化工学报 ›› 2021, Vol. 72 ›› Issue (2): 1156-1168.DOI: 10.11949/0438-1157.20200905
收稿日期:2020-07-06
修回日期:2020-08-27
出版日期:2021-02-05
发布日期:2021-02-05
通讯作者:
王玉军
作者简介:魏娟(1995—),女,硕士,基金资助:
WEI Juan( ),WANG Yujun(
),WANG Yujun( ),LUO Guangsheng
),LUO Guangsheng
Received:2020-07-06
Revised:2020-08-27
Online:2021-02-05
Published:2021-02-05
Contact:
WANG Yujun
摘要:
碳热还原氮化法是大规模制备高纯度氮化铝(AlN)粉体的主要方法,通过微反应器制备不同孔容的铝源,系统探究了前体孔容和微观形貌对AlN粉体产物的影响,并通过动力学模拟验证了筛选出的前体的活性。同时对氮化反应升温过程的影响也做了探究,最终通过对前体和焙烧升温过程的优化,得到纯度99%以上的AlN粉体,其平均粒径约为150 nm,O元素含量为0.55%。
中图分类号:
魏娟, 王玉军, 骆广生. 铝源孔容和焙烧升温过程对碳热还原法制备氮化铝粉体的影响[J]. 化工学报, 2021, 72(2): 1156-1168.
WEI Juan, WANG Yujun, LUO Guangsheng. Influence of pore volume and heating process on preparation of aluminum nitride powder by carbothermal reduction method[J]. CIESC Journal, 2021, 72(2): 1156-1168.
| 序号 | 组分 | 微反出口pH | 比表面积/(m2/g) | 孔容/(ml/g) | 平均孔径/nm | 产品氮化率 |
|---|---|---|---|---|---|---|
| 1 | AlOOH | 10.32 | 320.40 | 0.17 | 9.29 | 0.332 |
| AlOOH+C | 10.32 | 132.7 | 0.14 | 9.02 | 0.332 | |
| 2 | AlOOH | 5.09 | 251.55 | 0.38 | 6.04 | 0.367 |
| AlOOH+C | 5.09 | 88.90 | 0.19 | 8.59 | 0.367 | |
| 3 | AlOOH | 6.33 | 279.77 | 0.71 | 10.13 | 0.397 |
| AlOOH+C | 6.33 | 97.35 | 0.25 | 10.47 | 0.397 | |
| 4 | AlOOH | 8.37 | 343.73 | 0.95 | 11.07 | 0.403 |
| AlOOH+C | 8.37 | 86.67 | 0.23 | 10.82 | 0.403 | |
| 5 | AlOOH | 8.92 | 396.10 | 1.13 | 11.43 | 0.342 |
| AlOOH+C | 8.92 | 145.70 | 0.16 | 4.51 | 0.342 | |
| 6 | C | — | 318.91 | 0.92 | 11.56 | — |
表1 不同孔容的拟薄水铝石与炭黑混合前后的BET数据以及对应的产品氮化率(1400℃,3 h)
Table 1 BET data and nitrading rate of γ-AlOOH with different pore volumes before and after mixing with carbon black (1400℃, 3 h)
| 序号 | 组分 | 微反出口pH | 比表面积/(m2/g) | 孔容/(ml/g) | 平均孔径/nm | 产品氮化率 |
|---|---|---|---|---|---|---|
| 1 | AlOOH | 10.32 | 320.40 | 0.17 | 9.29 | 0.332 |
