化工学报 ›› 2022, Vol. 73 ›› Issue (8): 3381-3393.DOI: 10.11949/0438-1157.20220592
收稿日期:2022-04-27
修回日期:2022-08-22
出版日期:2022-08-05
发布日期:2022-09-06
通讯作者:
朱为宏
作者简介:胡宏龙(1995—),男,博士,huhlecust@foxmail.com
基金资助:
Honglong HU1( ), Zhigang ZHENG2, Weihong ZHU1(
), Zhigang ZHENG2, Weihong ZHU1( )
)
Received:2022-04-27
Revised:2022-08-22
Online:2022-08-05
Published:2022-09-06
Contact:
Weihong ZHU
摘要:
光控手性分子开关结合到液晶材料体系中,可以有效利用其光诱导的手性变化,实现远程光刺激液晶材料的自组装螺旋超结构。二芳基乙烯(DAE)是一类新型的、有前景的光致变色分子,作为智能光响应开关,在手性向列相液晶材料体系中表现出优异的性能。本文重点围绕结构设计,总结了一系列具备不同螺旋扭曲力(HTP)的手性DAE分子及其在液晶自组装螺旋结构中所产生的特定性能,如光可逆宽范围调控和光控手性反转。该类光控DAE手性向列相液晶体系在手性调控、光学显示、可调谐激光等领域具有巨大的应用潜力。最后讨论了该领域面临的挑战和机遇,并指出了未来可能的发展方向。
中图分类号:
胡宏龙, 郑致刚, 朱为宏. 基于光控二芳基乙烯的手性向列相液晶体系研究进展[J]. 化工学报, 2022, 73(8): 3381-3393.
Honglong HU, Zhigang ZHENG, Weihong ZHU. Progress of chiral nematic liquid-crystal systems with light-driven diarylethenes[J]. CIESC Journal, 2022, 73(8): 3381-3393.
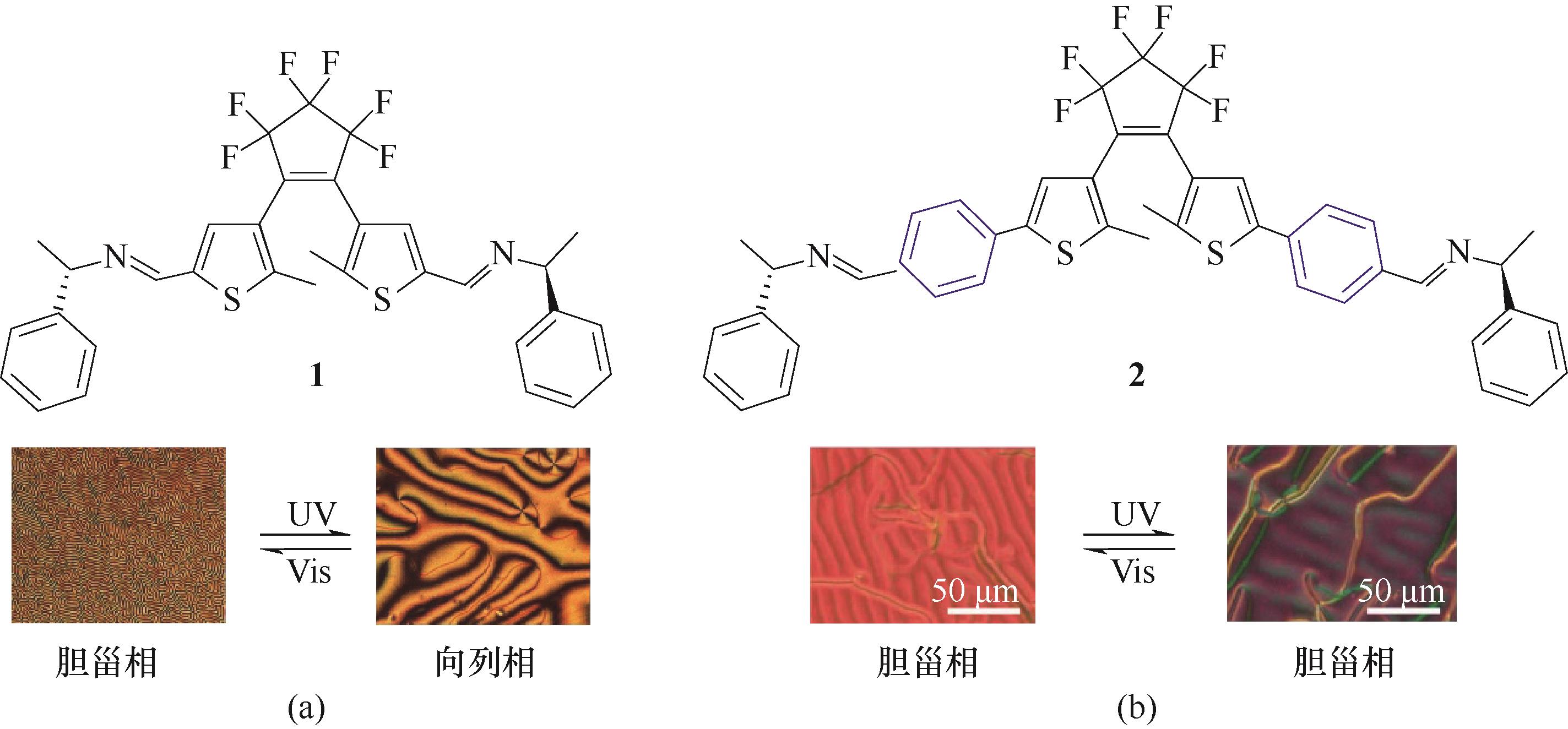
图3 手性DAE分子1 (a) [65]和分子2(b) [66]的化学结构及其对应的不同光照下的显微织构图
Fig.3 Chemical structures of chiral DAE molecule 1 (a) [65] and molecule 2 (b)[66] and their corresponding textures under different light irradiation
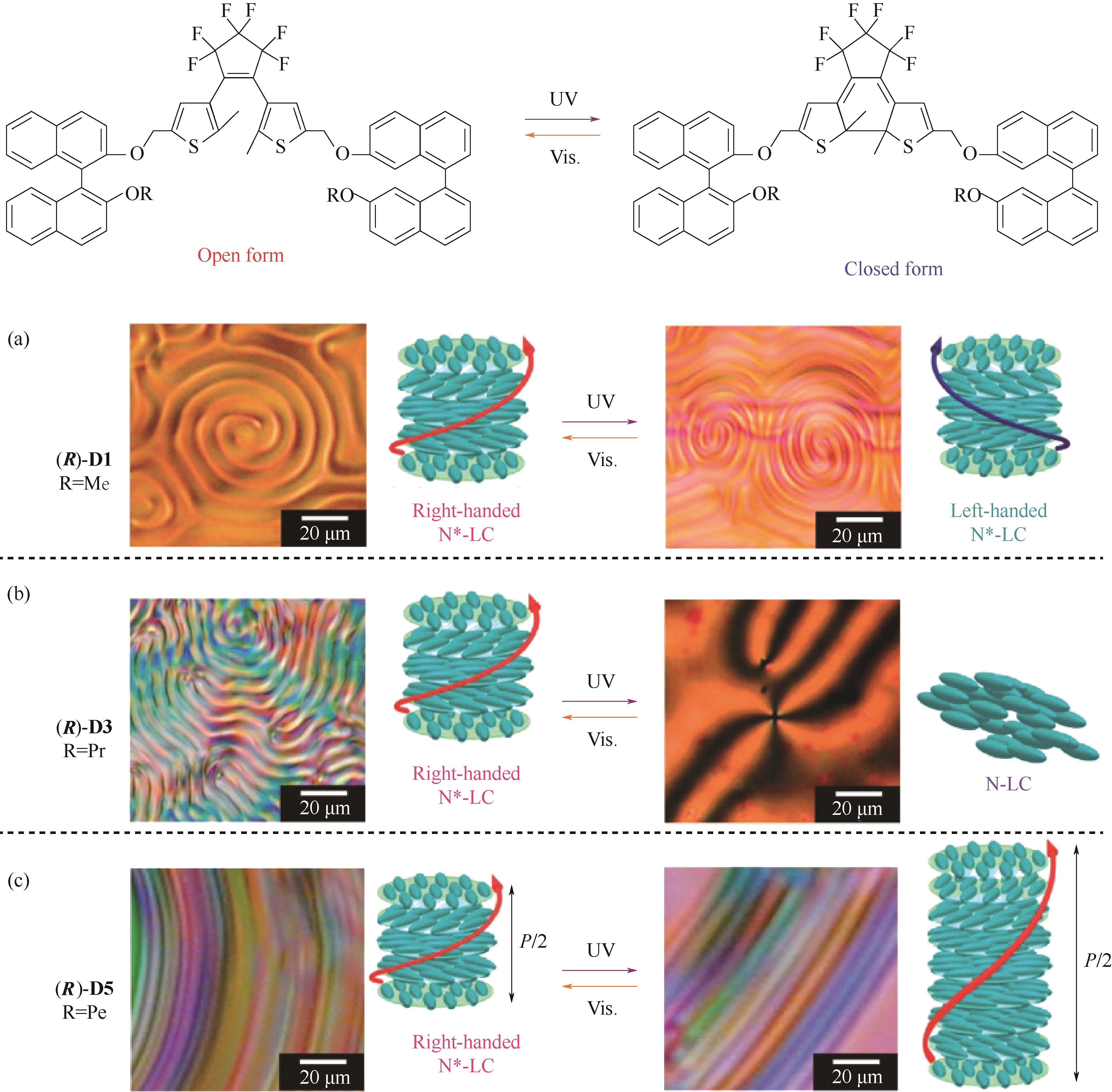
图4 手性DAE分子(R)-D1、(R)-D3、(R)-D5的化学结构及其对应的不同光照下的显微织构图[67]
Fig.4 Chemical structures of chiral DAE molecule (R)-D1, (R)-D3, (R)-D5, and their corresponding textures under different light irradiation[67]
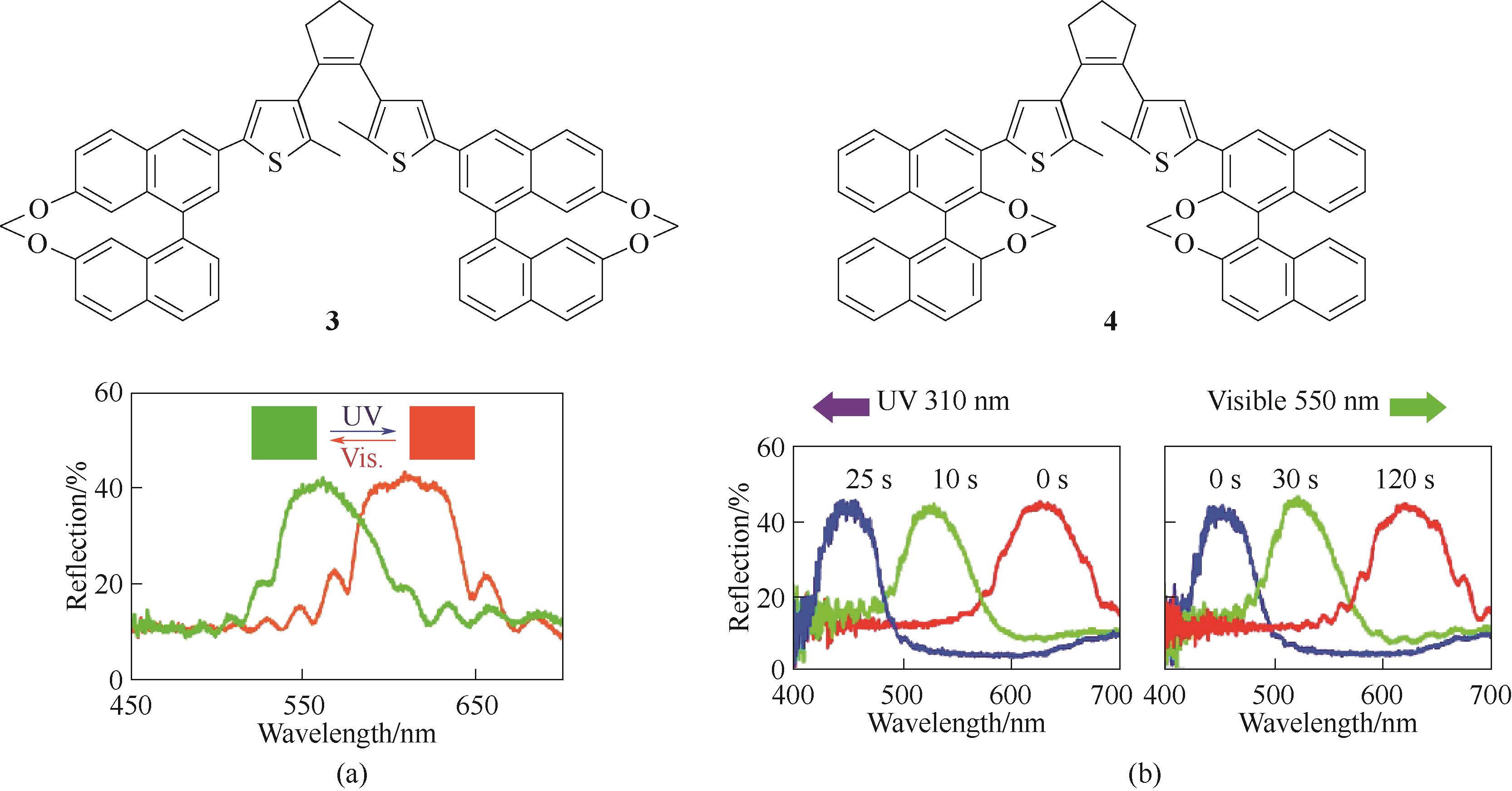
图5 手性DAE 分子3 (a) [68]和分子4 (b) [69]的化学结构及其对应的不同光照下的反射光谱图
Fig.5 Chemical structures of chiral DAE molecule 3 (a)[68] and molecule 4 (b)[69], and their corresponding reflection spectra under different light irradiation
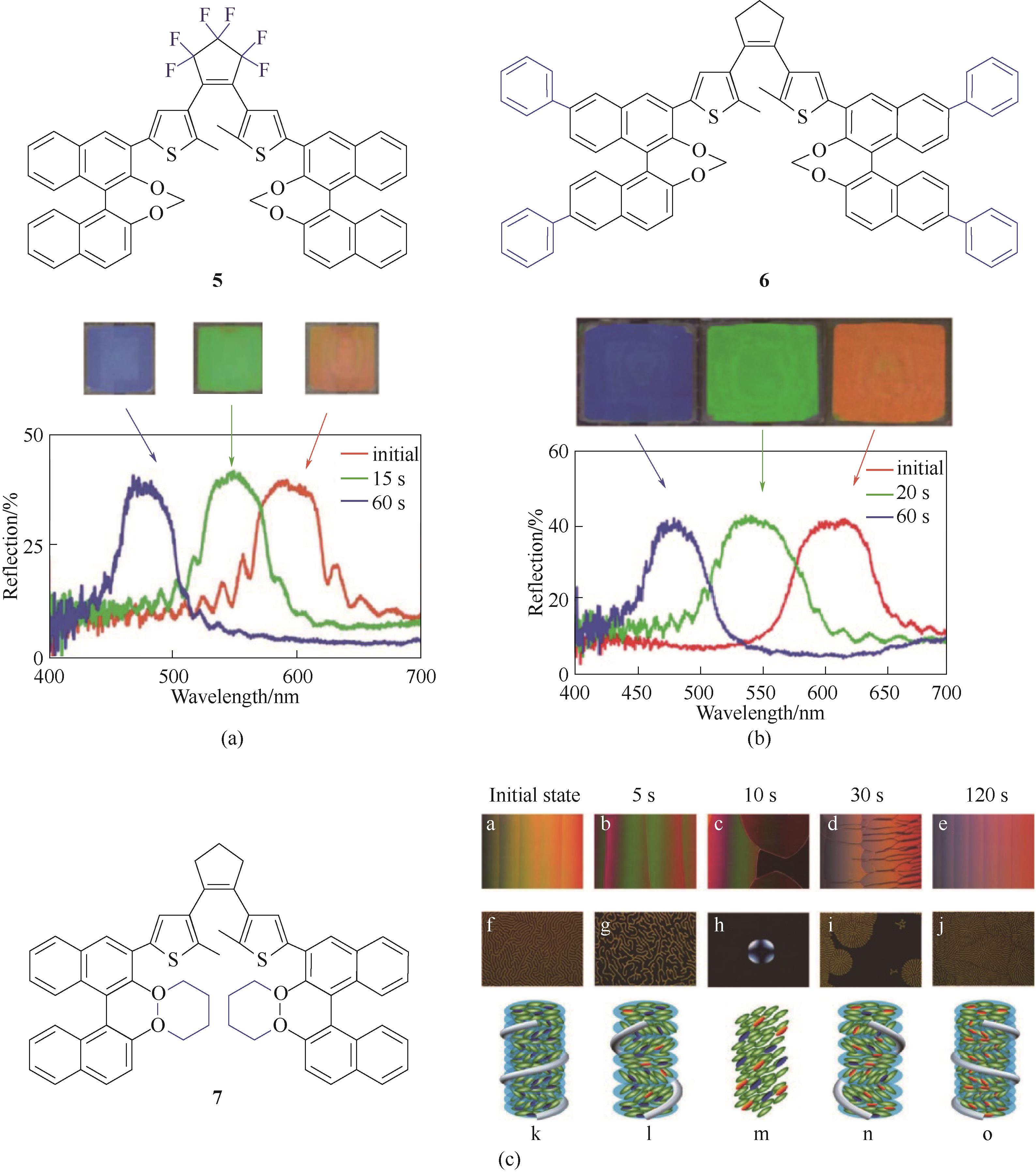
图6 手性DAE分子5(a) [70]、分子6(b) [71]、分子7(c) [72]的化学结构及其对应的不同光照下反射光谱图和手性反转图
Fig.6 Chemical structures of chiral DAE molecule 5 (a)[70], molecule 6 (b) [71] and molecule 7 (c) [72], and their corresponding reflection spectra and chirality inversion under different light irradiation

图7 手性DAE分子 (M)-1o和 (M)-2o的化学结构及其对应的不同光照下的反射光谱图[51]
Fig.7 Chemical structures of chiral DAE molecule (M)-1o and (M)-2o, and their corresponding reflection spectral under different light irradiation[51]

图8 胆甾相液晶螺旋方向调控:(a) 近红外光驱动的手性螺旋反转示意图[73];(b) 光电调控倾斜螺旋超结构体系反射光谱图[48];(c) 光驱动胆甾相螺旋轴三维调控[49]
Fig.8 Modulation of cholesteric liquid-crystal helical direction: (a) schematic illustration of near-infrared light driven chiral helix inversion[73]; (b) reflection spectra of the heliconical superstructures modulated by electric field and light[48]; (c) three-dimensional modulation of light-driven cholesteric helical axis[49]
