化工学报 ›› 2020, Vol. 71 ›› Issue (11): 5016-5024.DOI: 10.11949/0438-1157.20200906
收稿日期:2020-07-06
修回日期:2020-09-17
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
陶端健
作者简介:冷杰(1994—),男,硕士研究生,基金资助:
Jie LENG( ),Jingjing LUO,Chonghu SONG,Yan ZHOU,Zhangmin LI,Duanjian TAO(
),Jingjing LUO,Chonghu SONG,Yan ZHOU,Zhangmin LI,Duanjian TAO( )
)
Received:2020-07-06
Revised:2020-09-17
Online:2020-11-05
Published:2020-11-05
Contact:
Duanjian TAO
摘要:
以甲酰胺为氮源和碳源,乙酸钴为钴源,采用预先缩合/高温焙烧两步法制备了一系列Co-N-C催化剂,利用XRD、TEM、N2吸附-脱附以及XPS等表征了催化剂结构,考察了催化剂用于硝基苯与苯甲醇“一锅法”合成 N-亚苄基苯胺的催化性能及其稳定性。结果表明,焙烧温度和掺杂氮元素对Co-N-C催化剂性能影响显著,催化剂Co-N-C/800具有最好的催化性能,0.6 MPa氮气气氛下,温度140℃,反应12 h,硝基苯转化率达99%,N-亚苄基苯胺选择性达99%,催化剂重复使用5次,其活性无明显下降。
中图分类号:
冷杰,罗晶晶,宋崇虎,周言,李章敏,陶端健. Co-N-C催化剂的制备及“一锅法”合成N-亚苄基苯胺[J]. 化工学报, 2020, 71(11): 5016-5024.
Jie LENG,Jingjing LUO,Chonghu SONG,Yan ZHOU,Zhangmin LI,Duanjian TAO. Preparation of Co-N-C catalysts and “one-pot method” for synthesis of N-benzyl aniline[J]. CIESC Journal, 2020, 71(11): 5016-5024.
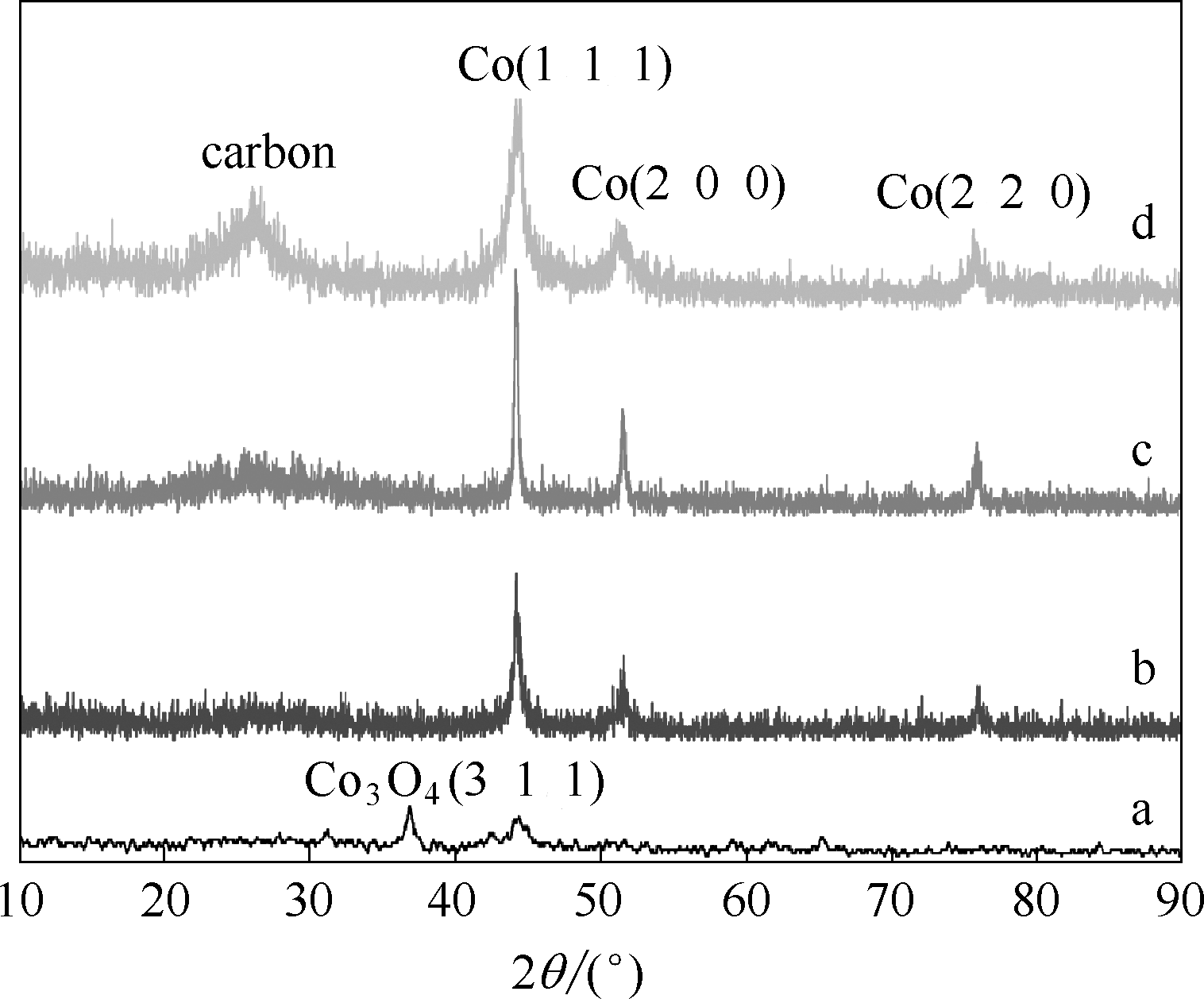
图1 催化剂Co-N-C/600(a)、Co-N-C/800(b)、Co-N-C/1000(c)、Co-N-C/800(酸洗) (d) 的XRD谱图
Fig.1 XRD patterns of catalysts Co-N-C/600 (a), Co-N-C/800 (b), Co-N-C/1000 (c) and Co-N-C/800 (acid wash) (d)
| Catalyst | SBET①/(m2/g) | V②/(cm3/g) | D③/nm |
|---|---|---|---|
| Co-N-C/600 | 95 | 0.19 | 7.2 |
| Co-N-C/800 | 299 | 0.28 | 4.3 |
| Co-N-C/1000 | 47 | 0.10 | 7.5 |
表1 催化剂Co-N-C的孔隙结构参数
Table 1 Textural properties of Co-N-C catalysts
| Catalyst | SBET①/(m2/g) | V②/(cm3/g) | D③/nm |
|---|---|---|---|
| Co-N-C/600 | 95 | 0.19 | 7.2 |
| Co-N-C/800 | 299 | 0.28 | 4.3 |
| Co-N-C/1000 | 47 | 0.10 | 7.5 |
| 催化剂 | 1转化率/ % | 3产率/ % | 4产率/ % |
|---|---|---|---|
| Co-N-C/800 | 99 | 98 | <1 |
| Co-N-C/600 | 54 | 53 | <1 |
| Co-N-C/1000 | 63 | 62 | <1 |
| Co-N-C/800(酸洗) | 33 | 33 | - |
| Co-C/800 | 2 | 2 | - |
表2 催化剂性能测试结果
Table 2 The results of catalyst performance
| 催化剂 | 1转化率/ % | 3产率/ % | 4产率/ % |
|---|---|---|---|
| Co-N-C/800 | 99 | 98 | <1 |
| Co-N-C/600 | 54 | 53 | <1 |
| Co-N-C/1000 | 63 | 62 | <1 |
| Co-N-C/800(酸洗) | 33 | 33 | - |
| Co-C/800 | 2 | 2 | - |
| 催化剂 | 反应条件 | 1转化率/% | 3产率/% |
|---|---|---|---|
| Co-N-C/800 | 0.05 g催化剂;5 ml二甲基甲酰胺;140℃ | 33 | 33 |
| Co-N-C/800 | 0.05 g催化剂;5 ml甲苯;140℃ | 69 | 68 |
