化工学报 ›› 2019, Vol. 70 ›› Issue (9): 3267-3274.DOI: 10.11949/0438-1157.20190244
收稿日期:2019-03-15
修回日期:2019-05-25
出版日期:2019-09-05
发布日期:2019-09-05
通讯作者:
桑世华
作者简介:聂国亮(1987—),男,博士研究生,基金资助:
Guoliang NIE1,2( ),Shihua SANG1,2(
),Shihua SANG1,2( ),Ruizhi CUI1,2
),Ruizhi CUI1,2
Received:2019-03-15
Revised:2019-05-25
Online:2019-09-05
Published:2019-09-05
Contact:
Shihua SANG
摘要:
针对四川盆地地下卤水富含钾、溴资源的特点,采用等温溶解平衡法研究了五元体系NaBr-KBr-MgBr2-CaBr2-H2O在298 K和323 K时的相平衡关系,测定了该五元体系在相应温度条件下平衡溶液的溶解度,根据相平衡实验数据绘制相应的干盐相图(KBr饱和)。研究结果表明:该五元体系在298 K和323 K条件下(KBr饱和)均有复盐生成,其中298 K温度下有复盐KBr·MgBr2·6H2O生成,相图中含有两个共饱点,五条单变量曲线和四个结晶区(KBr·MgBr2·6H2O、NaBr·2H2O、NaBr、CaBr2·6H2O的结晶区);323 K温度下有两种复盐KBr·MgBr2·6H2O、2MgBr2·CaBr2·12H2O生成,相图中含有三个共饱点,七条单变量曲线和五个结晶区(KBr·MgBr2·6H2O、NaBr·2H2O、NaBr、CaBr2·4H2O、2MgBr2·CaBr2·12H2O的结晶区)。同时,对该五元体系在两个不同温度下的相图、KBr图以及水含量图进行了对比分析。
中图分类号:
聂国亮, 桑世华, 崔瑞芝. 298 K和323 K条件下五元体系NaBr-KBr-MgBr2-CaBr2-H2O相平衡研究[J]. 化工学报, 2019, 70(9): 3267-3274.
Guoliang NIE, Shihua SANG, Ruizhi CUI. Phase equilibria in quinary system NaBr-KBr-MgBr2-CaBr2-H2O at 298 K and 323 K[J]. CIESC Journal, 2019, 70(9): 3267-3274.
| No. | Composition of liquid phase w(B)×100 | J?necke index of dry salt/(g/(100 g salt)) | Equilibrium solids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(NaBr) +J(MgBr2)+J(CaBr2) = 100 | |||||||||
| w(NaBr) | w(KBr) | w(MgBr2) | w(CaBr2) | J(NaBr) | J(MgBr2) | J(H2O) | J(KBr) | ||
| 1, G1 | 0.00 | 0.61 | 4.66 | 56.05 | 0.00 | 7.68 | 63.71 | 1.00 | CB6 + KMB6 + KB |
| 2 | 0.43 | 1.04 | 4.64 | 55.69 | 0.71 | 7.64 | 62.87 | 1.71 | CB6 + KMB6 + KB |
| 3, X1 | 0.73 | 1.48 | 4.62 | 55.21 | 1.21 | 7.63 | 62.68 | 2.44 | CB6+KMB6+NB+KB |
| 4, E1 | 0.74 | 1.50 | 0.00 | 57.76 | 1.26 | 0.00 | 68.38 | 2.56 | CB6 + NB + KB |
| 5 | 0.74 | 1.50 | 1.21 | 56.98 | 1.26 | 2.05 | 67.15 | 2.55 | CB6 + NB + KB |
| 6 | 0.74 | 1.50 | 2.56 | 56.01 | 1.25 | 4.32 | 66.08 | 2.53 | CB6 + NB + KB |
| 7 | 0.74 | 1.49 | 3.66 | 55.89 | 1.23 | 6.07 | 63.39 | 2.47 | CB6 + NB + KB |
| 8, N1 | 8.06 | 3.81 | 36.13 | 0.00 | 18.24 | 81.76 | 117.67 | 8.62 | KMB6 + NB2 + KB |
| 9 | 7.40 | 3.64 | 32.35 | 2.90 | 17.35 | 75.85 | 125.93 | 8.53 | KMB6 + NB2 + KB |
| 10 | 6.98 | 3.50 | 30.30 | 4.83 | 16.58 | 71.95 | 129.16 | 8.31 | KMB6 + NB2 + KB |
| 11, Y1 | 6.66 | 3.31 | 28.10 | 6.97 | 15.96 | 67.34 | 131.70 | 7.93 | KMB6+NB2+NB+KB |
| 12 | 5.92 | 3.01 | 25.82 | 11.24 | 13.77 | 60.07 | 125.66 | 7.00 | KMB + NB + KB |
| 13 | 5.29 | 2.74 | 22.67 | 17.72 | 11.58 | 49.63 | 112.92 | 6.00 | KMB6 + NB + KB |
| 14 | 4.61 | 2.33 | 19.30 | 23.47 | 9.73 | 40.73 | 106.14 | 4.92 | KMB6 + NB + KB |
| 15 | 3.65 | 2.01 | 15.27 | 30.90 | 7.33 | 30.65 | 96.69 | 4.03 | KMB6 + NB + KB |
| 16 | 1.98 | 1.79 | 9.45 | 41.02 | 3.78 | 18.02 | 87.24 | 3.41 | KMB6 + NB + KB |
