化工学报 ›› 2023, Vol. 74 ›› Issue (7): 2753-2772.DOI: 10.11949/0438-1157.20230174
张琦钰1( ), 高利军1, 苏宇航1, 马晓博1, 王翊丞2, 张亚婷2, 胡超1(
), 高利军1, 苏宇航1, 马晓博1, 王翊丞2, 张亚婷2, 胡超1( )
)
收稿日期:2023-02-28
修回日期:2023-07-03
出版日期:2023-07-05
发布日期:2023-08-31
通讯作者:
胡超
作者简介:张琦钰(1992—),男,硕士研究生,ted_zhang_cup@163.com
基金资助:
Qiyu ZHANG1( ), Lijun GAO1, Yuhang SU1, Xiaobo MA1, Yicheng WANG2, Yating ZHANG2, Chao HU1(
), Lijun GAO1, Yuhang SU1, Xiaobo MA1, Yicheng WANG2, Yating ZHANG2, Chao HU1( )
)
Received:2023-02-28
Revised:2023-07-03
Online:2023-07-05
Published:2023-08-31
Contact:
Chao HU
摘要:
利用电化学还原技术将二氧化碳(CO2)转化为高能燃料或高值化学品是提升CO2利用附加值、缓解CO2排放压力的有效途径,也是风电、水电和太阳能等绿色能源的转化与存储方式之一,对间歇性电能“削峰填谷”意义重大。实现高效电化学还原CO2的关键之一在于创制高性能的电催化材料。综述了碳基催化材料在电化学还原CO2方面的研究进展,系统探讨了本征缺陷碳材料、掺杂碳材料、碳基复合材料和整体式碳材料的结构特点以及与电催化还原CO2性能的构效关系,并在此基础之上,展望了碳基催化材料在电化学还原CO2领域中的挑战和未来发展。
中图分类号:
张琦钰, 高利军, 苏宇航, 马晓博, 王翊丞, 张亚婷, 胡超. 碳基催化材料在电化学还原二氧化碳中的研究进展[J]. 化工学报, 2023, 74(7): 2753-2772.
Qiyu ZHANG, Lijun GAO, Yuhang SU, Xiaobo MA, Yicheng WANG, Yating ZHANG, Chao HU. Recent advances in carbon-based catalysts for electrochemical reduction of carbon dioxide[J]. CIESC Journal, 2023, 74(7): 2753-2772.
| 产物 | k | m | n | |
|---|---|---|---|---|
| CO | 1 | 2 | 1 | -0.52 |
| HCOOH | 1 | 2 | 0 | -0.61 |
| HCHO | 1 | 4 | 1 | -0.51 |
| CH3OH | 1 | 6 | 1 | 0.02 |
| CH4 | 1 | 8 | 2 | 0.17 |
| C2H4 | 2 | 12 | 4 | 0.08 |
| CH3CH2OH | 2 | 12 | 3 | 0.09 |
表1 电化学CO2RR的阴极反应产物
Table 1 Cathodic products of electrochemical CO2RR
| 产物 | k | m | n | |
|---|---|---|---|---|
| CO | 1 | 2 | 1 | -0.52 |
| HCOOH | 1 | 2 | 0 | -0.61 |
| HCHO | 1 | 4 | 1 | -0.51 |
| CH3OH | 1 | 6 | 1 | 0.02 |
| CH4 | 1 | 8 | 2 | 0.17 |