| AlOOH+C | 10.32 | 132.7 | 0.14 | 9.02 | 0.332 | |
| 2 | AlOOH | 5.09 | 251.55 | 0.38 | 6.04 | 0.367 |
| AlOOH+C | 5.09 | 88.90 | 0.19 | 8.59 | 0.367 | |
| 3 | AlOOH | 6.33 | 279.77 | 0.71 | 10.13 | 0.397 |
| AlOOH+C | 6.33 | 97.35 | 0.25 | 10.47 | 0.397 | |
| 4 | AlOOH | 8.37 | 343.73 | 0.95 | 11.07 | 0.403 |
| AlOOH+C | 8.37 | 86.67 | 0.23 | 10.82 | 0.403 | |
| 5 | AlOOH | 8.92 | 396.10 | 1.13 | 11.43 | 0.342 |
| AlOOH+C | 8.92 | 145.70 | 0.16 | 4.51 | 0.342 | |
| 6 | C | — | 318.91 | 0.92 | 11.56 | — |
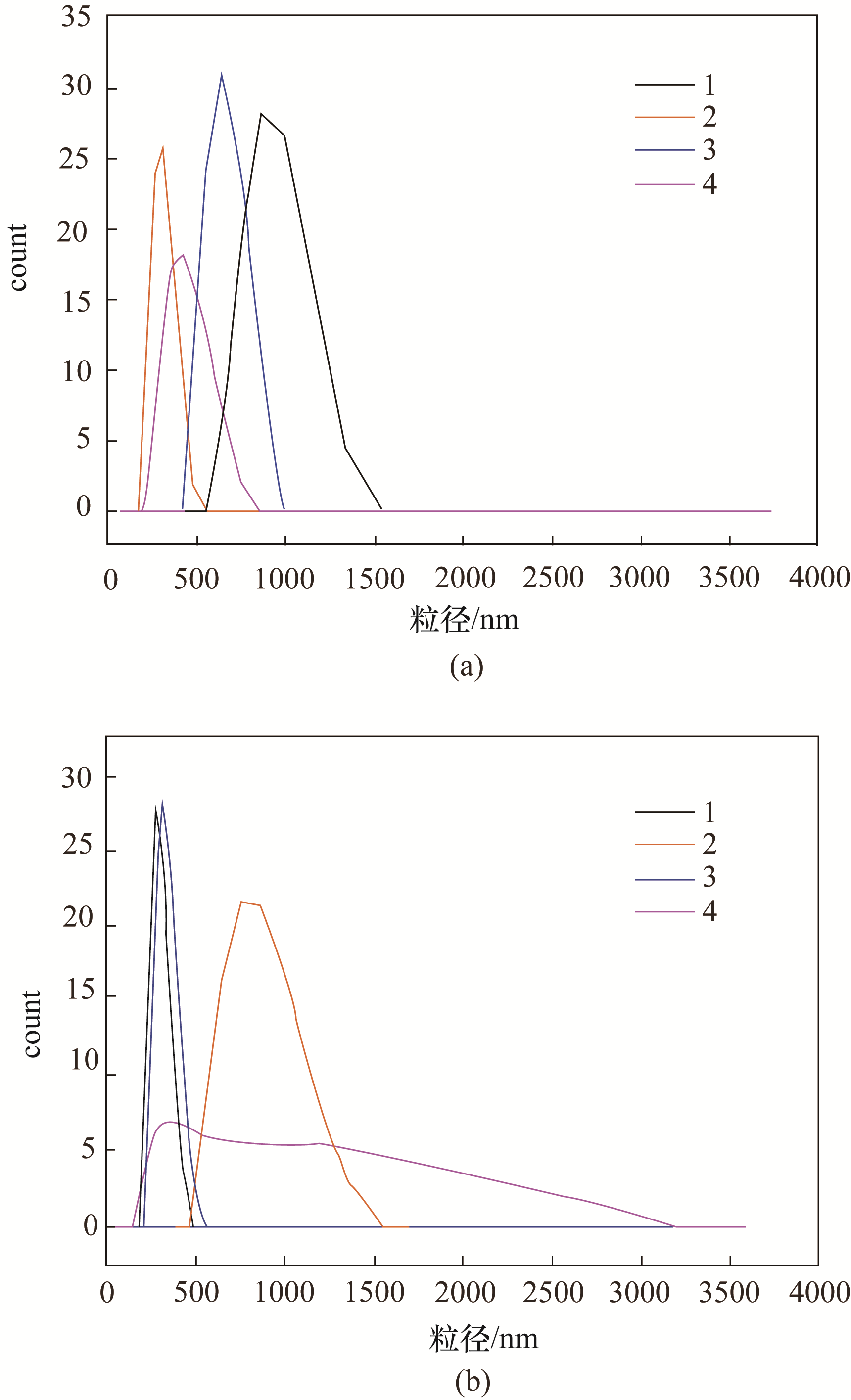
图5 γ-AlOOH(a)和其对应AlN产物(b)的粒度分布图(1~4分别对应表1中的1~4号拟薄水铝石)
Fig.5 Grain size distribution of γ-AlOOH (a) and AlN (b)(1—4 respectively correspond to 1—4 in table 1)
| 组分 | 编号 | 混合条件 | 比表面积/(m2/g) | 孔容/(ml/g) | 平均孔径/nm | 产品氮化率 |
|---|---|---|---|---|---|---|
| 纯AlOOH | a | 无处理 | 279.77 | 0.71 | 10.13 | — |
| b | 干磨10 min | 49.07 | 0.13 | 10.51 | — | |
| 纯炭黑 | c | 无处理 | 318.91 | 0.92 | 11.56 | — |
| AlOOH+C | 1 | 干磨3 min | 232.17 | 0.51 | 8.78 | 0.351 |
| 2 | 干磨10 min | 97.35 | 0.25 | 10.47 | 0.397 | |
| 3 | 干磨30 min | 56.32 | 0.19 | 13.5 | 0.321 | |
| 4 | 湿磨30 min | 191.6 | 0.42 | 8.79 | 0.412 | |
| 5 | 微反后混合 | 96.17 | 0.13 | 3.8 | 0.404 | |
| 6 | 微反中混合 | 49.97 | 0.1 | 3.81 | 0.571 |
表2 不同混合方式得到的前体BET数据以及对应的产品氮化率
Table 2 BET data and nitrading rate of different mixing methods
| 组分 | 编号 | 混合条件 | 比表面积/(m2/g) | 孔容/(ml/g) | 平均孔径/nm | 产品氮化率 |
|---|---|---|---|---|---|---|
| 纯AlOOH | a | 无处理 | 279.77 | 0.71 | 10.13 | — |
| b | 干磨10 min | 49.07 | 0.13 | 10.51 | — | |
| 纯炭黑 | c | 无处理 | 318.91 | 0.92 | 11.56 | — |
| AlOOH+C | 1 | 干磨3 min | 232.17 | 0.51 | 8.78 | 0.351 |
| 2 | 干磨10 min | 97.35 | 0.25 | 10.47 | 0.397 | |
| 3 | 干磨30 min | 56.32 | 0.19 | 13.5 | 0.321 | |
| 4 | 湿磨30 min | 191.6 | 0.42 | 8.79 | 0.412 | |
| 5 | 微反后混合 | 96.17 | 0.13 | 3.8 | 0.404 | |