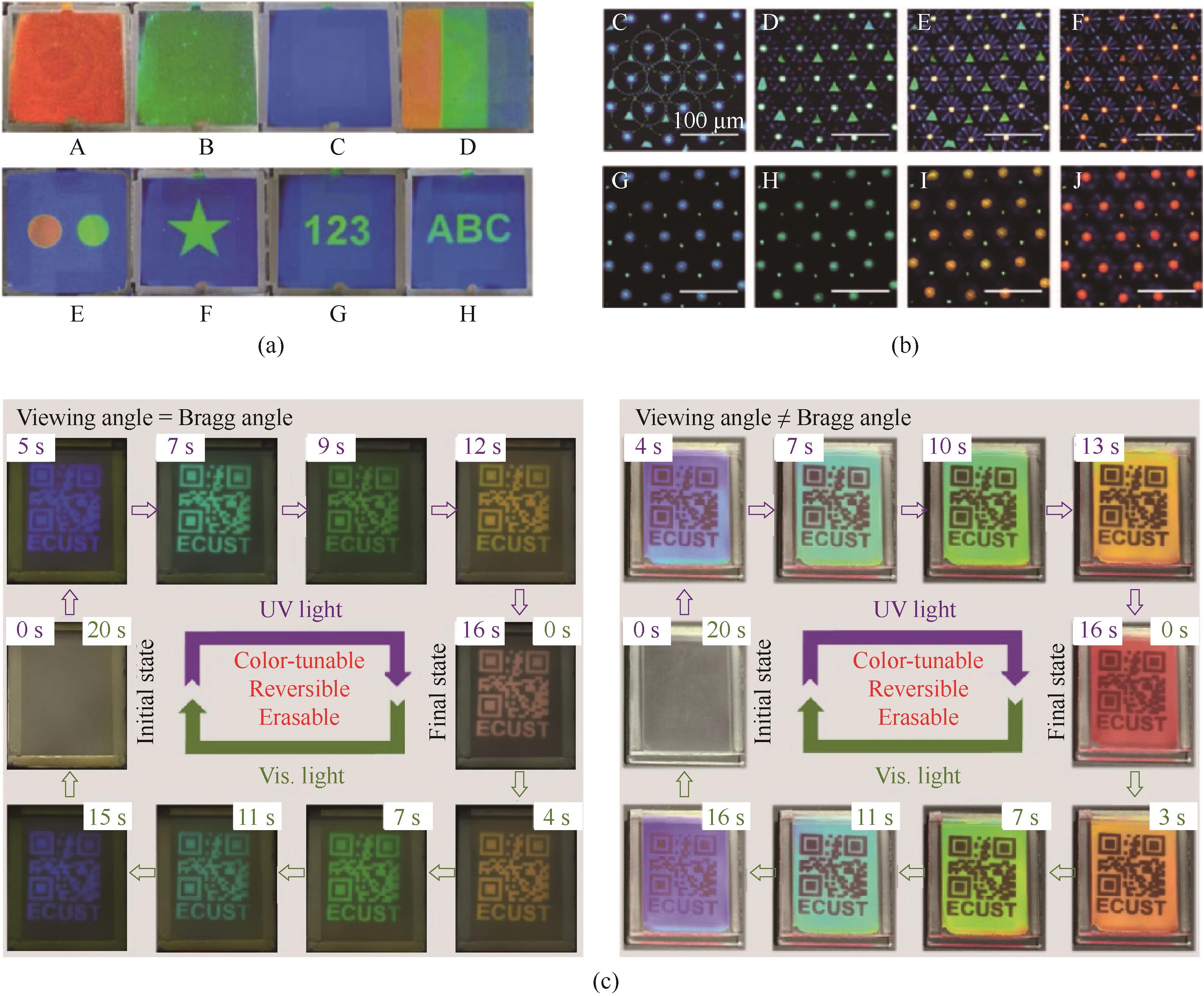
图9 多色图案显示:(a) RGB三色图案显示图[69]; (b) 液晶微滴反射模式偏振显微镜图像[50]; (c) 可逆、可擦、渐变、角度依赖的多重防伪技术[51]
Fig.9 Multicolor pattern display: (a) RGB three-color pattern display[69]; (b) polarization microscopy image of the liquid crystal droplet in reflection mode[50]; (c) reversible, erasable, gradient, angle-dependent multiple anti-counterfeiting technologies[51]

图10 光调谐激光发射:(a) 光电调控倾斜螺旋超结构体系激光光谱图[48];(b) 光调谐激光发射及其对应的反射光谱图[51];(c) 四维可调谐激光示意图[74]
Fig.10 Light modulated laser emission: (a) laser spectra of the heliconical superstructures modulated by electric field and light[48]; (b) light manipulated laser emission and its corresponding reflection spectra[51]; (c) schematic diagram of the quadri-dimensional manipulable laser[74]
| 1 | Yashima E, Ousaka N, Taura D, et al. Supramolecular helical systems: helical assemblies of small molecules, foldamers, and polymers with chiral amplification and their functions[J]. Chemical Reviews, 2016, 116(22): 13752-13990. |
| 2 | Sharma V, Crne M, Park J O, et al. Structural origin of circularly polarized iridescence in jeweled beetles[J]. Science, 2009, 325(5939): 449-451. |
| 3 | Chung W J, Oh J W, Kwak K, et al. Biomimetic self-templating supramolecular structures[J]. Nature, 2011, 478(7369): 364-368. |
| 4 | Geelhaar T, Griesar K, Reckmann B. 125 Years of liquid crystals—a scientific revolution in the home[J]. Angewandte Chemie International Edition, 2013, 52(34): 8798-8809. |
| 5 | Bremer M, Kirsch P, Klasen-Memmer M, et al. The TV in your pocket: development of liquid-crystal materials for the new millennium[J]. Angewandte Chemie International Edition, 2013, 52(34): 8880-8896. |
| 6 | Wöhrle T, Wurzbach I, Kirres J, et al. Discotic liquid crystals[J]. Chemical Reviews, 2016, 116(3): 1139-1241. |
| 7 | Akbari A, Sheath P, Martin S T, et al. Large-area graphene-based nanofiltration membranes by shear alignment of discotic nematic liquid crystals of graphene oxide[J]. Nature Communications, 2016, 7: 10891. |
| 8 | Yadav S P, Singh S. Carbon nanotube dispersion in nematic liquid crystals: an overview[J]. Progress in Materials Science, 2016, 80: 38-76. |
| 9 | Wei T, Chen P, Tang M J, et al. Liquid-crystal-mediated active waveguides toward programmable integrated optics[J]. Advanced Optical Materials, 2020, 8(10): 1902033. |
