supercapacitors[J]. Journal of Power Sources, 2020, 449: 227571. |
| 10 | Wang X L, Liu J, Hu Y F, et al. Oxygen vacancy-expedited ion diffusivity in transition-metal oxides for high-performance lithium-ion batteries[J]. Science China Materials, 2022, 65(6): 1421-1430. |
| 11 | Anh L T, Rai A K, Thi T V, et al. Enhanced electrochemical performance of novel K-doped Co3O4 as the anode material for secondary lithium-ion batteries[J]. Journal of Materials Chemistry A, 2014, 2(19): 6966-6975. |
| 12 | Wang J, Zhao H L, Yang Q, et al. Electrochemical characteristics of Li4- x Cu x Ti5O12 used as anode material for lithium-ion batteries[J]. Ionics, 2013, 19(3): 415-419. |
| 13 | Zheng Y, Wu X, Lan X X, et al. A spinel (FeNiCrMnMgAl)3O4 high entropy oxide as a cycling stable anode material for Li-ion batteries[J]. Processes, 2021, 10(1): 49. |
| 14 | Bérardan D, Franger S, Meena A K, et al. Room temperature lithium superionic conductivity in high entropy oxides[J]. Journal of Materials Chemistry A, 2016, 4(24): 9536-9541. |
| 15 | Yan J H, Wang D, Zhang X Y, et al. A high-entropy perovskite titanate lithium-ion battery anode[J]. Journal of Materials Science, 2020, 55(16): 6942-6951. |
| 16 | 李星, 瞿美臻, 于作龙. 锂离子电池负极材料Li4- x K x Ti5O12结构和电化学性能[J]. 无机化学学报, 2010, 26(2): 233-239. |
| Li X, Qu M Z, Yu Z L. Structural and electrochemical characteristics of Li4- x K x Ti5O12 as anode material for lithium-ion batteries[J]. Chinese Journal of Inorganic Chemistry, 2010, 26(2): 233-239. | |
| 17 | Qiu N, Chen H, Yang Z M, et al. A high entropy oxide (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O with superior lithium storage performance[J]. Journal of Alloys and Compounds, 2019, 777: 767-774. |
| 18 | Nguyen T X, Tsai C C, Patra J, et al. Co-free high entropy spinel oxide anode with controlled morphology and crystallinity for outstanding charge/discharge performance in lithium-ion batteries[J]. Chemical Engineering Journal, 2022, 430: 132658. |
| 19 | Rost C M, Sachet E, Borman T, et al. Entropy-stabilized oxides[J]. Nature Communications, 2015, 6: 8485. |
| 20 | Sarkar A, Wang Q S, Schiele A, et al. High-entropy oxides: fundamental aspects and electrochemical properties[J]. Advanced Materials, 2019, 31(26): 1806236. |
| 21 | Nguyen T X, Patra J, Chang J K, et al. High entropy spinel oxide nanoparticles for superior lithiation-delithiation performance[J]. Journal of Materials Chemistry A, 2020, 8(36): 18963-18973. |
| 22 | Xiao B, Wu G, Wang T D, et al. High-entropy oxides as advanced anode materials for long-life lithium-ion batteries[J]. Nano Energy, 2022, 95: 106962. |
| 23 | Wang D, Jiang S D, Duan C Q, et al. Spinel-structured high entropy oxide (FeCoNiCrMn)3O4 as anode towards superior lithium storage performance[J]. Journal of Alloys and Compounds, 2020, 844: 156158. |
| 24 | Xiang H Z, Xie H X, Chen Y X, et al. Porous spinel-type (Al0.2CoCrFeMnNi)0.58O4- δ high-entropy oxide as a novel high-performance anode material for lithium-ion batteries[J]. Journal of Materials Science, 2021, 56(13): 8127-8142. |
| 25 | Mao A Q, Xiang H Z, Zhang Z G, et al. Solution combustion synthesis and magnetic property of rock-salt (Co0.2Cu0.2Mg0.2Ni0.2Zn0.2)O high-entropy oxide nanocrystalline powder[J]. Journal of Magnetism and Magnetic Materials, 2019, 484: 245-252. |
| 26 | 项厚政, 谢鸿翔, 李文超, 等. 尖晶石型高熵氧化物的制备和电化学性能[J]. 高等学校化学学报, 2020, 41(8): 1801-1809. |
| Xiang H Z, Xie H X, Li W C, et al. Synthesis and electrochemical performance of spinel-type high-entropy oxides[J]. Chemical Journal of Chinese Universities, 2020, 41(8): 1801-1809. | |
| 27 | Bérardan D, Franger S, Dragoe D, et al. Colossal dielectric constant in high entropy oxides[J]. Physica Status Solidi (RRL) - Rapid Research Letters, 2016, 10(4): 328-333. |
| 28 | Chen H, Qiu N, Wu B Z, et al. Tunable pseudocapacitive contribution by dimension control in nanocrystalline-constructed (Mg0.2Co0.2Ni0.2Cu0.2Zn0.2)O solid solutions to achieve superior lithium-storage properties[J]. RSC Advances, 2019, 9(50): 28908-28915. |