| 6 | 微反中混合 | 49.97 | 0.1 | 3.81 | 0.571 |
| 混合方式 | O/%(质量) | C/%(质量) |
|---|---|---|
| 干磨10 min | 0.62 | 0.063 |
| 微反应器中混合 | 0.57 | 0.114 |
表3 不同混合方式得到的AlN产品的O、C杂质含量
Table 3 O and C content of AlN by different mixing methods
| 混合方式 | O/%(质量) | C/%(质量) |
|---|---|---|
| 干磨10 min | 0.62 | 0.063 |
| 微反应器中混合 | 0.57 | 0.114 |
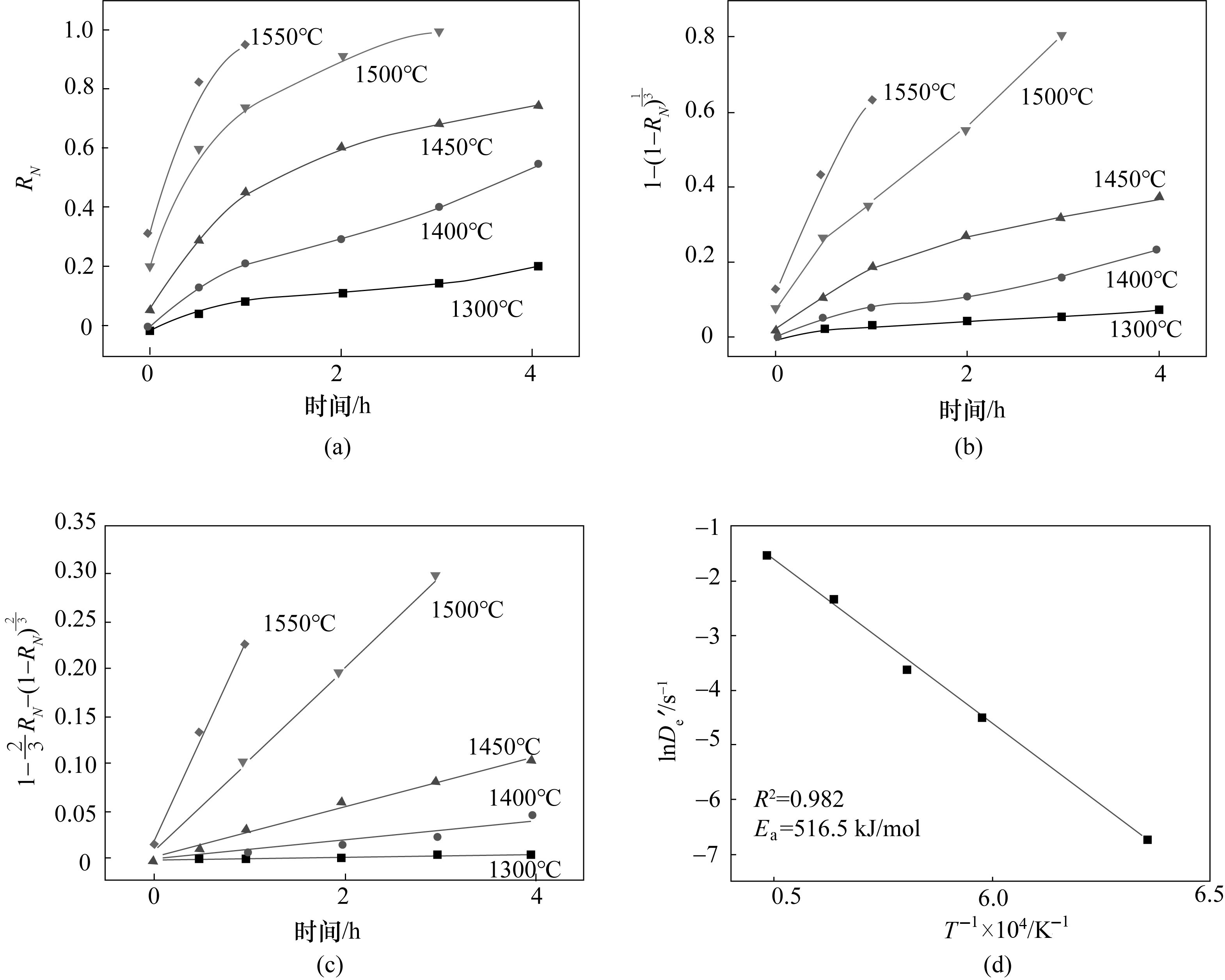
图13 不同温度下RN(a)、1-(1-RN)1/3(b)、1-2/3RN-(1-RN)2/3(c)随反应时间的变化,lnDe'对T-1拟合直线(d)
Fig.13 Plots of RN (a), 1-(1-RN)1/3 (b), 1-2/3RN-(1-RN)2/3 (c) against reaction time. Arrhenius plots of lnDe' against T-1 (d)
| 反应机理 | R2 | ||||
|---|---|---|---|---|---|
| 1300℃ | 1400℃ | 1450℃ | 1500℃ | 1550℃ | |
| 表面化学反应控制 | 0.940 | 0.982 | 0.940 | 0.942 | 0.983 |
| 内扩散控制 | 0.932 | 0.976 | 0.994 | 0.998 | 0.996 |
表4 表面化学反应控制和内扩散控制条件下的线性拟合R2值
Table 4 Linear fitting R2 values under surface chemical reaction control and internal diffusion control
| 反应机理 | R2 | ||||
|---|---|---|---|---|---|
| 1300℃ | 1400℃ | 1450℃ | 1500℃ | 1550℃ | |
| 表面化学反应控制 | 0.940 | 0.982 | 0.940 | 0.942 | 0.983 |
| 内扩散控制 | 0.932 | 0.976 | 0.994 | 0.998 | 0.996 |
| 1 | Sheppard L M. Aluminum nitride - a versatile but challenging material [J]. American Ceramic Society Bulletin, 1990, 69(11): 1801-1812. |
| 2 | 严光能, 邓先友, 林金堵. 高导热氮化铝基板在航空工业的应用研究[J]. 印制电路信息, 2017, 25(10): 32-37. |
| Yan G N, Deng X Y, Lin J D. The research of high-thermal-conductive aluminum nitride substrate in airport power electronics [J]. Printed Circuit Information, 2017, 25(10): 32-37. | |
| 3 | He X, Shi L, Guo Y, et al. Study on microstructure and dielectric properties of aluminum nitride ceramics [J]. Materials Characterization, 2015, 106, 404-410. |
| 4 | Son H W, Kim B N, Suzuki T S, et al. Fabrication of translucent AlN ceramics with MgF2 additive by spark plasma sintering [J]. Journal of the American Ceramic Society, 2018, 101(10): 4430-4433. |