| Co-N-C/800 | 0.05 g催化剂;5 ml乙醇;140℃ | 67 | 66 |
| Co-N-C/800 | 0.05 g催化剂;5 ml环己烷;140℃ | 78 | 76 |
| Co-N-C/800 | 0.05 g催化剂;5 ml辛烷;140℃ | 89 | 88 |
| Co-N-C/800 | 0.10 g催化剂;5 ml辛烷;140℃ | 99 | 98 |
| Co-N-C/800 | 0.15 g催化剂;5 ml辛烷;140℃ | 99 | 99 |
| Co-N-C/800 | 0.10 g催化剂;5 ml辛烷;120℃ | 61 | 60 |
| Co-N-C/800① | 0.15 g催化剂;5 ml辛烷;140℃ | 73 | 72 |
| Co-N-C/800② | 0.15 g催化剂;5 ml辛烷;140℃ | 89 | 87 |
表3 反应条件优化结果
Table 3 Optimization results of reaction conditions
| 催化剂 | 反应条件 | 1转化率/% | 3产率/% |
|---|---|---|---|
| Co-N-C/800 | 0.05 g催化剂;5 ml二甲基甲酰胺;140℃ | 33 | 33 |
| Co-N-C/800 | 0.05 g催化剂;5 ml甲苯;140℃ | 69 | 68 |
| Co-N-C/800 | 0.05 g催化剂;5 ml乙醇;140℃ | 67 | 66 |
| Co-N-C/800 | 0.05 g催化剂;5 ml环己烷;140℃ | 78 | 76 |
| Co-N-C/800 | 0.05 g催化剂;5 ml辛烷;140℃ | 89 | 88 |
| Co-N-C/800 | 0.10 g催化剂;5 ml辛烷;140℃ | 99 | 98 |
| Co-N-C/800 | 0.15 g催化剂;5 ml辛烷;140℃ | 99 | 99 |
| Co-N-C/800 | 0.10 g催化剂;5 ml辛烷;120℃ | 61 | 60 |
| Co-N-C/800① | 0.15 g催化剂;5 ml辛烷;140℃ | 73 | 72 |
| Co-N-C/800② | 0.15 g催化剂;5 ml辛烷;140℃ | 89 | 87 |
| 底物1a | 底物2a | 产物3a | 1a转化率/% | 3a产率/% |
|---|---|---|---|---|
 |  |  | 82 | 80 |
 |  |  | 97 | 95 |
 |  |  | 98 | 96 |
 |  |  | 100 | 98 |
 |  |  | 97 | 95 |
 |  |  | 91 | 90 |
 |  |  | 98 | 97 |
 |  |  | 98 | 97 |
 |  |  | 96 | 94 |
表4 反应底物拓展结果
Table 4 Results of other reaction substrates
| 底物1a | 底物2a | 产物3a | 1a转化率/% | 3a产率/% |
|---|---|---|---|---|
 |  |  | 82 | 80 |
 |  |  | 97 | 95 |
 |  |  | 98 | 96 |
 |  |  | 100 | 98 |
 |  |  | 97 | 95 |
 |  |  | 91 | 90 |
 |  |  | 98 | 97 |
 |  |  | 98 | 97 |
 |  |  | 96 | 94 |
| 催化剂 | 外加 碱源 | 反应条件 | 3产率/% | 参考文献 |
|---|---|---|---|---|
| Co-N-C/800 | — | N2,140℃,12 h,辛烷 | 98 | 本文 |
| Ru/N-C | NaOH | N2,130℃,24 h,甲苯 | 98 | [ |
| Ru/C | KOH | N2,160℃,22 h,甲苯 | 94.1 | [ |
| Cd0.78Zn0.22S | — | N2,50℃,2 h,三氟甲苯, 可见光 (λ > 420 nm) | 36.8 | [ |
| CdIn2S4 | — | N2,60℃,8 h,三氟甲苯, 可见光 (λ > 420 nm) | 32 | [ |
| Ni/CdS | — | N2,20℃,4 h, 可见光 (λ > 420 nm) | 34 | [ |
| Pd/HT | — | 空气,130℃,24 h,甲苯 | 93 | [ |
| Au–Pd/TiO2 | — | 氩气,160℃,3 h,1,3,5-三甲苯 | 87.1 | [ |
| Co/N-C-600 | — | 一氧化碳,130℃,10 h,水 | 89.7 | [ |
表5 本文与文献结果的对比
Table 5 Comparison of this work with the literatures
| 催化剂 | 外加 碱源 | 反应条件 | 3产率/% | 参考文献 |
|---|---|---|---|---|
| Co-N-C/800 | — | N2,140℃,12 h,辛烷 | 98 | 本文 |