| 17 | 1.33 | 1.57 | 6.31 | 49.32 | 2.33 | 11.08 | 72.81 | 2.76 | KMB6 + NB + KB |
| 18, F1 | 10.45 | 3.05 | 0.00 | 40.61 | 20.47 | 0.00 | 89.87 | 5.97 | NB + NB2 + KB |
| 19 | 10.02 | 3.07 | 1.99 | 37.31 | 20.32 | 4.03 | 96.53 | 6.22 | NB + NB2 + KB |
| 20 | 9.86 | 3.10 | 4.02 | 34.20 | 20.51 | 8.36 | 101.54 | 6.45 | NB + NB2 + KB |
| 21 | 9.30 | 3.13 | 6.72 | 29.90 | 20.25 | 14.63 | 110.95 | 6.82 | NB + NB2 + KB |
| 22 | 8.85 | 3.16 | 8.87 | 26.32 | 20.10 | 20.14 | 119.89 | 7.18 | NB + NB2 + KB |
| 23 | 8.46 | 3.18 | 12.70 | 21.79 | 19.70 | 29.57 | 125.42 | 7.40 | NB + NB2 + KB |
| 24 | 7.99 | 3.21 | 15.77 | 18.97 | 18.70 | 36.91 | 126.52 | 7.51 | NB + NB2 + KB |
| 25 | 7.47 | 3.25 | 19.76 | 14.86 | 17.75 | 46.95 | 129.86 | 7.72 | NB + NB2 + KB |
| 26 | 7.01 | 3.29 | 24.33 | 10.60 | 16.71 | 58.01 | 130.59 | 7.84 | NB + NB2 + KB |
表1 298 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O平衡液相组成(KBr饱和)
Table 1 Compositions of liquids in quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 298 K (saturated with KBr)
| No. | Composition of liquid phase w(B)×100 | J?necke index of dry salt/(g/(100 g salt)) | Equilibrium solids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(NaBr) +J(MgBr2)+J(CaBr2) = 100 | |||||||||
| w(NaBr) | w(KBr) | w(MgBr2) | w(CaBr2) | J(NaBr) | J(MgBr2) | J(H2O) | J(KBr) | ||
| 1, G1 | 0.00 | 0.61 | 4.66 | 56.05 | 0.00 | 7.68 | 63.71 | 1.00 | CB6 + KMB6 + KB |
| 2 | 0.43 | 1.04 | 4.64 | 55.69 | 0.71 | 7.64 | 62.87 | 1.71 | CB6 + KMB6 + KB |
| 3, X1 | 0.73 | 1.48 | 4.62 | 55.21 | 1.21 | 7.63 | 62.68 | 2.44 | CB6+KMB6+NB+KB |
| 4, E1 | 0.74 | 1.50 | 0.00 | 57.76 | 1.26 | 0.00 | 68.38 | 2.56 | CB6 + NB + KB |
| 5 | 0.74 | 1.50 | 1.21 | 56.98 | 1.26 | 2.05 | 67.15 | 2.55 | CB6 + NB + KB |
| 6 | 0.74 | 1.50 | 2.56 | 56.01 | 1.25 | 4.32 | 66.08 | 2.53 | CB6 + NB + KB |
| 7 | 0.74 | 1.49 | 3.66 | 55.89 | 1.23 | 6.07 | 63.39 | 2.47 | CB6 + NB + KB |
| 8, N1 | 8.06 | 3.81 | 36.13 | 0.00 | 18.24 | 81.76 | 117.67 | 8.62 | KMB6 + NB2 + KB |
| 9 | 7.40 | 3.64 | 32.35 | 2.90 | 17.35 | 75.85 | 125.93 | 8.53 | KMB6 + NB2 + KB |
| 10 | 6.98 | 3.50 | 30.30 | 4.83 | 16.58 | 71.95 | 129.16 | 8.31 | KMB6 + NB2 + KB |
| 11, Y1 | 6.66 | 3.31 | 28.10 | 6.97 | 15.96 | 67.34 | 131.70 | 7.93 | KMB6+NB2+NB+KB |
| 12 | 5.92 | 3.01 | 25.82 | 11.24 | 13.77 | 60.07 | 125.66 | 7.00 | KMB + NB + KB |
| 13 | 5.29 | 2.74 | 22.67 | 17.72 | 11.58 | 49.63 | 112.92 | 6.00 | KMB6 + NB + KB |
| 14 | 4.61 | 2.33 | 19.30 | 23.47 | 9.73 | 40.73 | 106.14 | 4.92 | KMB6 + NB + KB |
| 15 | 3.65 | 2.01 | 15.27 | 30.90 | 7.33 | 30.65 | 96.69 | 4.03 | KMB6 + NB + KB |
| 16 | 1.98 | 1.79 | 9.45 | 41.02 | 3.78 | 18.02 | 87.24 | 3.41 | KMB6 + NB + KB |
| 17 | 1.33 | 1.57 | 6.31 | 49.32 | 2.33 | 11.08 | 72.81 | 2.76 | KMB6 + NB + KB |
| 18, F1 | 10.45 | 3.05 | 0.00 | 40.61 | 20.47 | 0.00 | 89.87 | 5.97 | NB + NB2 + KB |
