化工学报 ›› 2025, Vol. 76 ›› Issue (9): 4872-4881.DOI: 10.11949/0438-1157.20250139
蒋智洪1,2( ), 雷骞1,2, 朱引军1,2, 雷志刚3, 陈洪林1,2(
), 雷骞1,2, 朱引军1,2, 雷志刚3, 陈洪林1,2( )
)
收稿日期:2025-02-17
修回日期:2025-05-30
出版日期:2025-09-25
发布日期:2025-10-23
通讯作者:
陈洪林
作者简介:蒋智洪(2000—),男,硕士研究生,jiangzhihong22@mails.ucas.ac.cn
基金资助:
Zhihong JIANG1,2( ), Qian LEI1,2, Yinjun ZHU1,2, Zhigang LEI3, Honglin CHEN1,2(
), Qian LEI1,2, Yinjun ZHU1,2, Zhigang LEI3, Honglin CHEN1,2( )
)
Received:2025-02-17
Revised:2025-05-30
Online:2025-09-25
Published:2025-10-23
Contact:
Honglin CHEN
摘要:
在Aspen Plus上采用UNIFAC方法构建了用于描述三聚甲醛体系的汽液平衡模型,结果表明本模型在温度处于343~423 K,甲醛浓度低于65%(质量分数,余同)以及三聚甲醛浓度低于80%时拥有良好的精度,相对平均偏差小于9.15%。同时拟合和估算了系统的密度、黏度和相变焓,与文献值相比,最大相对平均偏差不超过8.17%。在此基础上,对三聚甲醛生产工艺中的浓缩塔进行设计和优化,探讨了进料甲醇对三聚甲醛提浓塔的影响,发现含有甲醇时提浓塔的能耗更高。此外,对提浓塔进行水力学校核,以保证设计的完整性。
中图分类号:
蒋智洪, 雷骞, 朱引军, 雷志刚, 陈洪林. 三聚甲醛体系物性模型和提浓工艺研究[J]. 化工学报, 2025, 76(9): 4872-4881.
Zhihong JIANG, Qian LEI, Yinjun ZHU, Zhigang LEI, Honglin CHEN. Study on physical property model and enrichment process of trioxane system[J]. CIESC Journal, 2025, 76(9): 4872-4881.
| 反应 | A | B | C | D |
|---|---|---|---|---|
| 式(2) | -30.946 | 4819.0 | 3.7410 | -0.004534 |
| -30.941 | 5653.0 | 3.7410 | -0.004534 | |
| -30.933 | 5361.0 | 3.7410 | -0.004534 | |
| 式(4) | 1129.7 | -25100 | -198.40 | 0.3160 |
| 1129.0 | -25510 | -198.40 | 0.3160 | |
| 1129.0 | -25630 | -198.40 | 0.3160 |
表1 化学反应平衡常数ki 的相关参数[19]
Table 1 Related parameters of chemical reaction equilibrium constant ki[19]
| 反应 | A | B | C | D |
|---|---|---|---|---|
| 式(2) | -30.946 | 4819.0 | 3.7410 | -0.004534 |
| -30.941 | 5653.0 | 3.7410 | -0.004534 | |
| -30.933 | 5361.0 | 3.7410 | -0.004534 | |
| 式(4) | 1129.7 | -25100 | -198.40 | 0.3160 |
| 1129.0 | -25510 | -198.40 | 0.3160 | |
| 1129.0 | -25630 | -198.40 | 0.3160 |
| 物质 | 拆分基团 |
|---|---|
| CH2O | 1·CH2O |
| H2O | 1·H2O |
| HOCH2OH | 1·HOCH2OH |
| HO(CH2O) n H,n>1 | 1·H2O n·CH2O |
| CH3OH | 1·CH3OH |
| HOCH2OCH3 | 1·CH3O 1·CH2OH |
| HO(CH2O) n CH3,n>1 | 1·CH3O 1·CH2OH n-1·CH2O |
| (CH2O)3 | 1·(CH2O)3 |
表2 三聚甲醛溶液中各组分的UNIFAC基团[13]
Table 2 UNIFAC groups of components in trioxane solutions[13]
| 物质 | 拆分基团 |
|---|---|
| CH2O | 1·CH2O |
| H2O | 1·H2O |
| HOCH2OH | 1·HOCH2OH |