| 10 | Mitov M. Cholesteric liquid crystals with a broad light reflection band[J]. Advanced Materials, 2012, 24(47): 6260-6276. |
| 11 | Li Y F, Prince E, Cho S, et al. Periodic assembly of nanoparticle arrays in disclinations of cholesteric liquid crystals[J]. Proceedings of the National Academy of Sciences of the United States of America, 2017, 114(9): 2137-2142. |
| 12 | Ma L L, Liu C, Wu S B, et al. Programmable self-propelling actuators enabled by a dynamic helical medium[J]. Science Advances, 2021, 7(32): eabh3505. |
| 13 | Coursault D, Grand J, Zappone B, et al. Linear self-assembly of nanoparticles within liquid crystal defect arrays[J]. Advanced Materials, 2012, 24(11): 1461-1465. |
| 14 | Pelliser L, Manceau M, Lethiec C, et al. Alignment of rod-shaped single-photon emitters driven by line defects in liquid crystals[J]. Advanced Functional Materials, 2015, 25(11): 1719-1726. |
| 15 | Rožič B, Fresnais J, Molinaro C, et al. Oriented gold nanorods and gold nanorod chains within smectic liquid crystal topological defects[J]. ACS Nano, 2017, 11(7): 6728-6738. |
| 16 | Kim Y H, Yoon D K, Jeong H S, et al. Smectic liquid crystal defects for self-assembling of building blocks and their lithographic applications[J]. Advanced Functional Materials, 2011, 21(4): 610-627. |
| 17 | Pieraccini S, Masiero S, Ferrarini A, et al. Chirality transfer across length-scales in nematic liquid crystals: fundamentals and applications[J]. Chemical Society Reviews, 2011, 40(1): 258-271. |
| 18 | Lin S Y, Gutierrez-Cuevas K G, Zhang X F, et al. Fluorescent photochromic α-cyanodiarylethene molecular switches: an emerging and promising class of functional diarylethene[J]. Advanced Functional Materials, 2021, 31(7): 2007957. |
| 19 | Qin L, Liu X J, He K Y, et al. Geminate labels programmed by two-tone microdroplets combining structural and fluorescent color[J]. Nature Communications, 2021, 12: 699. |
| 20 | Mathews M, Zola R S, Hurley S, et al. Light-driven reversible handedness inversion in self-organized helical superstructures[J]. Journal of the American Chemical Society, 2010, 132(51): 18361-18366. |
| 21 | Qin L, Gu W, Wei J, et al. Piecewise phototuning of self-organized helical superstructures[J]. Advanced Materials, 2018, 30(8): 1704941. |