| 19 | 10.02 | 3.07 | 1.99 | 37.31 | 20.32 | 4.03 | 96.53 | 6.22 | NB + NB2 + KB |
| 20 | 9.86 | 3.10 | 4.02 | 34.20 | 20.51 | 8.36 | 101.54 | 6.45 | NB + NB2 + KB |
| 21 | 9.30 | 3.13 | 6.72 | 29.90 | 20.25 | 14.63 | 110.95 | 6.82 | NB + NB2 + KB |
| 22 | 8.85 | 3.16 | 8.87 | 26.32 | 20.10 | 20.14 | 119.89 | 7.18 | NB + NB2 + KB |
| 23 | 8.46 | 3.18 | 12.70 | 21.79 | 19.70 | 29.57 | 125.42 | 7.40 | NB + NB2 + KB |
| 24 | 7.99 | 3.21 | 15.77 | 18.97 | 18.70 | 36.91 | 126.52 | 7.51 | NB + NB2 + KB |
| 25 | 7.47 | 3.25 | 19.76 | 14.86 | 17.75 | 46.95 | 129.86 | 7.72 | NB + NB2 + KB |
| 26 | 7.01 | 3.29 | 24.33 | 10.60 | 16.71 | 58.01 | 130.59 | 7.84 | NB + NB2 + KB |
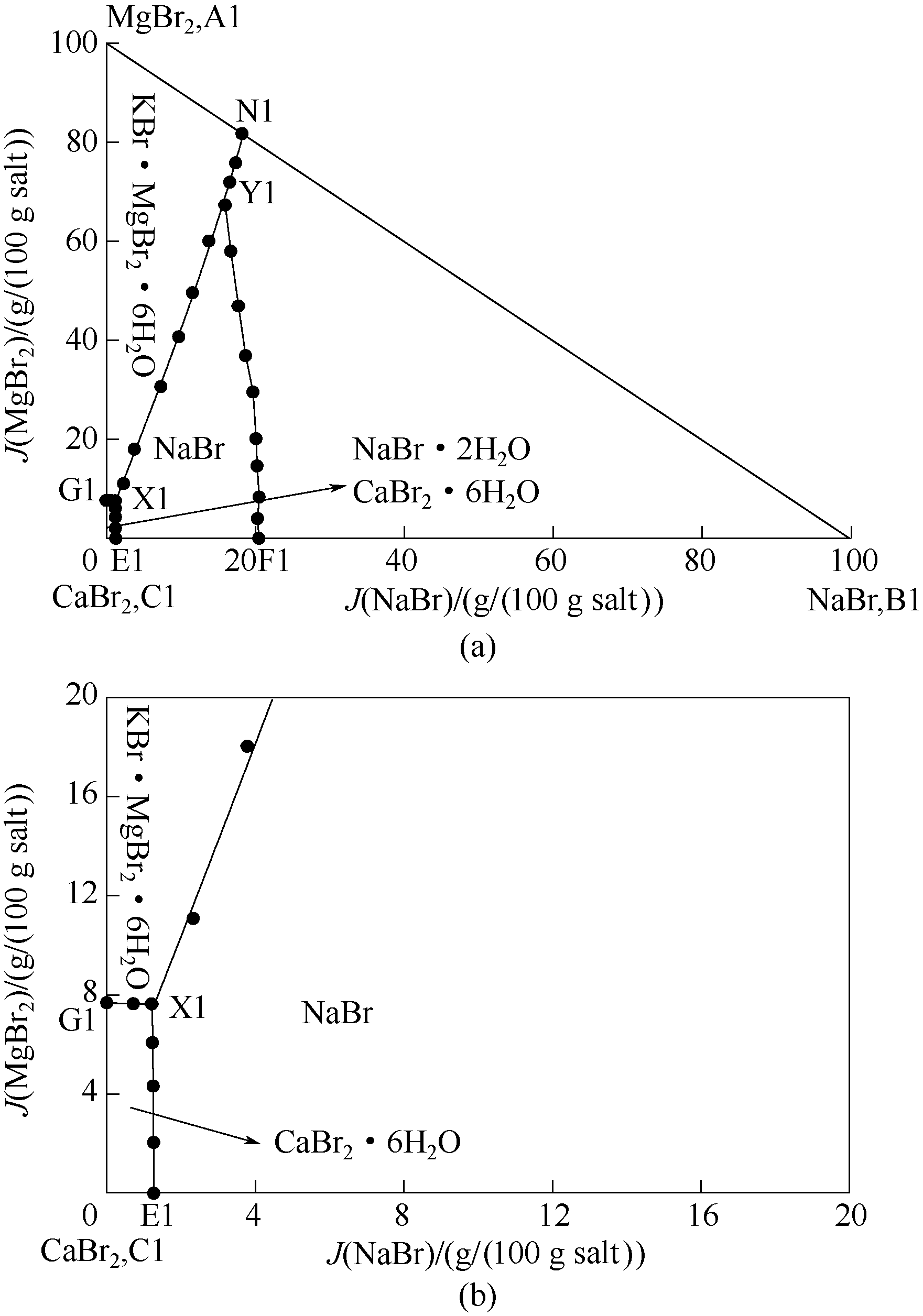
图1 298 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O相图(KBr饱和) (a)及局部放大图(b)
Fig.1 Equilibrium phase diagram of quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 298 K (saturated with KBr) (a) and partially enlarged diagram (b)
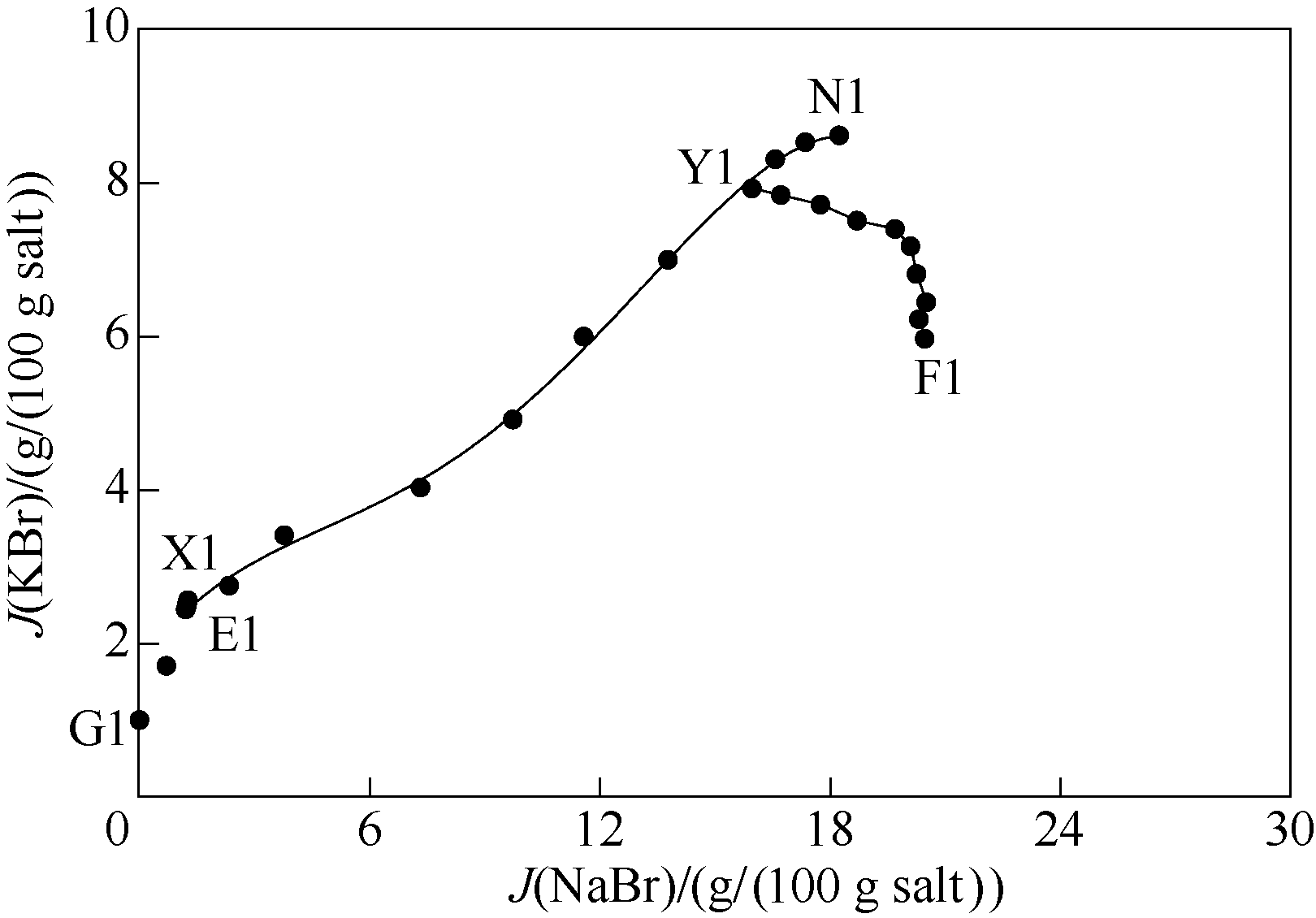
图2 298 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O KBr图 (KBr饱和)
Fig.2 KBr content diagram of quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 298 K (saturated with KBr)
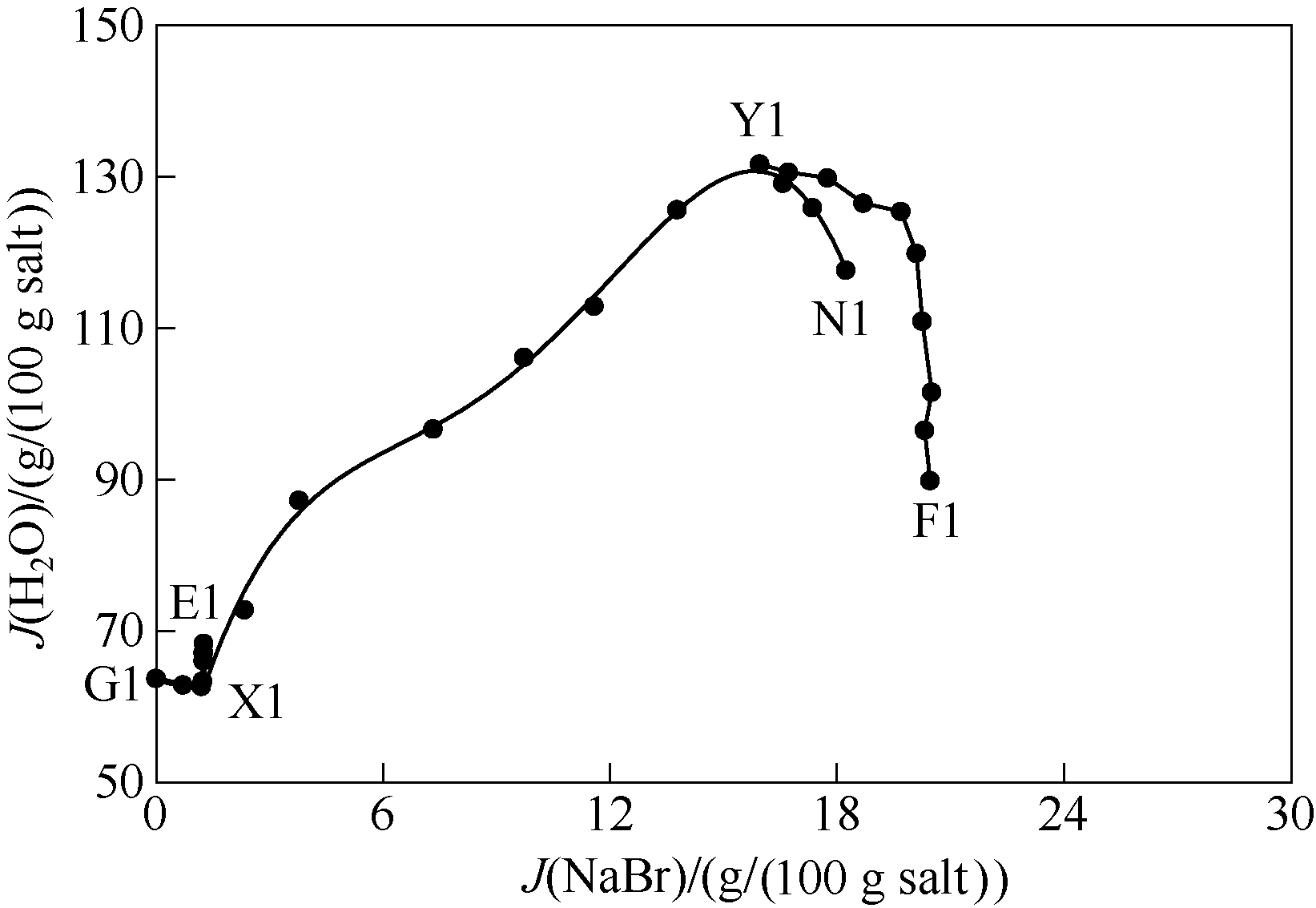
图3 298 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O水含量图(KBr饱和)
Fig.3 Water contents of saturated solutions in quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 298 K (saturated with KBr)
| No. | Composition of liquid phase w(B)×100 | J?necke index of dry salt/(g/(100 g salt)) | Equilibrium solids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(NaBr)+J(MgBr2)+J(CaBr2) = 100 | |||||||||
| w(NaBr) | w(KBr) | w(MgBr2) | w(CaBr2) | J(NaBr) | J(MgBr2) | J(H2O) | J(KBr) | ||
| 1, E2 | 0.00 | 0.49 | 7.16 | 61.85 | 0.00 | 10.38 | 44.20 | 0.71 | MCB12 + KMB6 + KB |
| 2 | 1.31 | 0.88 | 6.84 | 57.20 | 2.00 | 10.47 | 51.68 | 1.35 | MCB12 + KMB6 + KB |
| 3, Z2 | 2.18 | 1.09 | 6.49 | 53.32 | 3.52 | 10.47 | 59.56 | 1.76 | MCB13+KMB6+NB+KB |
| 4,G2 | 0.72 | 0.67 | 0.00 | 70.22 | 1.01 | 0.00 | 40.02 | 0.94 | CB4 + NB + KB |
| 5 | 0.70 | 0.61 | 1.21 | 66.98 | 1.02 | 1.76 | 44.27 | 0.89 | CB4 + NB + KB |
| 6, X2 | 0.65 | 0.54 | 3.22 | 63.51 | 0.96 | 4.78 | 47.61 | 0.80 | CB4+NB+MCB12+ KB |
| 7, N2 | 12.81 | 4.54 | 30.15 | 0.00 | 29.82 | 70.18 | 122.21 | 10.57 | MB6 + NB2 + KB |
| 8 | 12.11 | 4.32 | 28.15 | 2.90 | 28.06 | 65.22 | 121.69 | 10.01 | MB6 + NB2 + KB |
| 9 | 10.98 | 3.98 | 25.30 | 6.83 | 25.47 | 58.69 | 122.73 | 9.23 | MB6 + NB2 + KB |
| 10 | 10.22 | 3.64 | 23.10 | 9.97 | 23.61 | 53.36 | 122.59 | 8.41 | MB6 + NB2 + KB |
| 11, Y2 | 9.27 | 3.41 | 20.57 | 13.44 | 21.42 | 47.53 | 123.17 | 7.88 | NB+NB2+KMB6+KB |
| 12 | 7.99 | 3.21 | 16.77 | 18.87 | 18.31 | 38.44 | 121.84 | 7.36 | MB6 + KMB6 + KB |