| HO(CH2O) n H,n>1 | 1·H2O n·CH2O |
| CH3OH | 1·CH3OH |
| HOCH2OCH3 | 1·CH3O 1·CH2OH |
| HO(CH2O) n CH3,n>1 | 1·CH3O 1·CH2OH n-1·CH2O |
| (CH2O)3 | 1·(CH2O)3 |
| 基团 | 编号 | R | Q | 文献 |
|---|---|---|---|---|
| —OH | 1 | 1 | 1.2 | [ |
| —CH2O— | 2 | 0.9183 | 0.78 | [ |
| —CH2— | 3 | 0.6744 | 0.54 | [ |
| H2O | 4 | 0.92 | 1.4 | [ |
| HOCH2OH | 5 | 2.6744 | 2.94 | [ |
| (CH2O)3 | 6 | 2.754 | 3.3 | [ |
| CH3OH | 7 | 1.4311 | 1.432 | [ |
| —CH3O | 8 | 1.1459 | 1.088 | [ |
| —CH2OH | 9 | 1.2044 | 1.124 | [ |
表3 UNIFAC基团的体积参数R和表面积参数Q
Table 3 Size and surface parameters of UNIFAC group
| 基团 | 编号 | R | Q | 文献 |
|---|---|---|---|---|
| —OH | 1 | 1 | 1.2 | [ |
| —CH2O— | 2 | 0.9183 | 0.78 | [ |
| —CH2— | 3 | 0.6744 | 0.54 | [ |
| H2O | 4 | 0.92 | 1.4 | [ |
| HOCH2OH | 5 | 2.6744 | 2.94 | [ |
| (CH2O)3 | 6 | 2.754 | 3.3 | [ |
| CH3OH | 7 | 1.4311 | 1.432 | [ |
| —CH3O | 8 | 1.1459 | 1.088 | [ |
| —CH2OH | 9 | 1.2044 | 1.124 | [ |
| i | j | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | — | 28.06 | 156.4 | 353.5 | 353.5 | 28.06 | -137.1 | 112.8 | -137.1 |
| 2 | 237.7 | — | 83.36 | 867.8 | 189.2 | a26 | 238.4 | 0 | 238.4 |
| 3 | 986.5 | 251.5 | — | 1318 | 1318 | 251.5 | 697.2 | 447.8 | 697.2 |
| 4 | -229.1 | -254.5 | 300 | — | 189.5 | 80.63 | 289.6 | -219.3 | a49 |
| 5 | -229.1 | 59.2 | 300 | -191.8 | — | 80.63 | 289.6 | -142.4 | 289.6 |
| 6 | 237.7 | a62 | 83.36 | 379.4 | 379.4 | — | 239.6 | 0 | 392.20 |
| 7 | 249.1 | -128.6 | 16.5 | -181.0 | -181 | -16.67 | — | -128.6 | 0 |
| 8 | 1164.8 | 0 | 273 | 423.8 | 774.8 | 0 | 238.4 | — | 238.4 |
| 9 | 249.1 | -128.6 | 16.5 | a94 | -181 | -187.7 | 0 | -128.6 | — |
表4 UNIFAC基团的相互作用参数aij[19]
Table 4 UNIFAC group interaction parameters aij[19]
| i | j | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | — | 28.06 | 156.4 | 353.5 | 353.5 | 28.06 | -137.1 | 112.8 | -137.1 |
| 2 | 237.7 | — | 83.36 | 867.8 | 189.2 | a26 | 238.4 | 0 | 238.4 |
| 3 | 986.5 | 251.5 | — | 1318 | 1318 | 251.5 | 697.2 | 447.8 | 697.2 |
| 4 | -229.1 | -254.5 | 300 | — | 189.5 | 80.63 | 289.6 | -219.3 | a49 |
| 5 | -229.1 | 59.2 | 300 | -191.8 | — | 80.63 | 289.6 | -142.4 | 289.6 |
| 6 | 237.7 | a62 | 83.36 | 379.4 | 379.4 | — | 239.6 | 0 | 392.20 |