| 22 | Pijper D, Jongejan M G M, Meetsma A, et al. Light-controlled supramolecular helicity of a liquid crystalline phase using a helical polymer functionalized with a single chiroptical molecular switch[J]. Journal of the American Chemical Society, 2008, 130(13): 4541-4552. |
| 23 | Bisoyi H K, Li Q. Light-driven liquid crystalline materials: from photo-induced phase transitions and property modulations to applications[J]. Chemical Reviews, 2016, 116(24): 15089-15166. |
| 24 | Wang Y, Li Q. Light-driven chiral molecular switches or motors in liquid crystals[J]. Advanced Materials, 2012, 24(15): 1926-1945. |
| 25 | Tamaoki N, Song S, Moriyama M, et al. Rewritable full-color recording in a photon mode[J]. Advanced Materials, 2000, 12(2): 94-97. |
| 26 | Wang H, Bisoyi H K, Urbas A M, et al. Reversible circularly polarized reflection in a self-organized helical superstructure enabled by a visible-light-driven axially chiral molecular switch[J]. Journal of the American Chemical Society, 2019, 141(20): 8078-8082. |
| 27 | Eelkema R, Pollard M M, Katsonis N, et al. Rotational reorganization of doped cholesteric liquid crystalline films[J]. Journal of the American Chemical Society, 2006, 128(44): 14397-14407. |
| 28 | White T J, Cazzell S A, Freer A S, et al. Widely tunable, photoinvertible cholesteric liquid crystals[J]. Advanced Materials, 2011, 23(11): 1389-1392. |
| 29 | Gerber P R. On the determination of the cholesteric screw sense by the grandjean-cano-method[J]. Zeitschrift Für Naturforschung A, 1980, 35(6): 619-622. |
| 30 | Heppke G, Oestreicher F. Notizen: Bestimmung des helixdrehsinnes cholesterischer phasen mit der grandjean-cano-methode/ Determination of the helical sense of cholesteric liquid crystals using the grandjean-cano method[J]. Zeitschrift Für Naturforschung A, 1977, 32(8): 899-902. |
| 31 | Heppke G, Oestreicher F. Determination of the cholesteric screw sense[J]. Molecular Crystals and Liquid Crystals, 1978, 41(9): 245-249. |
| 32 | Irie M, Mohri M. Thermally irreversible photochromic systems. Reversible photocyclization of diarylethene derivatives[J]. The Journal of Organic Chemistry, 1988, 53(4): 803-808. |
| 33 | Irie M, Fukaminato T, Matsuda K, et al. Photochromism of diarylethene molecules and crystals: memories, switches, and actuators[J]. Chemical Reviews, 2014, 114(24): 12174-12277. |
| 34 | Kathan M, Eisenreich F, Jurissek C, et al. Light-driven molecular trap enables bidirectional manipulation of dynamic covalent systems[J]. Nature Chemistry, 2018, 10(10): 1031-1036. |