| 5 | Jackson T B, Virkar A V, More K L, et al. High-thermal-conductivity aluminum nitride ceramics: the effect of thermodynamic, kinetic, and microstructural factors [J]. Journal of the American Ceramic Society, 1997, 80(6): 1421-1435. |
| 6 | Fei C L, Liu X L, Zhu B P, et al. AlN piezoelectric thin films for energy harvesting and acoustic devices [J]. Nano Energy, 2018, 51(1): 146-161. |
| 7 | 张浩, 崔嵩, 何金奇. 高性能氮化铝粉体技术发展现状[J]. 真空电子技术, 2015, (5): 14-18. |
| Zhang H, Cui S, He J Q. Technology development of high-performance AlN powders [J]. Vacuum Electronics, 2015, (5): 14-18. | |
| 8 | 王毅, 李东红, 张岩岩. 碳热还原法制备氮化铝的影响因素[J]. 真空电子技术, 2019, (3): 64-66+72. |
| Wang Y, Li D H, Zhang Y Y. Influencing factors of carbothermic reduction for AlN synthesis [J]. Vacuum Electronics, 2019, (3): 64-66+72. | |
| 9 | Cinar F S, Kizilirmak V, Derin B, et al. Carbothermal reduction - nitridation study of seydisehir alumina [J]. Silicates Industries, 2004, 69(7/8): 305-310. |
| 10 | 侯海兰, 冯月斌, 字富庭, 等. 碳热还原氮化法制备氮化铝的研究进展[J].粉末冶金工业, 2019, 29(1): 69-72. |
| Hou H L, Feng Y B, Zi F T, et al. Research progress in preparation of aluminum nitride by carbothermal reduction nitridation [J]. Powder Metallurgy Industry, 2019, 29(1): 69-72. | |
| 11 | 张笑. 氧化铝碳热还原氮化的机理研究[D]. 昆明: 昆明理工大学, 2017. |
| Zhang X. Research on mechanism of carbothermal reduction nitriding of alumina [D]. Kunming: Kunming University of Science and Technology, 2017. | |
| 12 | 马丁. 适合于导热基板用AlN粉体的制备与表征[D]. 北京: 北京交通大学, 2019. |
| Ma D. Preparation and characterization of AlN powders for thermally conductive substrates [D]. Beijing: Beijing Jiaotong University, 2019. | |
| 13 | 秦明礼, 曲选辉, 林健凉, 等. 原料对碳热还原法合成氮化铝粉末的影响[J].中南工业大学学报(自然科学版), 2002, (5): 505-508. |