| C2H4 | 2 | 12 | 4 | 0.08 |
| CH3CH2OH | 2 | 12 | 3 | 0.09 |
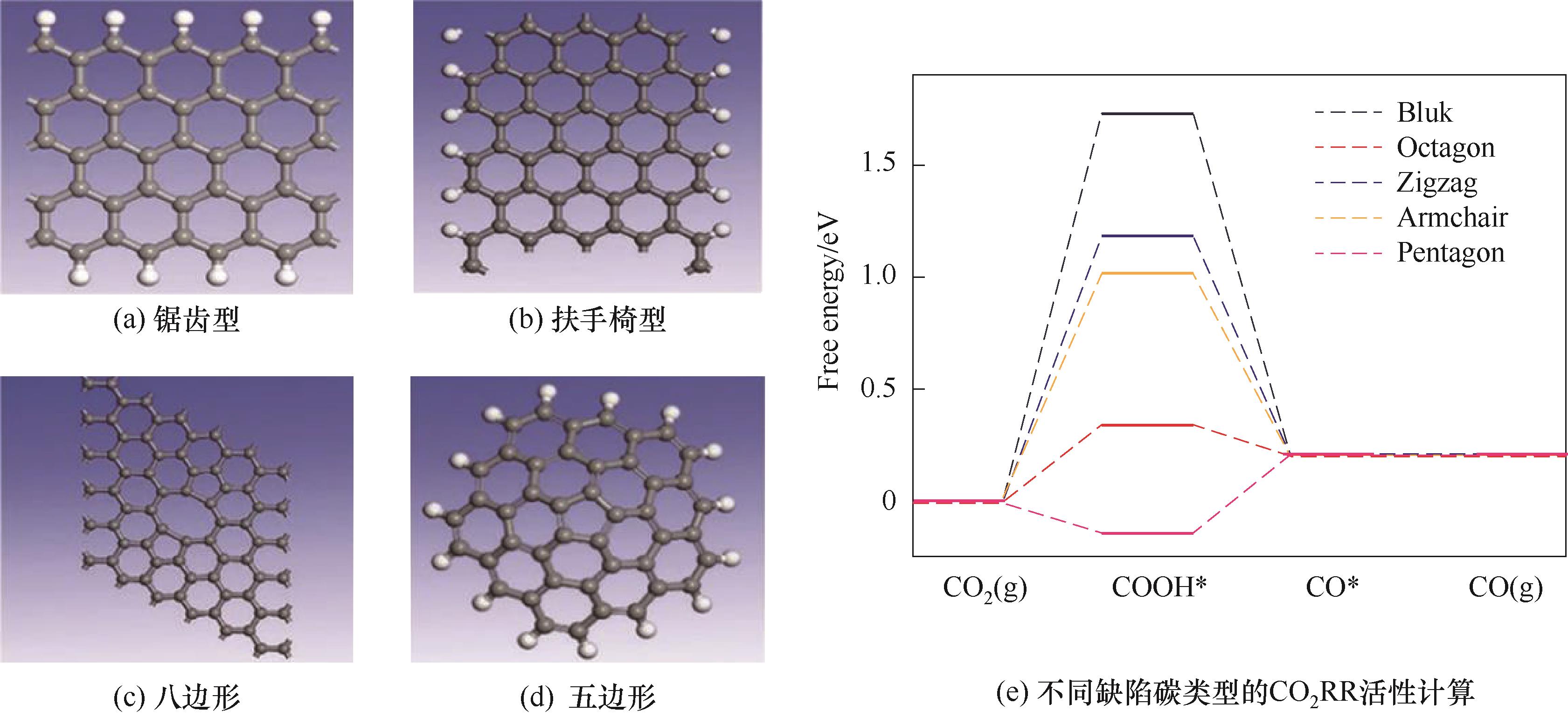
图3 四种不同缺陷类型结构示意图以及CO2RR活性计算[18]
Fig.3 Schematic diagrams of the structures and DFT calculations for CO2RR activities of four different defect types[18]

图4 杂原子掺杂在碳原子矩阵的示意图以及基于不同氮位点的DFT活性计算[46]
Fig.4 Schematic representation of heteroatom doping in carbon matrix and DFT calculation based on different nitrogen sites[46]

图5 氟掺杂碳催化剂的电镜照片、掺杂模型与不同位点的DFT理论计算[53]
Fig.5 Electron micrographs of F-doped carbon catalysts, and their doping models and DFT theoretical calculation for different sites[53]
| 催化剂 | 电解液 | FE/% | 主要产物 | 过电势/V | 活性中心 |
|---|---|---|---|---|---|
| FC[ | 0.1 mol·L-1 KHCO3 | 89.6 | CO | 0.51 | 氟原子和碳原子 |
| NDC[ | 0.5 mol·L-1 NaHCO3 | 83.7 | CO | 0.71 | 吡啶氮原子 |
| N/C-Cl-1100[ | 0.1 mol·L-1 KHCO3 | 99.5 | CO | 0.40 | 石墨氮原子 |
| CPSN[ | 0.1 mol·L-1 KHCO3 | 11.3 | CO | 0.88 | 碳原子 |
| BND[ | 0.1 mol·L-1 NaHCO3 | 93.2 | CH3CH2OH | 0.90 | 硼原子和氮原子 |
| NCNTs[ | 0.1 mol·L-1 KHCO3 | 约80 | CO | — | 吡啶氮原子和石墨氮原子 |
| NCNTs[ | 0.1 mol·L-1 KHCO3 | 约80 | CO | 0.26 | 吡啶氮原子和石墨氮原子 |
| NG[ | 0.1 mol·L-1 KHCO3 | 约85 | CO | 0.47 | 吡啶氮原子 |
| NG-T[ | 0.5 mol·L-1 KHCO3 | 95.0 | CO | 0.62 | 碳原子 |
| c-NC[ | 0.1 mol·L-1 KHCO3 | 77.0 | CH3CH2OH | 0.63 | 吡啶氮原子和吡咯氮原子 |
表2 非金属异原子掺杂碳材料在电催化CO2RR中的性能对比
Table 2 Performance comparisons of non-metallic heteroatom-doped carbon materials in electrochemical CO2RR
| 催化剂 | 电解液 | FE/% | 主要产物 | 过电势/V | 活性中心 |
|---|---|---|---|---|---|
| FC[ | 0.1 mol·L-1 KHCO3 | 89.6 | CO | 0.51 | 氟原子和碳原子 |
| NDC[ | 0.5 mol·L-1 NaHCO3 | 83.7 | CO | 0.71 | 吡啶氮原子 |