| Ru/N-C | NaOH | N2,130℃,24 h,甲苯 | 98 | [ |
| Ru/C | KOH | N2,160℃,22 h,甲苯 | 94.1 | [ |
| Cd0.78Zn0.22S | — | N2,50℃,2 h,三氟甲苯, 可见光 (λ > 420 nm) | 36.8 | [ |
| CdIn2S4 | — | N2,60℃,8 h,三氟甲苯, 可见光 (λ > 420 nm) | 32 | [ |
| Ni/CdS | — | N2,20℃,4 h, 可见光 (λ > 420 nm) | 34 | [ |
| Pd/HT | — | 空气,130℃,24 h,甲苯 | 93 | [ |
| Au–Pd/TiO2 | — | 氩气,160℃,3 h,1,3,5-三甲苯 | 87.1 | [ |
| Co/N-C-600 | — | 一氧化碳,130℃,10 h,水 | 89.7 | [ |
| 1 | 王树清, 高崇. 中间体N-亚苄基苯胺的合成研究[J]. 精细石油化工进展, 2006, 7(3): 45-47. |
| Wang S Q, Gao C. Study on synthesis of N-benzalaniline as itermediate[J]. Advances in Fine Petrochemicals., 2006, 7(3): 45-47. | |
| 2 | Naeimi H, Rabiei K. Montmorillonite as a heterogeneous catalyst in the efficient, mild and one pot synthesis of Schiff bases under solvent-free conditions[J]. J. Chin. Chem. Soc., 2012, 59: 208-212. |
| 3 | Wang K Z, Gao W B, Jiang P B, et al. Bi-functional catalyst of porous N-doped carbon with bimetallic FeCu for solvent-free resultant imines and hydrogenation of nitroarenes[J]. Mol. Catal., 2019, 465: 43–53. |
| 4 | Song T, Park J E, Chung Y K. Rhodium-catalyzed synthesis of imines and esters from benzyl alcohols and nitroarenes: change in catalyst reactivity depending on the presence or absence of the phosphine ligand[J]. J. Org. Chem., 2018, 83: 4197-4203. |
| 5 | Zhou P, Zhang Z H. One-pot reductive amination of carbonyl compounds with nitro compounds over the Co-Nxcatalyst by transfer hydrogenation[J]. ChemSusChem, 2017, 10(9): 1892-1897. |
| 6 | Guo B, Li H-X, Zhang S-Q, et al. C-N bond formation catalyzed by ruthenium nanoparticles supported on N-doped carbon via acceptorless dehydrogenation to secondary amines, imines, benzimidazoles and quinoxalines[J]. ChemCatChem, 2018, 10(24): 5627-5636. |
| 7 | 时洪涛, 刘迎新. Au-Ru/TiO2催化硝基苯和苯甲醛一锅法合成N-苄基苯胺[J]. 工业催化, 2012, 20(8): 61-64. |
| Shi H T, Liu Y X. One-pot synthesis of benzylaniline from nitrobenzene and benzaldehyde over Au-Ru/TiO2 catalyst[J]. Ind. Catal., 2012, 20(8): 61-64. | |
| 8 | Böhmer M, Kampert F, Peris E, et al. IrIII/AuI and RhIII/AuI heterobimetallic complexes as catalysts for the coupling of nitrobenzene and benzylic alcohol[J]. Organometallics, 2018, 37: 4092-4099. |
| 9 | 陆晓蕾, 张琳, 顾运江, 等. Ru/C催化剂上硝基苯与苯甲醇“一锅法” 合成N-亚苄基苯胺的研究[J].浙江化工, 2013, 44(10): 29-33. |