| 7 | 249.1 | -128.6 | 16.5 | -181.0 | -181 | -16.67 | — | -128.6 | 0 |
| 8 | 1164.8 | 0 | 273 | 423.8 | 774.8 | 0 | 238.4 | — | 238.4 |
| 9 | 249.1 | -128.6 | 16.5 | a94 | -181 | -187.7 | 0 | -128.6 | — |
| 物质 | Ai | Bi | Ci | 文献 |
|---|---|---|---|---|
| CH2O | 14.4625 | -2204.13 | -30.15 | [ |
| H2O | 16.2886 | -3816.44 | -46.13 | [ |
| CH3OH | 16.5725 | -3626.55 | -34.29 | [ |
| HOCH2OH | 17.4364 | -4762.07 | -51.2 | [ |
| HOCH2OCH3 | 19.5736 | -5646.71 | 0 | [ |
| (CH2O)3 | 14.3796 | -3099.47 | -68.92 | [ |
表5 纯组分蒸气压的参数
Table 5 Parameters of pure component vapor pressure
| 物质 | Ai | Bi | Ci | 文献 |
|---|---|---|---|---|
| CH2O | 14.4625 | -2204.13 | -30.15 | [ |
| H2O | 16.2886 | -3816.44 | -46.13 | [ |
| CH3OH | 16.5725 | -3626.55 | -34.29 | [ |
| HOCH2OH | 17.4364 | -4762.07 | -51.2 | [ |
| HOCH2OCH3 | 19.5736 | -5646.71 | 0 | [ |
| (CH2O)3 | 14.3796 | -3099.47 | -68.92 | [ |

图3 甲醛-水-甲醇溶液汽液平衡时的计算值与文献值对比
Fig.3 Comparison of calculated value and literature value in vapor-liquid equilibrium of formaldehyde-water-methanol solution
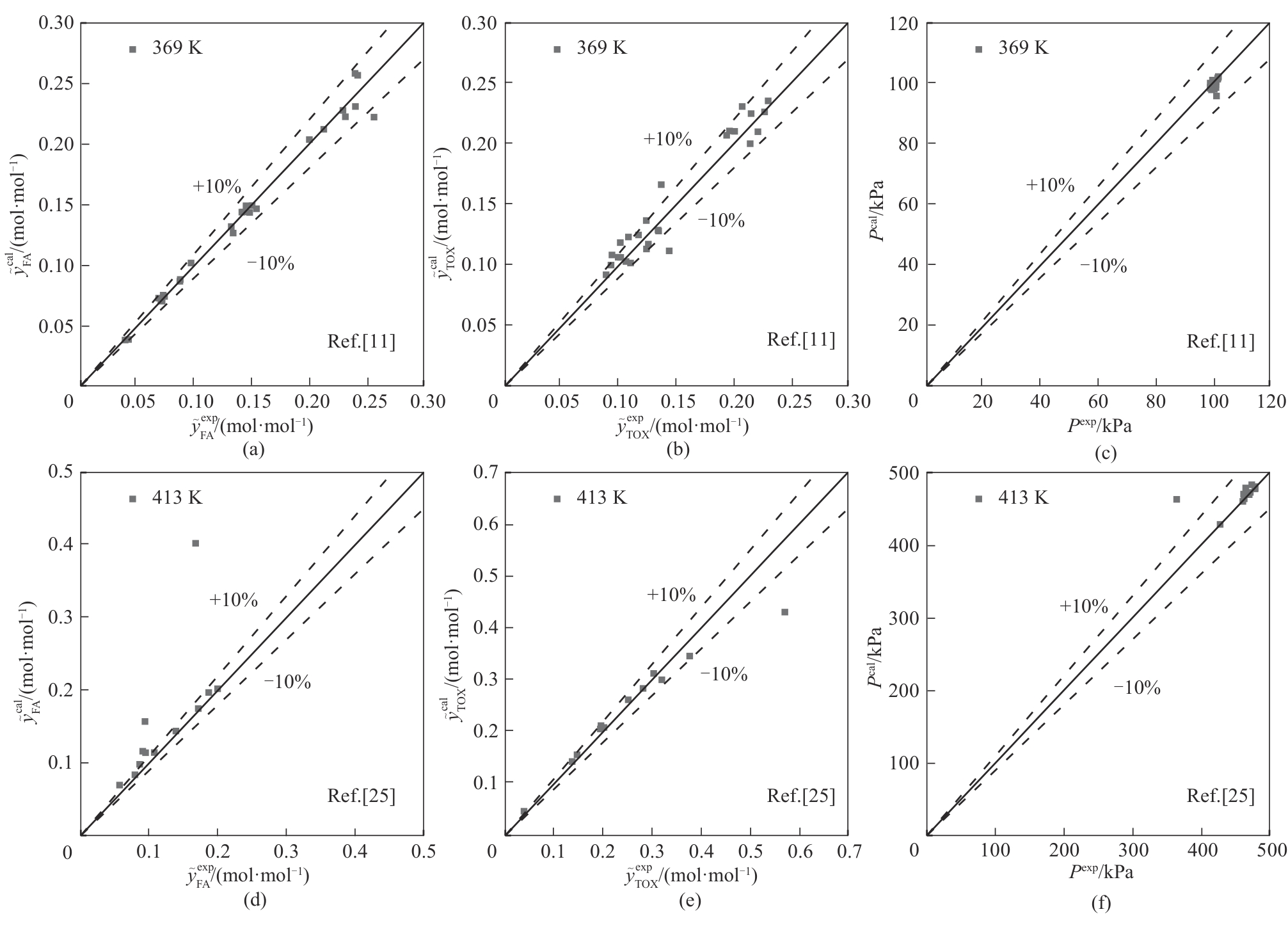
图4 甲醛-水-三聚甲醛溶液汽液平衡时的计算值与文献值对比
Fig.4 Comparison of calculated value and literature value in vapor-liquid equilibrium of formaldehyde-water- trioxane solution
| 模型 | 体系 | Δ | Δ | Δ | Δp/% |
|---|---|---|---|---|---|
| 本模型 | 甲醛-水 | 4.64 | — | — | 0.81 |
| 甲醛-水-甲醇 | 9.15 | — | 5.26 | 2.35 | |
| 甲醛-水-三聚甲醛 | 7.31 | 7.93 | — | 0.97 | |
| Bongartz等[ | 甲醛-水 | 3.51 | — | — | 1.20 |
| 甲醛-水-甲醇 | 14.04 | — | 10.97 | 3.03 | |
| 甲醛-水-三聚甲醛 | 19.38 | 31.16 | — | 5.95 | |
| Schemme等[ | 甲醛-水 | 14.61 | — | — | 4.63 |
| 甲醛-水-甲醇 | 5.91 | — | 7.09 | 3.59 | |
| 甲醛-水-三聚甲醛 | 11.91 | 8.72 | — | 2.08 |
表6 不同体系的相对平均偏差
Table 6 Relative average deviation of different systems
| 模型 | 体系 | Δ | Δ | Δ | Δp/% |
|---|---|---|---|---|---|
| 本模型 | 甲醛-水 | 4.64 | — | — | 0.81 |
| 甲醛-水-甲醇 | 9.15 | — | 5.26 | 2.35 | |
| 甲醛-水-三聚甲醛 | 7.31 | 7.93 | — | 0.97 | |
| Bongartz等[ | 甲醛-水 | 3.51 | — | — | 1.20 |
| 甲醛-水-甲醇 | 14.04 | — | 10.97 | 3.03 | |
| 甲醛-水-三聚甲醛 | 19.38 | 31.16 | — | 5.95 | |
| Schemme等[ | 甲醛-水 | 14.61 | — | — | 4.63 |
| 甲醛-水-甲醇 | 5.91 | — | 7.09 | 3.59 | |
| 甲醛-水-三聚甲醛 | 11.91 | 8.72 | — | 2.08 |