| 13 | 6.32 | 2.78 | 13.63 | 24.86 | 14.10 | 30.42 | 116.96 | 6.20 | MB6 + KMB6 + KB |
| 14 | 5.01 | 2.11 | 11.11 | 31.75 | 10.47 | 23.21 | 104.49 | 4.41 | MB6 + KMB6 + KB |
| 15 | 3.76 | 1.58 | 9.45 | 41.02 | 6.93 | 17.43 | 81.49 | 2.91 | MB6 + KMB6 + KB |
| 16, D2 | 45.93 | 8.15 | 0.00 | 2.48 | 94.88 | 0.00 | 89.73 | 16.84 | NB + NB2 +KB |
| 17 | 42.31 | 7.73 | 1.29 | 3.79 | 89.28 | 2.72 | 94.70 | 16.31 | NB + NB2 +KB |
| 18 | 39.77 | 7.21 | 2.87 | 4.56 | 84.26 | 6.08 | 96.59 | 15.28 | NB + NB2 +KB |
| 19 | 36.45 | 6.89 | 4.80 | 5.32 | 78.26 | 10.31 | 99.92 | 14.79 | NB + NB2 +KB |
| 20 | 32.21 | 6.41 | 6.21 | 6.78 | 71.26 | 13.74 | 107.06 | 14.18 | NB + NB2 +KB |
| 21 | 26.77 | 5.91 | 10.29 | 7.73 | 59.77 | 22.97 | 110.07 | 13.19 | NB + NB2 +KB |
| 22 | 23.34 | 5.04 | 12.39 | 8.69 | 52.54 | 27.89 | 113.78 | 11.35 | NB + NB2 +KB |
| 23 | 17.51 | 4.38 | 15.82 | 10.33 | 40.11 | 36.23 | 119.01 | 10.03 | NB + NB2 +KB |
| 24 | 14.67 | 3.97 | 17.82 | 11.09 | 33.66 | 40.89 | 120.35 | 9.11 | NB + NB2 +KB |
| 25, F2 | 0.00 | 0.52 | 3.26 | 63.39 | 0.00 | 4.89 | 49.26 | 0.78 | MCB12 + CB4 + KB |
| 26 | 0.33 | 0.52 | 3.25 | 63.44 | 0.49 | 4.85 | 48.43 | 0.78 | MCB12 + CB4 + KB |
表2 323 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O平衡液相组成(KBr饱和)
Table 2 Compositions of liquids in quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 323 K (saturated with KBr)
| No. | Composition of liquid phase w(B)×100 | J?necke index of dry salt/(g/(100 g salt)) | Equilibrium solids | ||||||
|---|---|---|---|---|---|---|---|---|---|
| J(NaBr)+J(MgBr2)+J(CaBr2) = 100 | |||||||||
| w(NaBr) | w(KBr) | w(MgBr2) | w(CaBr2) | J(NaBr) | J(MgBr2) | J(H2O) | J(KBr) | ||
| 1, E2 | 0.00 | 0.49 | 7.16 | 61.85 | 0.00 | 10.38 | 44.20 | 0.71 | MCB12 + KMB6 + KB |
| 2 | 1.31 | 0.88 | 6.84 | 57.20 | 2.00 | 10.47 | 51.68 | 1.35 | MCB12 + KMB6 + KB |
| 3, Z2 | 2.18 | 1.09 | 6.49 | 53.32 | 3.52 | 10.47 | 59.56 | 1.76 | MCB13+KMB6+NB+KB |
| 4,G2 | 0.72 | 0.67 | 0.00 | 70.22 | 1.01 | 0.00 | 40.02 | 0.94 | CB4 + NB + KB |
| 5 | 0.70 | 0.61 | 1.21 | 66.98 | 1.02 | 1.76 | 44.27 | 0.89 | CB4 + NB + KB |
| 6, X2 | 0.65 | 0.54 | 3.22 | 63.51 | 0.96 | 4.78 | 47.61 | 0.80 | CB4+NB+MCB12+ KB |
| 7, N2 | 12.81 | 4.54 | 30.15 | 0.00 | 29.82 | 70.18 | 122.21 | 10.57 | MB6 + NB2 + KB |
| 8 | 12.11 | 4.32 | 28.15 | 2.90 | 28.06 | 65.22 | 121.69 | 10.01 | MB6 + NB2 + KB |
| 9 | 10.98 | 3.98 | 25.30 | 6.83 | 25.47 | 58.69 | 122.73 | 9.23 | MB6 + NB2 + KB |
| 10 | 10.22 | 3.64 | 23.10 | 9.97 | 23.61 | 53.36 | 122.59 | 8.41 | MB6 + NB2 + KB |
| 11, Y2 | 9.27 | 3.41 | 20.57 | 13.44 | 21.42 | 47.53 | 123.17 | 7.88 | NB+NB2+KMB6+KB |
| 12 | 7.99 | 3.21 | 16.77 | 18.87 | 18.31 | 38.44 | 121.84 | 7.36 | MB6 + KMB6 + KB |