| Lu X L, Zhang L, Gu Y J, et al. One-pot synthesis of N-benzalaniline from nitrobenzene and benzyl alcohol over Ru/C Catalyst[J]. Zhejiang Chem. Ind., 2013, 44(10): 29-33. | |
| 10 | Zanardi A, Mata J A, Peris E. One-pot preparation of imines from nitroarenes by a tandem process with an Ir–Pd heterodimetallic catalyst[J]. Chem. Eur. J., 2010, 16: 10502-10506. |
| 11 | Zhang C H, Zhao P S, Zhang Z L. Co-N-C supported on SiO2: a facile, efficient catalyst for aerobic oxidation of amines to imines[J]. RSC Adv., 2017, 7: 47366-47372. |
| 12 | Hirakawa H, Katayama M, Shiraishi Y, et al. One-pot synthesis of imines from nitroaromatics and alcohols by tandem photocatalytic and catalytic reactions on degussa (Evonik) P25 titanium dioxide[J]. ACS Appl. Mater. Inter., 2015, 6(7): 3797-3806. |
| 13 | Cui X J, Zhang C G, Shi F, et al. Au/Ag-Mo nano-rods catalyzed reductive coupling of nitrobenzenes and alcohols using glycerol as the hydrogen source [J]. Chem. Commun., 2012, 48: 9391-9393. |
| 14 | Tang C H, He L, Liu Y M, et al. Direct one-pot reductive N-alkylation of nitroarenes by using alcohols with supported gold catalysts[J]. Chem. Eur. J., 2011, 17: 7172-7177. |
| 15 | Tang L, Sun H, Li Y F, et al. Highly active and selective synthesis of imines from alcohols and amines or nitroarenes catalyzed by Pd/DNA in water with dehydrogenation[J]. Green Chem., 2012, 14: 3423. |
| 16 | Chen J, Huang S J, Lin J, et al. Recyclable palladium catalyst for facile synthesis of imines from benzyl alcohols and nitroarenes[J]. Appl. Catal. A-Gen., 2014, 470: 1-7. |
| 17 | Zhou P, Yu C L, Jiang L, et al. One-pot reductive amination of carbonyl compounds with nitro compounds with CO/H2O as the hydrogen donor over non-noble cobalt catalyst[J]. J. Catal., 2017, 352: 264-273. |
| 18 | Li M S, Fernando C L, Keane M. Combined catalytic action of supported Cu and Au in imine production from coupled benzyl alcohol and nitrobenzene reactions[J]. Appl. Catal. A-Gen., 2018, 557: 145-153. |
| 19 | Sankar M, He Q, Dawson S, et al. Supported bimetallic nano-alloys as highly active catalysts for the one-pot tandem synthesis of imines and secondary amines from nitrobenzene and alcohols[J]. Catal. Sci. Technol., 2016, 6: 5473. |