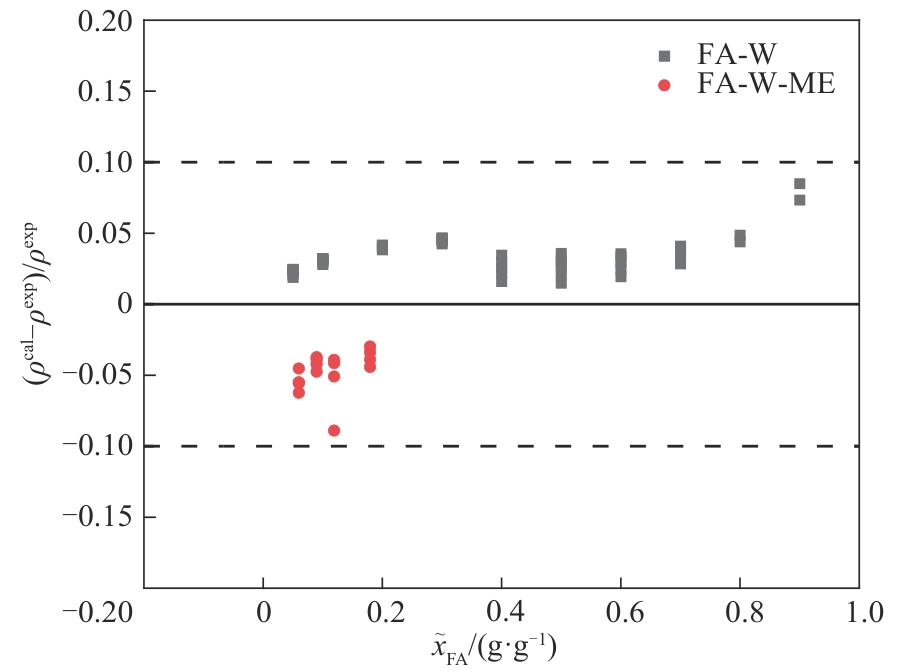
图5 甲醛-水(293~383 K)及甲醛-水-甲醇(283~333 K)体系的计算密度和文献密度[28]的相对偏差
Fig.5 Relative deviation of calculated density and literature density[28] for the formaldehyde-water (293—383 K) and formaldehyde-water-methanol (283—333 K) systems
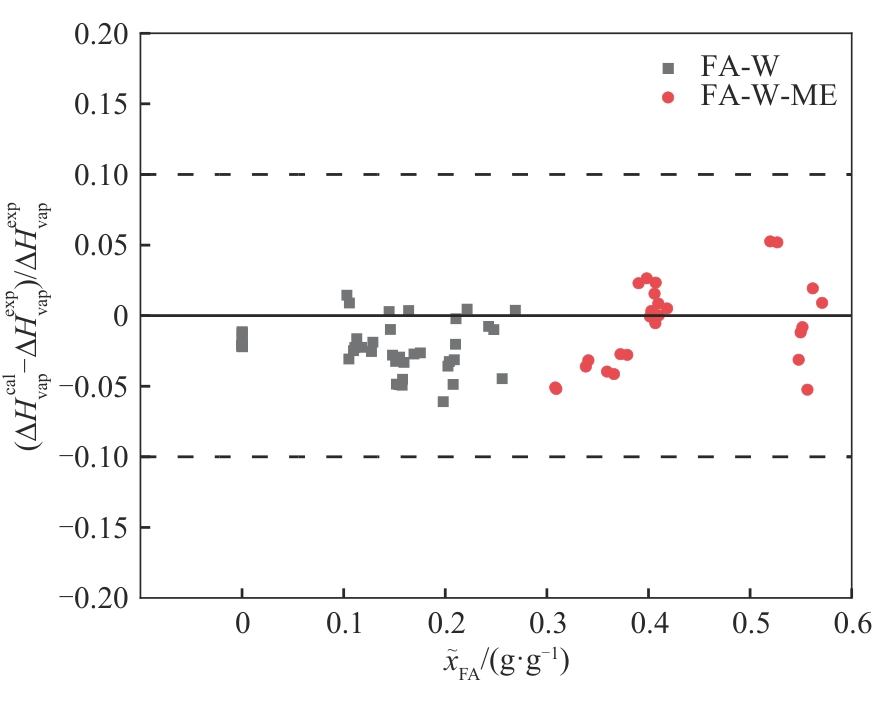
图6 甲醛-水(322~362 K)及甲醛-水-甲醇(311~332 K)体系相变焓的计算值和文献值[29]的相对偏差
Fig.6 Relative deviation of calculated enthalpy of phase transition and literature values[29] for the formaldehyde-water (322—362 K) and formaldehyde-water-methanol (311—332 K) systems
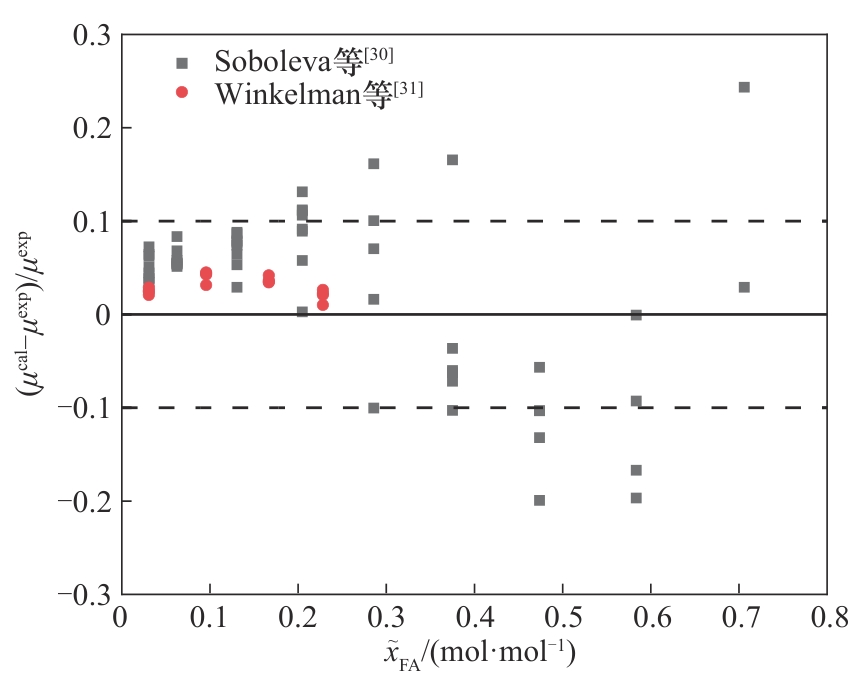
图7 甲醛-水(293~373 K)体系黏度的计算值与文献值的相对偏差
Fig.7 Relative deviation of calculated viscosity and literature values for the formaldehyde-water (293—373 K) system
| 进料 | 馏出物进料比/(g·g-1) | 回流比/(g·g-1) | 理论进料位置 | 理论塔板数 | 总能耗/kW |
|---|---|---|---|---|---|
| 不含甲醇 | 0.30 | 0.28 | 2 | 14 | 18.92 |
| 含甲醇 | 0.30 | 0.39 | 2 | 13 | 21.61 |
表7 提浓塔的操作条件
Table 7 Operating conditions of concentration column
| 进料 | 馏出物进料比/(g·g-1) | 回流比/(g·g-1) | 理论进料位置 | 理论塔板数 | 总能耗/kW |
|---|---|---|---|---|---|
| 不含甲醇 | 0.30 | 0.28 | 2 | 14 | 18.92 |
| 含甲醇 | 0.30 | 0.39 | 2 | 13 | 21.61 |
| 条件 | 流量/(kg·h-1) | 甲醛浓度/(g·g-1) | 水浓度/(g·g-1) | 甲醇浓度/(g·g-1) | 三聚甲醛浓度/(g·g-1) | |
|---|---|---|---|---|---|---|
| 含甲醇 | 进料 | 100 | 0.3933 | 0.4469 | 0.0107 | 0.1491 |
| 塔顶 | 29.52 | 0.1445 | 0.3355 | 0.0199 | 0.5001 | |
| 塔底 | 70.48 | 0.4975 | 0.4935 | 0.0069 | 0.0021 | |
| 不含甲醇 | 进料 | 100 | 0.3936 | 0.4548 | — | 0.1516 |
| 塔顶 | 30.02 | 0.1512 | 0.3487 | — | 0.5001 | |
| 塔底 | 69.98 | 0.4976 | 0.5003 | — | 0.0021 |
表8 提浓塔进出流量与组成
Table 8 Inlet and outlet flow rates and compositions of the concentration column
| 条件 | 流量/(kg·h-1) | 甲醛浓度/(g·g-1) | 水浓度/(g·g-1) | 甲醇浓度/(g·g-1) | 三聚甲醛浓度/(g·g-1) | |
|---|---|---|---|---|---|---|
| 含甲醇 | 进料 | 100 | 0.3933 | 0.4469 | 0.0107 | 0.1491 |
| 塔顶 | 29.52 | 0.1445 | 0.3355 | 0.0199 | 0.5001 | |
| 塔底 | 70.48 | 0.4975 | 0.4935 | 0.0069 | 0.0021 | |
| 不含甲醇 | 进料 | 100 | 0.3936 | 0.4548 | — | 0.1516 |
| 塔顶 | 30.02 | 0.1512 | 0.3487 | — | 0.5001 | |
| 塔底 | 69.98 | 0.4976 | 0.5003 | — | 0.0021 |
| 塔板 | 填料高度/m | 液泛率/% | 压降/kPa | 持液量/L |
|---|---|---|---|---|
| 2 | 0.5 | 77.45 | 0.14 | 9.90 |