| 13 | 6.32 | 2.78 | 13.63 | 24.86 | 14.10 | 30.42 | 116.96 | 6.20 | MB6 + KMB6 + KB |
| 14 | 5.01 | 2.11 | 11.11 | 31.75 | 10.47 | 23.21 | 104.49 | 4.41 | MB6 + KMB6 + KB |
| 15 | 3.76 | 1.58 | 9.45 | 41.02 | 6.93 | 17.43 | 81.49 | 2.91 | MB6 + KMB6 + KB |
| 16, D2 | 45.93 | 8.15 | 0.00 | 2.48 | 94.88 | 0.00 | 89.73 | 16.84 | NB + NB2 +KB |
| 17 | 42.31 | 7.73 | 1.29 | 3.79 | 89.28 | 2.72 | 94.70 | 16.31 | NB + NB2 +KB |
| 18 | 39.77 | 7.21 | 2.87 | 4.56 | 84.26 | 6.08 | 96.59 | 15.28 | NB + NB2 +KB |
| 19 | 36.45 | 6.89 | 4.80 | 5.32 | 78.26 | 10.31 | 99.92 | 14.79 | NB + NB2 +KB |
| 20 | 32.21 | 6.41 | 6.21 | 6.78 | 71.26 | 13.74 | 107.06 | 14.18 | NB + NB2 +KB |
| 21 | 26.77 | 5.91 | 10.29 | 7.73 | 59.77 | 22.97 | 110.07 | 13.19 | NB + NB2 +KB |
| 22 | 23.34 | 5.04 | 12.39 | 8.69 | 52.54 | 27.89 | 113.78 | 11.35 | NB + NB2 +KB |
| 23 | 17.51 | 4.38 | 15.82 | 10.33 | 40.11 | 36.23 | 119.01 | 10.03 | NB + NB2 +KB |
| 24 | 14.67 | 3.97 | 17.82 | 11.09 | 33.66 | 40.89 | 120.35 | 9.11 | NB + NB2 +KB |
| 25, F2 | 0.00 | 0.52 | 3.26 | 63.39 | 0.00 | 4.89 | 49.26 | 0.78 | MCB12 + CB4 + KB |
| 26 | 0.33 | 0.52 | 3.25 | 63.44 | 0.49 | 4.85 | 48.43 | 0.78 | MCB12 + CB4 + KB |
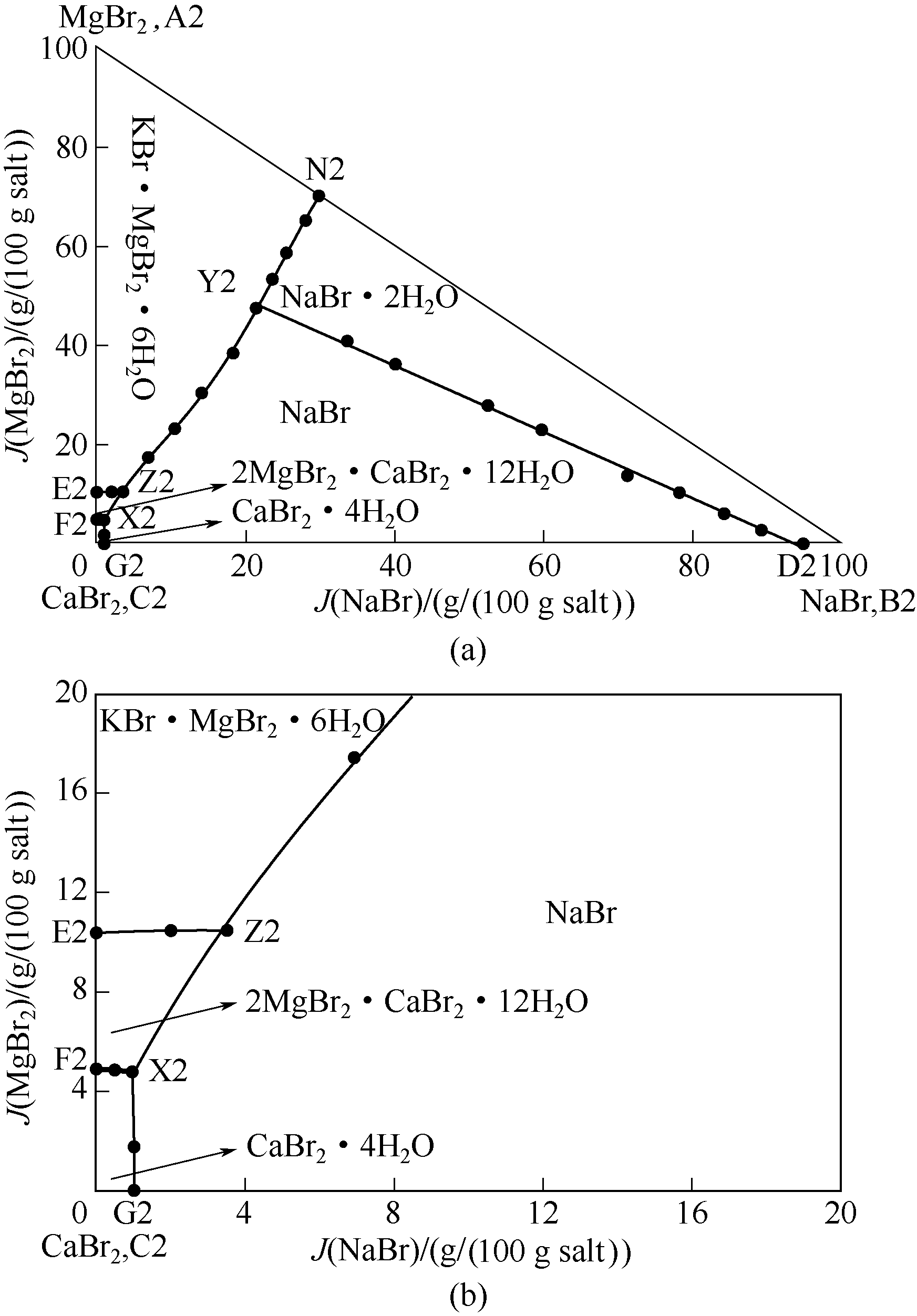
图4 323 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O相图(KBr饱和)(a)及局部放大图(b)
Fig.4 Equilibrium phase diagram of quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 323 K (saturated with KBr) (a) and partially enlarged diagram (b)
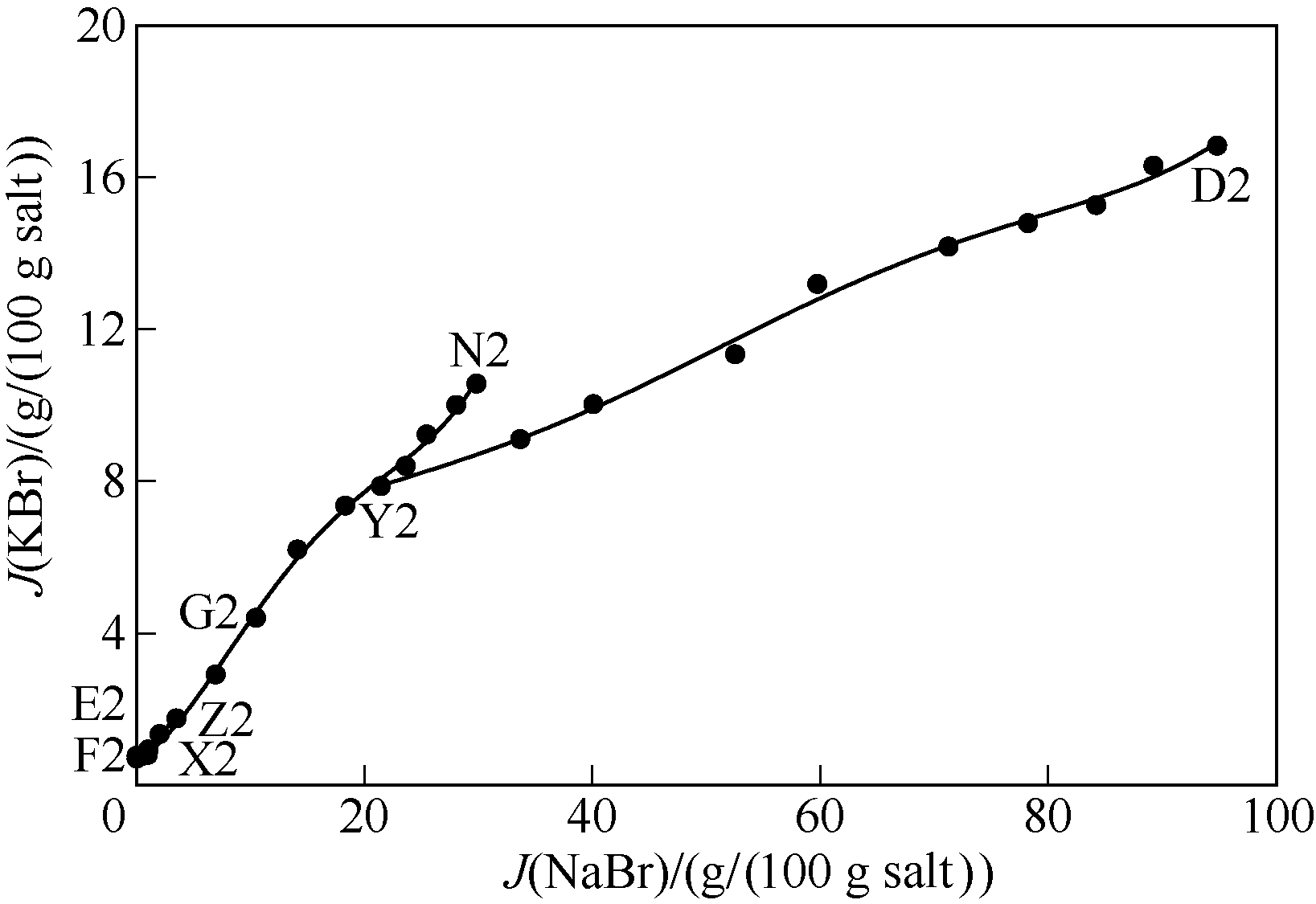
图5 323 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O KBr图 (KBr饱和)
Fig.5 KBr content diagram of quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 323 K (saturated with KBr)
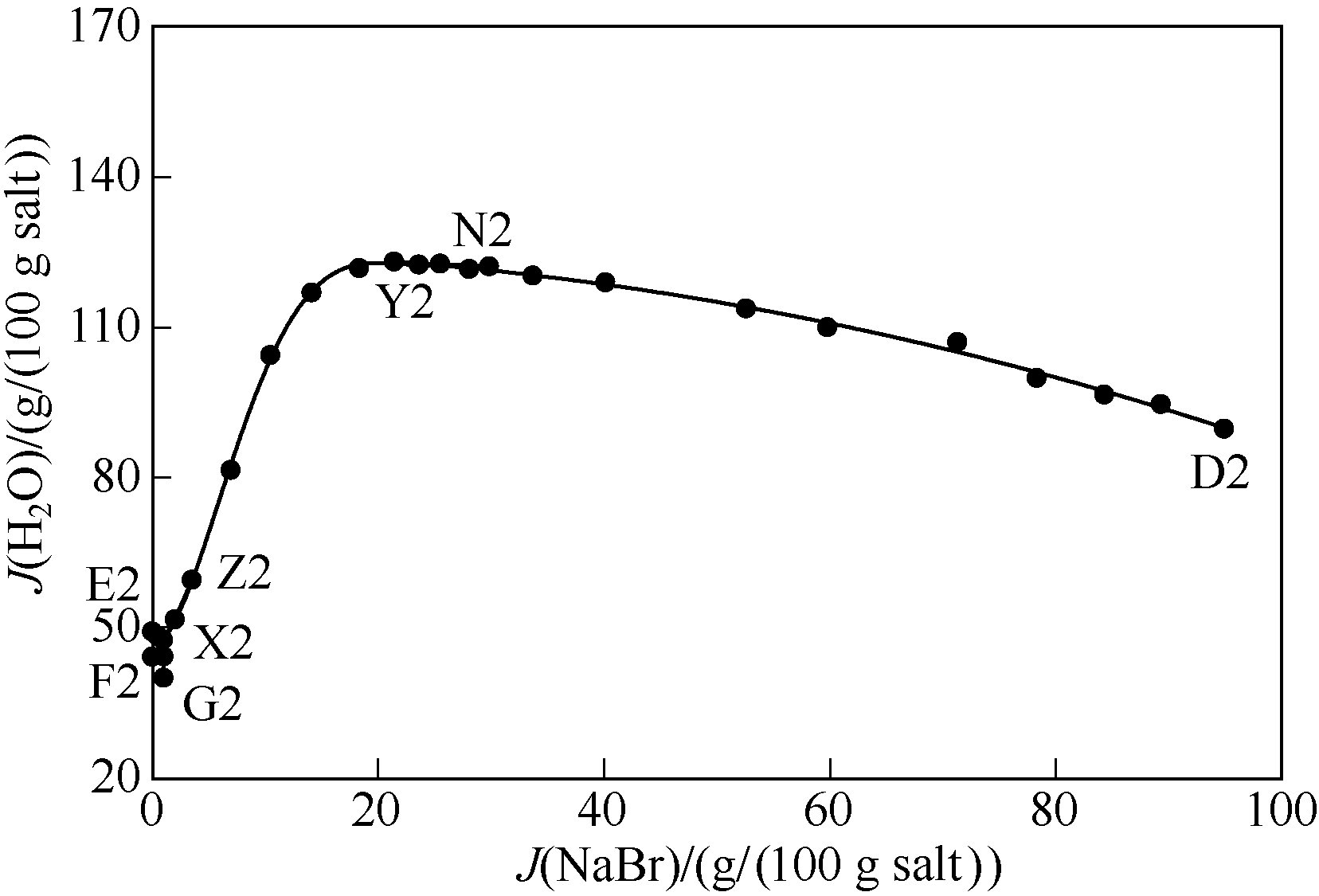
图6 323 K条件下五元体系NaBr-KBr-CaBr2-MgBr2-H2O水含量图(KBr饱和)
Fig.6 Water contents of saturated solutions in quinary system NaBr-KBr-CaBr2-MgBr2-H2O at 323 K (saturated with KBr)
| 1 | 郑绵平, 齐文, 张永生 . 中国钾盐地质资源现状与找钾方向初步分析[J]. 地质通报, 2006, 25(11): 1239-1246. |
| Zheng M P , Qi W , Zhang Y S . Present situation of potash resources and direction of potash search in China[J]. Geological Bulletin of China, 2006, 25(11): 1239-1246. | |
| 2 | 林耀庭 . 四川地下卤水资源优势及综合开发前景[J]. 盐湖研究, 2006, 14(4): 1-8. |
| Lin Y T . Resource advantages of the underground brines of Sichuan basin and the outlook of their comprehensive exploitation[J]. Journal of Salt Lake Research, 2006, 14(4): 1-8. | |
| 3 | 郑绵平, 侯献华, 于常青, 等 . 成盐理论引领我国找钾取得重要进展[J]. 地球学报, 2015, 36(2): 129-139. |
| Zheng M P , Hou X H , Yu C Q , et al . The leading role of salt formation theory in the breakthrough and important progress in potash deposit prospecting[J]. Acta Geoscientica Sinica, 2015, 36(2): 129-139. | |
| 4 | 侯献华, 樊馥, 郑绵平, 等 . 青海盐湖钾盐资源开发利用及产业发展[J]. 科技导报, 2017, 35(12): 67-71. |