| 20 | Wu Y H, Ye X G, Zhang S J, et al. Photocatalytic synthesis of Schiff base compounds in the coupled system of aromatic alcohols and nitrobenzene using CdXZn1-XS photocatalysts[J]. J. Catal., 2018, 359: 151-160. |
| 21 | Ye X G, Chen Y H, Ling C C, et al. One-pot synthesis of Schiff base compounds via photocatalytic reaction in the coupled system of aromatic alcohols and nitrobenzene using CdIn2S4 photocatalyst[J]. Dalton Trans., 2018, 47: 10915-10924. |
| 22 | Yu W W, Guo X W, Song C S, et al. Visible-light-initiated one-pot clean synthesis of nitrone from nitrobenzene and benzyl alcohol over CdS photocatalyst[J]. J. Catal., 2019, 370: 97-106. |
| 23 | Zhao X, Zhou Y, Tao D J, et al. Co-N-C catalysts synthesized by pyrolysis of Co-based deep eutectic solvents for aerobic oxidation of alcohols[J]. New J. Chem., 2018, 42: 15871-15878. |
| 24 | Dou Y B, Chen Y, Li J R, et al. Pd@ZIF-67 derived recyclable Pd-based catalysts with hierarchical pores for high-performance Heck reaction[J]. ACS Sustain. Chem. Eng., 2018, 6: 2103-2111. |
| 25 | Liu D, Yang P, Zhang H, et al. Direct reductive coupling of nitroarenes and alcohols catalysed by Co-N-C/CNT@AC[J]. Green Chem., 2019, 21: 2129-2137. |
| 26 | Zhao X, Zhou Y, Tao D J, et al. Ultralow loading cobalt-based nanocatalyst for benign and efficient aerobic oxidation of allylic alcohols and biobased olefins[J]. ACS Sustain. Chem. Eng., 2019, 2(7): 1901-1908. |
| 27 | An X C, Zhou Y, Tao D J, et al. Rapid capture and efficient removal of low-concentration SO2 in simulated flue gas by hypercrosslinked hollow nanotube ionic polymers[J]. Chem. Eng. J., 2020, 394: 124859. |
| 28 | Jie S S, Yang C Q, Chen Y, et al. Facile synthesis of ultrastable Co-N-C catalysts using cobalt porphyrin and peptides as precursors for selective oxidation of ethylbenzene[J]. Mol. Catal., 2018, 458: 1-8. |
| 29 | Sun T G, Xu L B, Li S Y, et al. Cobalt-nitrogen-doped ordered macro-/mesoporous carbon for highly efficient oxygen reduction reaction[J]. Appl. Catal. B-Environ., 2016, 193: 1-8. |
| 30 | Lan G J, Wang Y, Yang Y, et al. Flour-derived N-doped mesoporous carbon extrudate as superior metal-free catalysts for acetylene hydrochlorination[J]. Chem. Commun., 2018, 54: 623. |