| 35 | Qi J, Chen C, Zhang X Y, et al. Light-driven transformable optical agent with adaptive functions for boosting cancer surgery outcomes[J]. Nature Communications, 2018, 9: 1848. |
| 36 | Han J L, Zhang J, Zhao T H, et al. Photoswitchable photon upconversion from turn-on mode fluorescent diarylethenes[J]. CCS Chemistry, 2021, 3(1): 665-674. |
| 37 | Celani P, Ottani S, Olivucci M, et al. What happens during the picosecond lifetime of 2A1 cyclohexa-1,3-diene? A CAS-SCF study of the cyclohexadiene/hexatriene photochemical interconversion[J]. Journal of the American Chemical Society, 1994, 116(22): 10141-10151. |
| 38 | Fukaminato T, Hirose T, Doi T, et al. Molecular design strategy toward diarylethenes that photoswitch with visible light[J]. Journal of the American Chemical Society, 2014, 136(49): 17145-17154. |
| 39 | Hebner T S, Podgórski M, Mavila S, et al. Shape permanence in diarylethene-functionalized liquid-crystal elastomers facilitated by thiol-anhydride dynamic chemistry[J]. Angewandte Chemie International Edition, 2022, 61(11): e202116522. |
| 40 | Hou L L, Zhang X Y, Cotella G F, et al. Optically switchable organic light-emitting transistors[J]. Nature Nanotechnology, 2019, 14(4): 347-353. |
| 41 | Zhang J H, Wang H P, Zhang L Y, et al. Coordinative-to-covalent transformation, isomerization dynamics, and logic gate application of dithienylethene based photochromic cages[J]. Chemical Science, 2020, 11(33): 8885-8894. |
| 42 | Cheng H B, Zhang S C, Bai E Y, et al. Future-oriented advanced diarylethene photoswitches: from molecular design to spontaneous assembly systems[J]. Advanced Materials, 2022, 34(16): 2108289. |
| 43 | Datta S, Takahashi S, Yagai S. Nanoengineering of curved supramolecular polymers: toward single-chain mesoscale materials[J]. Accounts of Materials Research, 2022, 3(2): 259-271. |
| 44 | Li R J, Tessarolo J, Lee H, et al. Multi-stimuli control over assembly and guest binding in metallo-supramolecular hosts based on dithienylethene photoswitches[J]. Journal of the American Chemical Society, 2021, 143(10): 3865-3873. |
| 45 | Okada D, Lin Z H, Huang J S, et al. Optical microresonator arrays of fluorescence-switchable diarylethenes with unreplicable spectral fingerprints[J]. Materials Horizons, 2020, 7(7): 1801-1808. |
| 46 | Li Z Q, Wang G N, Ye Y X, et al. Loading photochromic molecules into a luminescent metal-organic framework for information anticounterfeiting[J]. Angewandte Chemie International Edition, 2019, 58(50): 18025-18031. |