| 29 | 肖雪春, 有俊达, 赵阳, 等. 基于溶液燃烧法制备多孔结构材料的实验设计[J]. 实验科学与技术, 2021, 19(1): 70-74. |
| Xiao X C, You J D, Zhao Y, et al. Porous structural materials synthesized by one-step solution combustion method[J]. Experiment Science and Technology, 2021, 19(1): 70-74. | |
| 30 | 吉可明, 孟凡会, 李忠. 溶液燃烧法制备无机材料研究进展[J]. 现代化工, 2014, 34(5): 22-25, 27. |
| Ji K M, Meng F H, Li Z. Recent advance in inorganic material prepared by solution combustion synthesis[J]. Modern Chemical Industry, 2014, 34(5): 22-25, 27. | |
| 31 | An Q Y, Zhang P F, Xiong F Y, et al. Three-dimensional porous V2O5 hierarchical octahedrons with adjustable pore architectures for long-life lithium batteries[J]. Nano Research, 2015, 8(2): 481-490. |
| 32 | Fang D L, Zhao Y C, Wang S S, et al. Highly efficient synthesis of nano-Si anode material for Li-ion batteries by a ball-milling assisted low-temperature aluminothermic reduction[J]. Electrochimica Acta, 2020, 330: 135346. |
| 33 | Zou F, Hu X, Li Z, et al. MOF-derived porous ZnO/ZnFe2O4/C octahedra with hollow interiors for high-rate lithium-ion batteries[J]. Advanced Materials, 2014, 26(38): 6622-6628. |
| 34 | Zhang Y Y, Chen P, Wang Q Y, et al. High-capacity and kinetically accelerated lithium storage in MoO3 enabled by oxygen vacancies and heterostructure[J]. Advanced Energy Materials, 2021, 11(31): 2101712. |
| 35 | Gu Y J, Li Y, Chen Y B, et al. Enhanced oxygen vacancies in nanostructured LiNi0.5Mn1.5O4- δ with a P4332 space group[J]. International Journal of Electrochemical Science, 2017, 12: 9523-9532. |
| 36 | Tang Z K, Xue Y F, Teobaldi G, et al. The oxygen vacancy in Li-ion battery cathode materials[J]. Nanoscale Horizons, 2020, 5(11): 1453-1466. |
| 37 | Cui Y, Xiao K F, Bedford N M, et al. Refilling nitrogen to oxygen vacancies in ultrafine tungsten oxide clusters for superior lithium storage[J]. Advanced Energy Materials, 2019, 9(37): 1902148. |
| 38 | Sarkar A, Velasco L, Wang D, et al. High entropy oxides for reversible energy storage[J]. Nature Communications, 2018, 9(1): 1-9. |
| 39 | Li Z Y, Zhang C K, Liu C F, et al. Enhanced electrochemical properties of Sn-doped V2O5 as a cathode material for lithium ion batteries[J]. Electrochimica Acta, 2016, 222: 1831-1838. |
| 40 | Sarkar A, Djenadic R, Usharani N J, et al. Nanocrystalline multicomponent entropy stabilised transition metal oxides[J]. Journal of the European Ceramic Society, 2017, 37(2): 747-754. |
| 41 | Wang J, Polleux J, Lim J, et al. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles[J]. The Journal of Physical Chemistry C, 2007, 111(40): 14925-14931. |
| 42 | 李延伟, 李世玉, 谢志平, 等. 电化学沉积制备V2O5薄膜电极的表面结构及储钠性能[J]. 化工学报, 2016, 67(11): 4771-4778. |
| Li Y W, Li S Y, Xie Z P, et al. Surface morphology and sodium storage performance of V2O5 thin film electrode prepared by CTAB assisted electrodeposition[J]. CIESC Journal, 2016, 67(11): 4771-4778. |
| [1] | 毕丽森, 刘斌, 胡恒祥, 曾涛, 李卓睿, 宋健飞, 吴翰铭. 粗糙界面上纳米液滴蒸发模式的分子动力学研究[J]. 化工学报, 2023, 74(S1): 172-178. |
| [2] | 于宏鑫, 邵双全. 水结晶过程的分子动力学模拟分析[J]. 化工学报, 2023, 74(S1): 250-258. |
| [3] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [4] | 程成, 段钟弟, 孙浩然, 胡海涛, 薛鸿祥. 表面微结构对析晶沉积特性影响的格子Boltzmann模拟[J]. 化工学报, 2023, 74(S1): 74-86. |
| [5] | 肖明堃, 杨光, 黄永华, 吴静怡. 浸没孔液氧气泡动力学数值研究[J]. 化工学报, 2023, 74(S1): 87-95. |
| [6] | 范孝雄, 郝丽芳, 范垂钢, 李松庚. LaMnO3/生物炭催化剂低温NH3-SCR催化脱硝性能研究[J]. 化工学报, 2023, 74(9): 3821-3830. |
| [7] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [8] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [9] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [10] | 曾如宾, 沈中杰, 梁钦锋, 许建良, 代正华, 刘海峰. 基于分子动力学模拟的Fe2O3纳米颗粒烧结机制研究[J]. 化工学报, 2023, 74(8): 3353-3365. |
| [11] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [12] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [13] | 杨越, 张丹, 郑巨淦, 涂茂萍, 杨庆忠. NaCl水溶液喷射闪蒸-掺混蒸发的实验研究[J]. 化工学报, 2023, 74(8): 3279-3291. |
| [14] | 汪林正, 陆俞冰, 张睿智, 罗永浩. 基于分子动力学模拟的VOCs热氧化特性分析[J]. 化工学报, 2023, 74(8): 3242-3255. |
| [15] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号