| 31 | Liu W G, Zhang L L, Yan W S, et al. Single-atom dispersed Co-N-C catalyst: structure identification and performance for hydrogenative coupling of nitroarenes[J]. Chem. Sci., 2016, 7: 5758-5764. |
| 32 | Cui X L, Liang K, Tian M, et al. Cobalt nanoparticles supported on N-doped mesoporous carbon as a highly efficient catalyst for the synthesis of aromatic amines[J]. J. Coll. Inter. Sci., 2017, 501: 231-240. |
| 33 | Yu P, Wang L, Sun F, et al. Co nanoislands rooted on Co-N-C nanosheets as efficient oxygen electrocatalyst for Zn-air batteries[J]. Adv. Mater., 2019, 31(30): 1901666. |
| 34 | Chen Y Z, Wang C, Wu Z Y, et al. From bimetallic metal-organic framework to porous carbon: high surface area and multicomponent active dopants for excellent electrocatalysis[J]. Adv. Mater., 2015, 27: 5010-5016. |
| [1] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [2] | 陈韶云, 徐东, 陈龙, 张禹, 张远方, 尤庆亮, 胡成龙, 陈建. 单层聚苯胺微球阵列结构的制备及其吸附性能[J]. 化工学报, 2023, 74(5): 2228-2238. |
| [3] | 徐东, 田杜, 陈龙, 张禹, 尤庆亮, 胡成龙, 陈韶云, 陈建. 聚苯胺/二氧化锰/聚吡咯复合纳米球的制备及其电化学储能性[J]. 化工学报, 2023, 74(3): 1379-1389. |
| [4] | 党迎喜, 谈朋, 刘晓勤, 孙林兵. 辐射冷却和太阳能加热驱动的CO2变温捕获[J]. 化工学报, 2023, 74(1): 469-478. |
| [5] | 徐珂, 史国强, 薛冬峰. 无机杂化钙钛矿团簇材料:介尺度钙钛矿材料发光性质研究[J]. 化工学报, 2022, 73(6): 2748-2756. |
| [6] | 陈婷, 胡泽浩, 秦喆, 陈园虹, 徐彦乔, 林坚, 谢志翔. 有机相微波合成AgInS2量子点及其白光发光二极管应用研究[J]. 化工学报, 2022, 73(11): 5167-5176. |
| [7] | 侯旺君, 闫翎鹏, 曹哲勇, 郑静霞, 杨永珍. 煤基零维纳米碳材料的合成、性能及其在能源转换和存储应用中的研究进展[J]. 化工学报, 2022, 73(11): 4791-4813. |
| [8] | 吴诗德, 易峰, 平丹, 张逸飞, 郝健, 刘国际, 方少明. NH4Cl辅助热解制备镍-氮-碳纳米管催化剂及其电还原CO2性能[J]. 化工学报, 2022, 73(10): 4484-4497. |
| [9] | 王欢, 符方宝, 李琼, 席跃宾, 杨东杰. 木质素碳纳米材料制备及在催化中的应用研究进展[J]. 化工学报, 2021, 72(9): 4445-4457. |
| [10] | 马生贵, 田博文, 周雨薇, 陈琳, 江霞, 高涛. 氮掺杂Stone-Wales缺陷石墨烯吸附H2S的密度泛函理论研究[J]. 化工学报, 2021, 72(9): 4496-4503. |
| [11] | 李宇明, 刘梓烨, 张启扬, 王雅君, 崔国庆, 姜桂元, 贺德华. 氮掺杂碳材料的制备及其在催化领域中的应用[J]. 化工学报, 2021, 72(8): 3919-3932. |
| [12] | 彭启, 贾力, 丁艺, 张永欣, 党超, 银了飞. 受限微结构对低表面张力液滴合并弹跳的影响[J]. 化工学报, 2021, 72(4): 1920-1929. |
| [13] | 赵玉海, 罗英武. 可逆失活自由基界面聚合[J]. 化工学报, 2021, 72(2): 653-668. |
| [14] | 张毅舟, 吴籼虹, 王治宇, 邱介山. 镶嵌单层MoS2的生物质基硼氮共掺杂碳纳米片合成与储钠性能[J]. 化工学报, 2021, 72(12): 6371-6379. |
| [15] | 丁鼎, 陆文多, 侯璐, 陆安慧. 纤维状BPO4/SiO2催化剂的制备及其丙烷氧化脱氢性能[J]. 化工学报, 2021, 72(11): 5590-5597. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号