| 47 | Yokoyama Y, Hosoda N, Osano Y T, et al. Absolute stereochemistry and CD spectra of resolved enantiomers of the colored form of a photochromic dithienylethene[J]. Chemistry Letters, 1998, 27(11): 1093-1094. |
| 48 | Yuan C L, Huang W B, Zheng Z G, et al. Stimulated transformation of soft helix among helicoidal, heliconical, and their inverse helices[J]. Science Advances, 2019, 5(10): eaax9501. |
| 49 | Zheng Z G, Li Y N, Bisoyi H K, et al. Three-dimensional control of the helical axis of a chiral nematic liquid crystal by light[J]. Nature, 2016, 531(7594): 352-356. |
| 50 | Fan J, Li Y N, Bisoyi H K, et al. Light-directing omnidirectional circularly polarized reflection from liquid-crystal droplets[J]. Angewandte Chemie International Edition, 2015, 54(7): 2160-2164. |
| 51 | Zheng Z G, Hu H L, Zhang Z P, et al. Digital photoprogramming of liquid-crystal superstructures featuring intrinsic chiral photoswitches[J]. Nature Photonics, 2022, 16(3): 226-234. |
| 52 | Yang H, Li M Q, Li C, et al. Unraveling dual aggregation-induced emission behavior in steric-hindrance photochromic system for super resolution imaging[J]. Angewandte Chemie International Edition, 2020, 59(22): 8560-8570. |
| 53 | Li M Q, Chen L J, Cai Y S, et al. Light-driven chiral switching of supramolecular metallacycles with photoreversibility[J]. Chem, 2019, 5(3): 634-648. |
| 54 | Li Y N, Wang M F, White T J, et al. Azoarenes with opposite chiral configurations: light-driven reversible handedness inversion in self-organized helical superstructures[J]. Angewandte Chemie International Edition, 2013, 52(34): 8925-8929. |
| 55 | Wang L, Dong H, Li Y N, et al. Reversible near-infrared light directed reflection in a self-organized helical superstructure loaded with upconversion nanoparticles[J]. Journal of the American Chemical Society, 2014, 136(12): 4480-4483. |
| 56 | Wang H, Bisoyi H K, Wang L, et al. Photochemically and thermally driven full-color reflection in a self-organized helical superstructure enabled by a halogen-bonded chiral molecular switch[J]. Angewandte Chemie International Edition, 2018, 57(6): 1627-1631. |
| 57 | Qin L, Wei J, Yu Y L. Photostationary RGB selective reflection from self-organized helical superstructures for continuous photopatterning[J]. Advanced Optical Materials, 2019, 7(18): 1900430. |