| 3 | 1.0 | 75.78 | 0.13 | 9.80 |
| 4 | 1.5 | 73.91 | 0.12 | 9.70 |
| 5 | 2.0 | 72.02 | 0.11 | 9.60 |
| 6 | 2.5 | 70.23 | 0.10 | 9.51 |
| 7 | 3.0 | 68.69 | 0.09 | 9.44 |
| 8 | 3.5 | 67.43 | 0.09 | 9.37 |
| 9 | 4.0 | 66.45 | 0.08 | 9.33 |
| 10 | 4.5 | 65.72 | 0.08 | 9.29 |
| 11 | 5.0 | 65.15 | 0.08 | 9.27 |
| 12 | 5.5 | 64.65 | 0.08 | 9.27 |
表9 提浓塔的水力学设计
Table 9 Hydraulic design of concentration column
| 塔板 | 填料高度/m | 液泛率/% | 压降/kPa | 持液量/L |
|---|---|---|---|---|
| 2 | 0.5 | 77.45 | 0.14 | 9.90 |
| 3 | 1.0 | 75.78 | 0.13 | 9.80 |
| 4 | 1.5 | 73.91 | 0.12 | 9.70 |
| 5 | 2.0 | 72.02 | 0.11 | 9.60 |
| 6 | 2.5 | 70.23 | 0.10 | 9.51 |
| 7 | 3.0 | 68.69 | 0.09 | 9.44 |
| 8 | 3.5 | 67.43 | 0.09 | 9.37 |
| 9 | 4.0 | 66.45 | 0.08 | 9.33 |
| 10 | 4.5 | 65.72 | 0.08 | 9.29 |
| 11 | 5.0 | 65.15 | 0.08 | 9.27 |
| 12 | 5.5 | 64.65 | 0.08 | 9.27 |
| [1] | Masamoto J, Hamanaka K, Yoshida K, et al. Synthesis of trioxane using heteropolyacids as catalyst[J]. Angewandte Chemie International Edition, 2000, 39(12): 2102-2104. |
| [2] | Curioni A, Sprik M, Andreoni W, et al. Density functional theory-based molecular dynamics simulation of acid-catalyzed chemical reactions in liquid trioxane[J]. Journal of the American Chemical Society, 1997, 119(31): 7218-7229. |
| [3] | Curioni A, Andreoni W, Hutter J, et al. Density-functional-theory-based molecular dynamics study of 1,3,5-trioxane and 1,3-dioxolane protolysis[J]. Journal of the American Chemical Society, 1994, 116(25): 11251-11255. |
| [4] | Ma W T, Hu Y F, Qi J G, et al. Acid-catalyzed synthesis of trioxane in aprotic media[J]. Industrial & Engineering Chemistry Research, 2017, 56(24): 6910-6915. |
| [5] | Pei X P, Li H, Zhang Z S, et al. Process intensification for energy efficient reactive distillation of trioxane production from aqueous formaldehyde[J]. Chemical Engineering and Processing-Process Intensification, 2022, 175: 108914. |
| [6] | 陈杰, 王洪波, 程锐. 离子液法与硫酸法生产三聚甲醛(TOX)工艺技术对比[J]. 化工技术与开发, 2013, 42(6): 60-62. |
| Chen J, Wang H B, Cheng R. Comparison between ionic liquid method and sulfuric acid method of trioxane preparation[J]. Technology & Development of Chemical Industry, 2013, 42(6): 60-62. | |