| Hou X H , Fan F , Zheng M P , et al . Development and utilization of potash resources of saline lakes in Qinghai[J]. Science & Technology Review, 2017, 35(12): 67-71. | |
| 5 | 李文光 . 我国钾盐资源的开发利用[J]. 盐湖研究, 1994, 2(3): 65-68. |
| Li W G . Comprehensive utilization of our potash resource[J]. Journal of Salt Lake Research, 1994, 2(3): 65-68. | |
| 6 | 林耀庭 . 我国卤水溴资源及其开发前景展望[J]. 盐湖研究, 2000, 8(2): 59-67. |
| Lin Y T . Bromine resource in brines and its exploitation prospect[J]. Journal of Salt Lake Research, 2000, 8(2): 59-67. | |
| 7 | 朱建华, 马淑芬, 刘红研 . 溴素生产应用现状分析及展望[J]. 矿产综合利用, 2004, (2): 36-41. |
| Zhu J H , Ma S F , Liu H Y . Analysis and prospects for present status of production and utilization of bromine[J]. Multipurpose Utilization of Mineral Resources, 2004, (2): 36-41. | |
| 8 | 陈向楠, 王海增 . 溴素资源与产业发展分析[J]. 盐业与化工, 2013, 42(6): 4-7. |
| Chen X N , Wang H Z . Bromine resource and analysis of the industry development[J]. Journal of Salt and Chemical Industry, 2013, 42(6): 4-7. | |
| 9 | Balarew C , Christov C , Valyashko V , et al . Thermodynamics of formation of Carnallite type double salts[J]. Journal of Solution Chemistry, 1993, 22: 173-181. |
| 10 | Christov C . Study of bromide salts solubility in the (m 1KBr + m 2CaBr2)(aq) system at T = 323.15 K. Thermodynamic model of solution behaviour and (solid + liquid) equilibria in theternaries (m 1KBr + m 2CaBr2)(aq), and (m 1MgBr2 + m 2CaBr2)(aq), and in the quinary (Na + K + Mg + Ca + Br + H2O) systems to high concentration and temperature[J]. J. Chem. Thermodynamics, 2012, 55: 7-22. |
| 11 | 翁延博, 王静康, 尹秋响, 等 . NaCl-NaBr-H2O三组分物系的固液平衡[J]. 石油化工, 2007, 36(4): 358-361. |
| Weng Y B , Wang J K , Yin Q X , et al . Solid-liquid equilibrium of NaCl-NaBr-H2O ternary system[J]. Petrochemical Technology, 2007, 36(4): 358-361. | |
| 12 | 翁延博, 王彦飞, 王静康, 等 . K+/Cl-, Br--H2O三元体系298 K, 313 K, 333 K时的相平衡[J]. 高校化学工程学报, 2007, 21(4): 695-699. |
| Weng Y B , Wang Y F , Wang J K , et al . Phase diagram for the ternary system of K+/Cl-, Br--H2O at 298 K, 313 K and 333 K[J]. Journal of Chemical Engineering of Chinese Universities, 2007, 21(4): 695-699. | |
| 13 | Christov C . Isopiestic investigation of the osmotic coefficients of MgBr2(aq) and study of bromide salts solubility in the (m 1KBr + m 2MgBr2)(aq) system at T = 323.15 K. Thermodynamic model of solution behaviour and (solid + liquid) equilibria in the MgBr2(aq), and (m 1KBr + m 2MgBr2)(aq) systems to high concentration and temperature[J]. J. Chem. Thermodynamics, 2011, 43: 344-353. |
| 14 | Hu J X , Sang S H , Zhou M F , et al . Phase equilibria in the ternary systems KBr-MgBr2-H2O and NaBr-MgBr2-H2O at 348.15K[J]. Fluid Phase Equilibria, 2015, 392: 127-131. |
| 15 | 桑世华, 殷辉安, 倪师军, 等 . 三元体系KBr-K2B4O7-H2O在298 K的相平衡研究[J]. 成都理工大学学报(自然科学版), 2006, 33(4): 414-416. |