| 58 | Zhang Q, Qu D H, Tian H, et al. Bottom-up: can supramolecular tools deliver responsiveness from molecular motors to macroscopic materials? [J]. Matter, 2020, 3(2): 355-370. |
| 59 | Eelkema R, Pollard M M, Vicario J, et al. Nanomotor rotates microscale objects[J]. Nature, 2006, 440(7081): 163. |
| 60 | Chen W C, Lee Y W, Chen C T. Diastereoselective, synergistic dual-mode optical switch with integrated chirochromic helicene and photochromic bis-azobenzene moieties[J]. Organic Letters, 2010, 12(7): 1472-1475. |
| 61 | Yamaguchi T, Inagawa T, Nakazumi H, et al. Photoswitching of helical twisting power of a chiral diarylethene dopant: pitch change in a chiral nematic liquid crystal[J]. Chemistry of Materials, 2000, 12(4): 869-871. |
| 62 | Yamaguchi T, Inagawa T, Nakazumi H, et al. Photoinduced pitch changes in chiral nematic liquid crystals formed by doping with chiral diarylethene[J]. Journal of Materials Chemistry, 2001, 11(10): 2453-2458. |
| 63 | Li Y N, Urbas A, Li Q. Synthesis and characterization of light-driven dithienylcyclopentene switches with axial chirality[J]. The Journal of Organic Chemistry, 2011, 76(17): 7148-7156. |
| 64 | Rameshbabu K, Urbas A, Li Q. Synthesis and characterization of thermally irreversible photochromic cholesteric liquid crystals[J]. The Journal of Physical Chemistry B. 2011, 115(13): 3409-3415. |
| 65 | Denekamp C, Feringa B L. Optically active diarylethenes for multimode photoswitching between liquid-crystalline phases[J]. Advanced Materials, 1998, 10(14): 1080-1082. |
| 66 | van Leeuwen T, Pijper T C, Areephong J, et al. Reversible photochemical control of cholesteric liquid crystals with a diamine-based diarylethene chiroptical switch[J]. Journal of Materials Chemistry, 2011, 21(9): 3142-3146. |
| 67 | Hayasaka H, Miyashita T, Nakayama M, et al. Dynamic photoswitching of helical inversion in liquid crystals containing photoresponsive axially chiral dopants[J]. Journal of the American Chemical Society, 2012, 134(8): 3758-3765. |
| 68 | Li Y N, Li Q. Photochemically reversible and thermally stable axially chiral diarylethene switches[J]. Organic Letters, 2012, 14(17): 4362-4365. |
| 69 | Li Y N, Urbas A, Li Q. Reversible light-directed red, green, and blue reflection with thermal stability enabled by a self-organized helical superstructure[J]. Journal of the American Chemical Society, 2012, 134(23): 9573-9576. |