| [7] | 关键, 林陵, 曾崇余. PW12/AC催化剂在合成三聚甲醛中的催化性能研究[J]. 天然气化工, 2005, 30(4): 19-22. |
| Guan J, Lin L, Zeng C Y. Trioxane synthesis from formaldehyde over the supperted PW12/AC catalyst[J]. Natural Gas Chemical Industry, 2005, 30(4): 19-22. | |
| [8] | Qi J G, Hu Y F, Ma W T, et al. The reactions that determine the yield and selectivity of 1,3,5-trioxane[J]. Chemical Engineering Journal, 2018, 331: 311-316. |
| [9] | Augé J, Gil R. A convenient solvent-free preparation of 1,3,5-trioxanes[J]. Tetrahedron Letters, 2002, 43(44): 7919-7920. |
| [10] | 范娟娟, 王士明, 刘剡. 三聚甲醛生产装置工艺优化研究[J]. 当代化工研究, 2019(3): 142-143. |
| Fan J J, Wang S M, Liu Y. Research on process optimization of trioxane production unit[J]. Modern Chemical Research, 2019(3): 142-143. | |
| [11] | Maurer G. Vapor-liquid equilibrium of formaldehyde-and water-containing multicomponent mixtures[J]. AIChE Journal, 1986, 32(6): 932-948. |
| [12] | Grützner T, Hasse H, Lang N, et al. Development of a new industrial process for trioxane production[J]. Chemical Engineering Science, 2007, 62(18/19/20): 5613-5620. |
| [13] | Hasse H, Hahnenstein I, Maurer G. Revised vapor-liquid equilibrium model for multicomponent formaldehyde mixtures[J]. AIChE Journal, 1990, 36(12): 1807-1814. |
| [14] | Schemme S, Meschede S, Köller M, et al. Property data estimation for hemiformals, methylene glycols and polyoxymethylene dimethyl ethers and process optimization in formaldehyde synthesis[J]. Energies, 2020, 13(13): 3401. |
| [15] | Schmitz N, Friebel A, von Harbou E V, et al. Liquid-liquid equilibrium in binary and ternary mixtures containing formaldehyde, water, methanol, methylal, and poly(oxymethylene) dimethyl ethers[J]. Fluid Phase Equilibria, 2016, 425: 127-135. |
| [16] | Fredenslund A, Jones R L, Prausnitz J M. Group-contribution estimation of activity coefficients in nonideal liquid mixtures[J]. AIChE Journal, 1975, 21(6): 1086-1099. |
| [17] | Albert M. Thermodynamische eigenschaften formaldehydhaltiger mischungen[D]. Kaiserslautern: Technische Universität Kaiserslautern, 1999. |
| [18] | Kuhnert C. Dampf-flüssigkeits-gleichgewichte in mehrkomponentigen formaldhydhaltigen [formaldehydhaltigen] systemen[D]. Kaiserslautern: Technische Universität Kaiserslautern, 2004. |