| N/C-Cl-1100[ | 0.1 mol·L-1 KHCO3 | 99.5 | CO | 0.40 | 石墨氮原子 |
| CPSN[ | 0.1 mol·L-1 KHCO3 | 11.3 | CO | 0.88 | 碳原子 |
| BND[ | 0.1 mol·L-1 NaHCO3 | 93.2 | CH3CH2OH | 0.90 | 硼原子和氮原子 |
| NCNTs[ | 0.1 mol·L-1 KHCO3 | 约80 | CO | — | 吡啶氮原子和石墨氮原子 |
| NCNTs[ | 0.1 mol·L-1 KHCO3 | 约80 | CO | 0.26 | 吡啶氮原子和石墨氮原子 |
| NG[ | 0.1 mol·L-1 KHCO3 | 约85 | CO | 0.47 | 吡啶氮原子 |
| NG-T[ | 0.5 mol·L-1 KHCO3 | 95.0 | CO | 0.62 | 碳原子 |
| c-NC[ | 0.1 mol·L-1 KHCO3 | 77.0 | CH3CH2OH | 0.63 | 吡啶氮原子和吡咯氮原子 |

图6 单原子Ni掺杂石墨烯(A-Ni-NG)的表征,性能测试及活性中心演变示意图[59]N-G—不含金属的氮掺杂石墨烯;Ni-NG—含Ni纳米颗粒的氮掺杂石墨烯;A-Ni-NSG,A-Ni-NG—含硫与不含硫的单原子Ni掺杂石墨烯
Fig.6 Structural characterization of single atom Ni doped graphene and its performance and structural evolution in CO2RR process [59]
| 催化剂 | 电解液 | FE/% | 电流密度/(mA·cm–2) | 主要产物 | 过电势/V | 活性中心 |
|---|---|---|---|---|---|---|
| Ni2+@NG[ | 0.5 mol·L-1 KHCO3 | 92 | 10.2 | CO | 0.58 | |
| NiSA/NP[ | 0.5 mol·L-1 NaHCO3 | 99 | 131 | CO | 0.7 | |
| P-NiSA/PCFM[ | 0.5 mol·L-1 KHCO3 | 95 | 56.1 | CO | 0.6 | |
| Ni-N-C[ | 0.1 mol·L-1 KHCO3 | 97.9 | 12.6 | CO | 0.67 | Ni-N x |
| NC-CNTs (Ni)[ | 0.1 mol·L-1 KHCO3 | 90 | 10 | CO | 0.9 | |
| A-Ni-NSG[ | 0.5 mol·L-1 KHCO3 | 97 | 22 | CO | 0.4 | |
| NiSA-N-CNTs[ | 0.5 mol·L-1 KHCO3 | 91.3 | 23.5 | CO | 0.6 | |
| Ni-N3-V SAC[ | 0.5 mol·L-1 KHCO3 | 94 | 65 | CO | 0.7 | |
| C-Zn x Ni y ZIF-8[ | 1 mol·L-1 KHCO3 | 97.8 | 71.5 | CO | 0.53 | Ni-N2 |
| Ni-N4-C[ | 0.1 mol·L-1 KHCO3 | 99 | 36.2 | CO | 0.71 | |
| Ni-NG[ | 0.5 mol·L-1 KHCO3 | 95 | 11 | CO | 0.62 | Ni-N x |
| Ni-NCB[ | 0.5 mol·L-1 KHCO3 | 99 | 22 | CO | 0.58 | Ni-N x |
| FeN4Cl/NC[ | 0.5 mol·L-1 KHCO3 | 90.5 | 10.8 | CO | 0.49 | |