| Sang S H , Yin H A , Ni S J , et al . A study on equilibrium solubilities and properties of solutions in the ternary system K2B4O7-KBr-H2O at 298 K[J]. Journal of Chengdu University of Technology (Science & Technology Edition), 2006, 33(4): 414-416. | |
| 16 | 赵向阳, 桑世华, 孙明亮 . 三元体系K2B4O7-KBr-H2O在323 K的相平衡研究[J]. 盐湖研究, 2011, 19(1): 35-39. |
| Zhao X Y , Sang S H , Sun M L . Phase equilibrium of the ternary system K2B4O7-KBr-H2O at 323 K[J]. Journal of Salt Lake Research, 2011, 19(1): 35-39. | |
| 17 | 桑世华, 李婷, 崔瑞芝 . 三元水盐体系KBr-K2B4O7-H2O 348 K相平衡研究[J]. 盐湖研究, 2013, 21(2): 29-32. |
| Sang S H , Li T , Cui R Z . A study on phase equilibria in the ternary salt-water system KBr-K2B4O7-H2O at 348 K[J]. Journal of Salt Lake Research, 2013, 21(2): 29-32. | |
| 18 | Cui R Z , Sang S H , Hu Y X , et al . Phase equilibria in the ternary systems KBr-K2B4O7-H2O and KCl-K2B4O7-H2O at 373 K[J]. Acta Geologica Sinica, 2013, 87(6): 1668-1673. |
| 19 | 桑世华, 崔瑞芝, 胡咏霞 . 三元体系NaBr-Na2SO4-H2O 和 NaBr-KBr-H2O 373 K 相平衡[J].高校化学工程学报, 2014, 28(5): 939-943. |
| Sang S H , Cui R Z , Hu Y X . Phase equilibria of two ternary systems NaBr-Na2SO4-H2O and NaBr-KBr-H2O at 373 K[J]. Journal of Chemical Engineering of Chinese Universities, 2014, 28(5): 939-943. | |
| 20 | Cui R Z , Sang S H , Li D W , et al . Measurements and calculations of solid-liquid equilibria in the quaternary system NaBr-KBr-CaBr2-H2O at 298 K[J]. Computer Coupling of Phase Diagrams and Thermochemistry, 2015, 49: 120-126. |
| 21 | Cui R Z , Wei C , Wang W , et al . Phase diagram of quaternary system NaBr-KBr-CaBr2-H2O at 323 K[J]. Russian Journal of Physical Chemistry A, 2018, 92: 475-481. |
| 22 | Cui R Z , Wang Z C , Xu J S , et al . Measurements and calculations of solid-liquid equilibria in the quaternary system KBr-CaBr2-MgBr2-H2O at (298 and 323) K[J]. Fluid Phase Equilibria, 2017, 450: 140-148. |
| 23 | Liu Q , Gao Y Y , Sang S H , et al . Solid-liquid equilibria in the quaternary systems NaBr-SrBr2-MgBr2-H2O and KBr-SrBr2-MgBr2-H2O at 323 K[J]. Journal of Chemical & Engineering Data, 2017, 62: 1264-1268. |
| 24 | Cui R Z , Nie G L , Sang S H , et al . Measurements of (solid + liquid) phase equilibria in the quaternary system NaBr + KBr + SrBr2 + H2O and two subsystems NaBr + SrBr2 +H2O and KBr + SrBr2 + H2O at T = 323 K[J]. Journal of Chemical & Engineering Data, 2017, 62: 3187-3192. |
| 25 | 王丹, 桑世华, 曾晓晓, 等 . KCl-KBr-K2SO4-H2O四组分物系323 K相平衡[J]. 石油化工, 2011, 40(3): 285-288. |
| Wang D , Sang S H , Zeng X X , et al . Phase equilibrium of KCl-KBr-K2SO4-H2O quaternary system at 323 K[J]. Petrochemical Technology, 2011, 40(3): 285-288. | |
| 26 | Zhang K J , Sang S H , Li T , et al . Liquid-solid equilibria in the quaternary system KCl-KBr-K2SO4-H2O at 348 K[J]. Journal of Chemical & Engineering Data, 2013, 58: 115-117. |