| Qin M L, Qu X H, Lin J L, et al. Effect of starting materials on the synthesis of aluminum nitride powders by carbothermal reduction method[J]. J. Cent. South. Univ. Technol., 2002, (5): 505-508. | |
| 14 | Kimura I, Ichiya K, Ishii M, et al. Synthesis of fine AlN powder by a floating nitridation technique using an N2/NH3 gas mixture [J]. Journal of Materials Science Letters, 1989, 8(3): 303-304. |
| 15 | Tsuge A, Inoue H, Kasori M, et al. Raw-material effect on AlN powder synthesis from Al2O3 carbothermal reduction [J]. Journal of Materials Science, 1990, 25(5): 2359-2361. |
| 16 | Mylinh D T, Yoon D H, Kim C Y. Aluminum nitride formation from aluminum oxide/phenol resin solid-gel mixture by carbothermal reduction nitridation method [J]. Archives of Metallurgy and Materials, 2015, 60(2): 1551-1555. |
| 17 | Pathak L C, Ray A K, Das S, et al. Carbothermal synthesis of nanocrystalline aluminum nitride powders [J]. Journal of the American Ceramic Society, 1999, 82(1): 257-260. |
| 18 | Xi S Q, Liu X K, Li P L, et al. AlN ceramics synthesized by carbothermal reduction of mechanical activated Al2O3 [J]. Journal of Alloys and Compounds, 2008, 457(1/2): 452-456. |
| 19 | 刘新宽, 马明亮, 周敬恩, 等. 高能球磨对碳热还原合成氮化铝的作用[J]. 兵器材料科学与工程, 1999, (3): 23-27. |
| Liu X K, Ma M L, Zhou J E. Effects of high energy ball milling on ain snythesis from Al2O3 by carbothermal reduction nitridation[J]. Ordnance Material Science and Engineering, 1999, (3): 24-28. | |
| 20 | Mao X X, Li J, Zhang H L, et al. Synthesis of AlN powder by carbothermal reduction-nitridation of alumina/carbon black foam [J]. Journal of Inorganic Materials, 2017, 32(10): 1115-1120. |
| 21 | Chikami H, Fukushima J, Hayashi Y, et al. Kinetics of microwave synthesis of AlN by carbothermal-reduction-nitridation at low temperature [J]. Journal of the American Ceramic Society, 2018, 101(11): 4905-4910. |