| 70 | Li Y N, Wang M F, Urbas A, et al. A photoswitchable and thermally stable axially chiral dithienylperfluorocyclopentene dopant with high helical twisting power[J]. Journal of Materials Chemistry C, 2013, 1(25): 3917-3923. |
| 71 | Li Y N, Wang M F, Wang H, et al. Rationally designed axially chiral diarylethene switches with high helical twisting power[J]. Chemistry - A European Journal, 2014, 20(49): 16286-16292. |
| 72 | Li Y N, Xue C M, Wang M F, et al. Photodynamic chiral molecular switches with thermal stability: from reflection wavelength tuning to handedness inversion of self-organized helical superstructures[J]. Angewandte Chemie International Edition, 2013, 52(51): 13703-13707. |
| 73 | Wang L, Dong H, Li Y N, et al. Luminescence-driven reversible handedness inversion of self-organized helical superstructures enabled by a novel near-infrared light nanotransducer[J]. Advanced Materials, 2015, 27(12): 2065-2069. |
| 74 | Hu H L, Liu B H, Li M Q, et al. A quadri-dimensional manipulable laser with an intrinsic chiral photoswitch[J]. Advanced Materials, 2022, 34(15): 2110170. |
| [1] | 汤晓玲, 王嘉瑞, 朱玄烨, 郑仁朝. 基于Pickering乳液的卤醇脱卤酶催化合成手性环氧氯丙烷[J]. 化工学报, 2023, 74(7): 2926-2934. |
| [2] | 邹树平, 姜镇涛, 王志才, 柳志强, 郑裕国. 环氧化物水解酶交联细胞聚集体催化合成(R)-环氧氯丙烷[J]. 化工学报, 2020, 71(9): 4238-4245. |
| [3] | 徐超, 薛誉, 陈虹月, 胡燚. 手性脯氨酸类离子液体化学修饰猪胰脂肪酶催化性能研究[J]. 化工学报, 2019, 70(6): 2221-2228. |
| [4] | 王耀国, 赵绍磊, 杨一纯, 龚俊波, 王静康, 汤伟伟. 手性药物结晶拆分的研究进展[J]. 化工学报, 2019, 70(10): 3651-3662. |
| [5] | 吴聂, 万里鹰, 李爱妹, 肖春平. 乙烯基对苯二甲酸类甲壳型液晶高分子的形状记忆性能[J]. 化工学报, 2018, 69(5): 2282-2289. |
| [6] | 罗飞, 翁西伦, 鲍宗必, 杨亦文, 任其龙. 高稳定性硅基杂化纤维素衍生物手性固定相的制备及其色谱拆分性能[J]. 化工学报, 2015, 66(11): 4520-4525. |
| [7] | 张诗曼, 张复实, 邓爱明. 刚性与柔性连接的骨架型二芳烯聚合物[J]. 化工学报, 2015, 66(11): 4716-4721. |
| [8] | 彭益强,张曙伟. 手性化合物酶法制备中辅酶再生体系的构建与应用进展[J]. 化工进展, 2014, 33(07): 1826-1831. |
| [9] | 周勤丽,孟 枭,徐 刚,杨立荣. 基于化学修饰法探讨脂肪酶对手性伯醇的识别机理[J]. 化工进展, 2013, 32(11): 2695-2700. |
| [10] | 梁亚琴1,2,胡志勇1,曹端林1,梁 栋1. 手性Gemini表面活性剂的研究进展[J]. 化工进展, 2013, 32(07): 1649-1655. |
| [11] | 徐倩倩,苏宝根,鲍宗必,邢华斌,杨亦文,任其龙. 西酞普兰对映体的手性色谱拆分及吸附平衡[J]. 化工学报, 2012, 63(4): 1095-1101. |
| [12] | 何年志,张学勤,肖美添. 手性修饰催化剂在多相不对称氢化中的研究进展[J]. 化工进展, 2012, 31(12): 2694-2701. |
| [13] | 刘会景1,霍翠婷2,邱敏清2. 液晶显示上游材料中国专利分析[J]. 化工进展, 2012, 31(11): 2589-2592. |
| [14] | 杨 洁,叶代勇. 纳米纤维素晶须表面接枝及其液晶性能研究进展[J]. 化工进展, 2012, 31(09): 1990-1997. |
| [15] | 王 俊,陈 帅,李翠勤,杨 光. 手性树枝状大分子催化剂的合成及其在不对称催化中的应用进展[J]. 化工进展, 2012, 31(02 ): 322-330. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号