| 27 | Cui R Z , Sang S H , Hu Y X . Solid-liquid equilibria in the quaternary systems KCl-KBr-K2B4O7-H2O and KCl-KBr-K2SO4-H2O at 373 K[J]. Journal of Chemical & Engineering Data, 2013, 58: 477-481. |
| 28 | Sang S H , Cui R Z , Hu J W , et al . Measurements of the solid-liquid equilibria in the quaternary system NaCl-NaBr-Na2SO4-H2O at 323 K [J]. Journal of Solution Chemistry, 2013, 42: 1633-1640. |
| 29 | Hu Y X , Sang S H , Cui R Z , et al . Solid-liquid equilibria in the quaternary system KCl-KBr-K2B4O7-H2O at 323 K[J]. Journal of Chemical & Engineering Data, 2014, 59: 1886-1891. |
| 30 | Sang S H , Zhang H , Zhong S Y , et al . Experimental study of the solubilities of salts in the systems Na2B4O7-NaBr-H2O and Na2B4O7-Na2SO4-NaBr-H2O at 323 K[J]. Fluid Phase Equilibria, 2014, 361: 171-174. |
| 31 | 崔瑞芝, 桑世华, 李婷, 等 . 五元体系KCl-KBr-K2SO4-K2B4O7-H2O 323 K和348 K的相平衡[J].化工学报, 2013, 64(3): 827-833. |
| Cui R Z , Sang S H , Li T , et al . Phase equilibria in quinary system KCl-KBr-K2SO4-K2B4O7-H2O at 323 K and 348 K[J]. CIESC Journal, 2013, 64(3): 827-833. | |
| 32 | 林耀庭, 姚有成, 康正华, 等 . 四川宣达盐盆富钾富矿卤水地球化学特征及资源意义研究[J]. 盐湖研究, 2004, 12(1): 8-18. |
| Lin Y T , Yao Y C , Kang Z H , et al . Study on the geochemical characteristics and resource significance of the highly mineralized potassium rich brine in the Sichuan Xuanda salt basin[J]. Journal of Salt Lake Research, 2004, 12(1): 8-18. |
| [1] | 胡超, 董玉明, 张伟, 张红玲, 周鹏, 徐红彬. 浓硫酸活化五氧化二钒制备高浓度全钒液流电池正极电解液[J]. 化工学报, 2023, 74(S1): 338-345. |
| [2] | 盛冰纯, 于建国, 林森. 铝基锂吸附剂分离高钠型地下卤水锂资源过程研究[J]. 化工学报, 2023, 74(8): 3375-3385. |
| [3] | 于旭东, 李琪, 陈念粗, 杜理, 任思颖, 曾英. 三元体系KCl + CaCl2 + H2O 298.2、323.2及348.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(8): 3256-3265. |
| [4] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [5] | 高靖博, 孙强, 李青, 王逸伟, 郭绪强. 考虑水合物结构转变的含氢气体水合物相平衡模型[J]. 化工学报, 2023, 74(2): 666-673. |
| [6] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [7] | 蔡进, 王晓辉, 汤涵, 陈光进, 孙长宇. TBAB水溶液体系中半笼型水合物的相平衡预测模型[J]. 化工学报, 2023, 74(1): 408-415. |
| [8] | 周桓, 张梦丽, 郝晴, 吴思, 李杰, 徐存兵. 硫酸镁型光卤石转化钾盐镁矾的过程机制与动态规律[J]. 化工学报, 2022, 73(9): 3841-3850. |
| [9] | 刘潜, 张香兰, 李志平, 李玉龙, 韩梦醒. 油酚分离过程低共熔溶剂的筛选及萃取性能研究[J]. 化工学报, 2022, 73(9): 3915-3928. |
| [10] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [11] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| [12] | 吴子睿, 孙瑞, 石凌峰, 田华, 王轩, 舒歌群. CO2混合工质的气液相平衡的混合规则对比与预测研究[J]. 化工学报, 2022, 73(4): 1483-1492. |
| [13] | 孙裕坤, 杨焘, 吴江涛. R32+R1234yf+R1234ze(E)混合制冷剂气液相平衡实验研究[J]. 化工学报, 2022, 73(3): 1063-1071. |
| [14] | 许昊, 陈伟, 李邹路. 以[Li(TX-7)]SCN/H2O为工质对的第二类热泵特性研究[J]. 化工学报, 2022, 73(2): 577-586. |
| [15] | 高腾飞, 李国选, 雷志刚. 从催化裂化柴油中分离联苯的溶剂筛选:实验和计算热力学[J]. 化工学报, 2022, 73(12): 5314-5323. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号