| 22 | 马艳红, 陈玮. 溶胶-凝胶法制备氮化铝粉体[J]. 真空电子技术, 2009, (4): 78-80. |
| Ma Y H, Chen W. Preparation of AlN by the sol-gel method [J]. Vacuum Electronics, 2009, (4): 78-80. | |
| 23 | 李亚伟, 李楠, 袁润章. Al2O3-C-N2系统反应热力学初步探讨[J]. 硅酸盐通报, 2001, (4): 3-8. |
| Li Y W, Li N, Yuan R Z. Thermodynamical discussion of the reaction evolution in Al2O3-C-N-2 system [J]. Bulletin of the Chinese Ceramic Society, 2001, (4): 3-8. | |
| 24 | Yu J K, Ueno S, Li H X, et al. Improvement of graphitization of isotropic carbon by Al2O3 formed from aluminium chelate compound [J]. Journal of the European Ceramic Society, 1999, 19(16): 2843-2848. |
| 25 | 傅仁利, 于洪珍, 张月铭, 等. 碳热还原法合成氮化铝的机理与热力学条件[J]. 中国矿业大学学报, 2002, (5): 415-420. |
| Fu R L, Yu H Z, Zhang Y M, et al. Mechanisms and thermodynamics conditions of carbothermal reduction nitridation reactions [J]. Journal of China University of Mining and Technology, 2002, (5): 415-420. | |
| 26 | Shen C Y, Qiu T, Lu Y M. Morphology and reaction mechanism of AIN fibers synthesized by carbothermal reduction [J]. Key Engineering Materials, 2005, 280/283(2): 1399-1402. |
| 27 | Suehiro T, Hirosaki N, Terao R, et al. Synthesis of aluminum nitride nanopowder by gas-reduction-nitridation method [J]. Journal of the American Ceramic Society, 2003, 86(6): 1046-1048. |
| 28 | 匡加才. AlN粉体及陶瓷的制备、结构与性能研究[D]. 长沙: 国防科学技术大学, 2004. |
| Kuang J C. Processing, microstructure and thermal properties of AlN ceramics and AlN powder prepared by modified carbothermal reduction method[D]. Changsha: National University of Defense Technology, 2004. | |
| 29 | Lefort P, Tetard D, Tristant P. Formation of aluminium carbide by carbothermal reduction of alumina - role of the gaseous aluminium phase [J]. Journal of the European Ceramic Society, 1993, 12(2): 123-129. |