| [19] | Schmitz N, Breitkreuz C F, Ströfer E, et al. Vapor-liquid equilibrium and distillation of mixtures containing formaldehdye and poly(oxymethylene) dimethyl ethers[J]. Chemical Engineering and Processing -Process Intensification, 2018, 131: 116-124. |
| [20] | Bai Z M, Liu H H, Liu Y S, et al. Prediction of the vapor-liquid equilibrium of chemical reactive systems containing formaldehyde using the COSMO-RS method[J]. Fluid Phase Equilibria, 2016, 415: 125-133. |
| [21] | Breitkreuz C F, Dyga M, Forte E, et al. Conceptual design of a crystallization-based trioxane production process[J]. Chemical Engineering and Processing-Process Intensification, 2022, 171: 108710. |
| [22] | Albert M, Hahnenstein I, Hasse H, et al. Vapor-liquid equilibrium of formaldehyde mixtures: new data and model revision[J]. AIChE Journal, 1996, 42(6): 1741-1752. |
| [23] | Albert M, García B C, Kuhnert C, et al. Vapor-liquid equilibrium of aqueous solutions of formaldehyde and methanol[J]. AIChE Journal, 2000, 46(8): 1676-1687. |
| [24] | Albert M, García B C, Kreiter C, et al. et al. Vapor-liquid and chemical equilibria of formaldehyde-water mixtures[J]. AIChE Journal, 1999, 45(9): 2024-2033. |
| [25] | Albert M, Hasse H, Kuhnert C, et al. New experimental results for the vapor-liquid equilibrium of the binary system (trioxane+ water) and the ternary system (formaldehyde+ trioxane+ water)[J]. Journal of Chemical & Engineering Data, 2005, 50(4): 1218-1223. |
| [26] | Kuhnert C, Albert M, Breyer S, et al. Phase equilibrium in formaldehyde containing multicomponent mixtures: experimental results for fluid phase equilibria of (formaldehyde+(water or methanol)+ methylal)) and (formaldehyde+ water+ methanol+ methylal) and comparison with predictions[J]. Industrial & Engineering Chemistry Research, 2006, 45(14): 5155-5164. |
| [27] | Bongartz D, Burre J, Mitsos A. Production of oxymethylene dimethyl ethers from hydrogen and carbon dioxide(part Ⅰ): Modeling and analysis for OME1 [J]. Industrial & Engineering Chemistry Research, 2019, 58(12): 4881-4889. |
| [28] | Dyga M, Keller A, Hasse H. Density of solutions of formaldehyde in water and alcohols[J]. AIChE Journal, 2022, 68(4): e17573. |