| 30 | Wan Y, Liu Y, Wang Y, et al. Preparation of large-pore-volume gamma-alumina vanofibers with a varrow pore size distribution in a membrane sispersion microreactor [J]. Industrial & Engineering Chemistry Research, 2017, 56(31): 8888-8894. |
| 31 | Galvez M E, Frei A, Halmann M, et al. Ammonia production via a two-step Al2O3/AlN thermochemical cycle. 2. Kinetic analysis [J]. Industrial & Engineering Chemistry Research, 2007, 46(7): 2047-2053. |
| [1] | 吴文涛, 褚良永, 张玲洁, 谭伟民, 沈丽明, 暴宁钟. 腰果酚生物基自愈合微胶囊的高效制备工艺研究[J]. 化工学报, 2023, 74(7): 3103-3115. |
| [2] | 王志龙, 杨烨, 赵真真, 田涛, 赵桐, 崔亚辉. 搅拌时间和混合顺序对锂离子电池正极浆料分散特性的影响[J]. 化工学报, 2023, 74(7): 3127-3138. |
| [3] | 朱风, 陈凯琳, 黄小凤, 鲍银珠, 李文斌, 刘嘉鑫, 吴玮强, 高王伟. KOH改性电石渣脱除羰基硫的性能研究[J]. 化工学报, 2023, 74(6): 2668-2679. |
| [4] | 衣思敏, 马亚丽, 刘伟强, 张金帅, 岳岩, 郑强, 贾松岩, 李雪. 微晶菱镁矿蒸氨及水化动力学研究[J]. 化工学报, 2023, 74(4): 1578-1586. |
| [5] | 张雪婷, 胡激江, 赵晶, 李伯耿. 高分子量聚丙二醇在微通道反应器中的制备[J]. 化工学报, 2023, 74(3): 1343-1351. |
| [6] | 章承浩, 罗京, 张吉松. 微反应器内基于氮氧自由基催化剂连续氧气/空气氧化反应的研究进展[J]. 化工学报, 2023, 74(2): 511-524. |
| [7] | 谢煜, 张民, 胡卫国, 王玉军, 骆广生. 利用膜分散微反应器高效溶解D-7-ACA的研究[J]. 化工学报, 2023, 74(2): 748-755. |
| [8] | 杨星宇, 马优, 朱春英, 付涛涛, 马友光. 梳状并行微通道内液液分布规律研究[J]. 化工学报, 2023, 74(2): 698-706. |
| [9] | 靳志远, 单国荣, 潘鹏举. AM/AMPS/SSS三元共聚物的制备及耐温耐盐性能[J]. 化工学报, 2023, 74(2): 916-923. |
| [10] | 付家崴, 陈帅帅, 方凯伦, 蒋新. 微反应器共沉淀反应制备铜锰催化剂[J]. 化工学报, 2023, 74(2): 776-783. |
| [11] | 张浩, 王子悦, 程钰洁, 何晓辉, 纪红兵. 单原子催化剂规模化制备的研究进展[J]. 化工学报, 2023, 74(1): 276-289. |
| [12] | 刘佳宁, 马嘉浩, 张军营, 程珏. 顺序双重热固化的硫醇-丙烯酸酯-环氧树脂三维网络的构建及性能[J]. 化工学报, 2022, 73(9): 4173-4186. |
| [13] | 李承威, 骆华勇, 张铭轩, 廖鹏, 方茜, 荣宏伟, 王竞茵. 氢氧化镧交联壳聚糖微球的微流控制备及其除磷性能[J]. 化工学报, 2022, 73(9): 3929-3939. |
| [14] | 张经纬, 周弋惟, 陈卓, 徐建鸿. 微反应器内的有机合成前沿进展[J]. 化工学报, 2022, 73(8): 3472-3482. |
| [15] | 侯跃辉, 刘璇, 廉应江, 韩梅, 尧超群, 陈光文. 超声微反应器内三硝基间苯三酚合成工艺研究[J]. 化工学报, 2022, 73(8): 3597-3607. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号