| [29] | Liu Y Q, Hasse H, Maurer G. Enthalpy change on vaporization of aqueous and methanolic formaldehyde solutions[J]. AIChE Journal, 1992, 38(11): 1693-1702. |
| [30] | Soboleva O, Blazhin Y M, Ogorodnikov S. Formaldehyde-water system(communication Ⅰ): Physicochemical properties of individual hydroxymethylene hydrates[J]. Zhurnal Pikladnoi Khimii, 1979, 52(7): 1519-1523. |
| [31] | Winkelman J G M, Beenackers A A C M. Correlations for the density and viscosity of aqueous formaldehyde solutions[J]. Industrial & Engineering Chemistry Research, 2000, 39(2): 557-562. |
| [32] | 中国科学院吉林应用化学研究所. 聚甲醛[M]. 北京: 燃料化学工业出版社, 1973: 38. |
| Jilin Institute of Applied Chemistry, Chinese Academy of Sciences. Polyformaldehyde[M]. Beijing: Fuel Chemical Industry Press, 1973: 38. |
| [1] | 刘豪, 王林, 丁昊, 耿嘉怡. R1150+R1234ze(E)二元体系223.15~253.15 K汽液相平衡研究[J]. 化工学报, 2025, 76(S1): 1-8. |
| [2] | 沙鑫权, 胡然, 丁磊, 蒋珍华, 吴亦农. 空间用单机两级有阀线性压缩机研制及测试[J]. 化工学报, 2025, 76(S1): 114-122. |
| [3] | 燕子腾, 詹飞龙, 丁国良. 空调用套管式分流器结构设计及分流效果验证[J]. 化工学报, 2025, 76(S1): 152-159. |
| [4] | 密晓光, 孙国刚, 程昊, 张晓慧. 印刷电路板式天然气冷却器性能仿真模型和验证[J]. 化工学报, 2025, 76(S1): 426-434. |
| [5] | 段浩磊, 陈浩远, 梁坤峰, 王林, 陈彬, 曹勇, 张晨光, 李硕鹏, 朱登宇, 何亚茹, 杨大鹏. 纯电动车热管理系统低GWP工质替代方案性能分析与综合评价[J]. 化工学报, 2025, 76(S1): 54-61. |
| [6] | 张文锋, 郭玮, 张新玉, 曹昊敏, 丁国良. 铝管铝翅片换热器模型开发及软件实现[J]. 化工学报, 2025, 76(S1): 84-92. |
| [7] | 丁昊, 王林, 刘豪. R290/R245fa汽液相平衡混合规则对比研究[J]. 化工学报, 2025, 76(S1): 9-16. |
| [8] | 王俊鹏, 冯佳琪, 张恩搏, 白博峰. 曲折式与阵列式迷宫阀芯结构内流动与空化特性研究[J]. 化工学报, 2025, 76(S1): 93-105. |
| [9] | 臧子晴, 李修真, 谈莹莹, 刘晓庆. 分凝器对两级分离自复叠制冷循环特性影响研究[J]. 化工学报, 2025, 76(S1): 17-25. |
| [10] | 赵子祥, 段钟弟, 孙浩然, 薛鸿祥. 大温差两相流动诱导水锤冲击的数值模型[J]. 化工学报, 2025, 76(S1): 170-180. |
| [11] | 黄灏, 王文, 贺隆坤. LNG船薄膜型液货舱预冷过程模拟与分析[J]. 化工学报, 2025, 76(S1): 187-194. |
| [12] | 汪思远, 刘国强, 熊通, 晏刚. 窗式空调器轴流风机的风速非均匀分布特性及其对冷凝器流路优化设计的影响规律[J]. 化工学报, 2025, 76(S1): 205-216. |
| [13] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [14] | 孙九春, 桑运龙, 王海涛, 贾浩, 朱艳. 泥水盾构仓体内射流对泥浆输送特性影响研究[J]. 化工学报, 2025, 76(S1): 246-257. |
| [15] | 石一帆, 柯钢, 陈浩, 黄孝胜, 叶芳, 李成娇, 郭航. 大型高低温环境实验室温度控制仿真[J]. 化工学报, 2025, 76(S1): 268-280. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号