| Fe/NG-750[ | 0.1 mol·L-1 KHCO3 | 80 | 2.63 | CO | 0.5 | |
| Fe-NC-S[ | 0.5 mol·L-1 KHCO3 | 93 | 4 | CO | 0.29 | |
| Cu–N2/GN[ | 0.1 mol·L-1 KHCO3 | 81 | 2.1 | CO | 0.4 | |
| Cu SAs/GDY[ | 0.1 mol·L-1 KHCO3 | 66 | 24 | CH4 | 1.47 | |
| In SAs/NC[ | 0.5 mol·L-1 KHCO3 | 96 | 8.87 | HCOOH | 0.53 | |
| Pd-NC[ | 0.5 mol·L-1 NaHCO3 | 55 | 2.2 | CO | 0.4 | |
| Single-atom Sn δ+[ | 0.25 mol·L-1 KHCO3 | 74.3 | 11.7 | HCOOH | 1.24 | |
| (Cl, N)-Mn/G[ | 0.5 mol·L-1 KHCO3 | 97 | 10 | CO | 0.49 | |
| Co1-N4[ | 0.1 mol·L-1 KHCO3 | 82 | 15.8 | CO | 0.7 | |
| Zn–N–G[ | 0.5 mol·L-1 KHCO3 | 91 | 11.2 | CO | 0.39 | |
| Cu/Pc-C[ | 0.5 mol·L-1 KCl | 25 | 2.8 | C2H4 | 1.68 | |
| Dual Cu SAC[ | 0.1 mol·L-1 KHCO3 | 91 | >90 | C2+ | — |
表3 单原子金属掺杂碳材料在电催化CO2RR 中的性能对比
Table 3 Performance comparisons of single-atom metal-doped carbon materials in electrochemical CO2RR
| 催化剂 | 电解液 | FE/% | 电流密度/(mA·cm–2) | 主要产物 | 过电势/V | 活性中心 |
|---|---|---|---|---|---|---|
| Ni2+@NG[ | 0.5 mol·L-1 KHCO3 | 92 | 10.2 | CO | 0.58 | |
| NiSA/NP[ | 0.5 mol·L-1 NaHCO3 | 99 | 131 | CO | 0.7 | |
| P-NiSA/PCFM[ | 0.5 mol·L-1 KHCO3 | 95 | 56.1 | CO | 0.6 | |
| Ni-N-C[ | 0.1 mol·L-1 KHCO3 | 97.9 | 12.6 | CO | 0.67 | Ni-N x |
| NC-CNTs (Ni)[ | 0.1 mol·L-1 KHCO3 | 90 | 10 | CO | 0.9 | |
| A-Ni-NSG[ | 0.5 mol·L-1 KHCO3 | 97 | 22 | CO | 0.4 | |
| NiSA-N-CNTs[ | 0.5 mol·L-1 KHCO3 | 91.3 | 23.5 | CO | 0.6 | |
| Ni-N3-V SAC[ | 0.5 mol·L-1 KHCO3 | 94 | 65 | CO | 0.7 | |
| C-Zn x Ni y ZIF-8[ | 1 mol·L-1 KHCO3 | 97.8 | 71.5 | CO | 0.53 | Ni-N2 |
| Ni-N4-C[ | 0.1 mol·L-1 KHCO3 | 99 | 36.2 | CO | 0.71 | |
| Ni-NG[ | 0.5 mol·L-1 KHCO3 | 95 | 11 | CO | 0.62 | Ni-N x |
| Ni-NCB[ | 0.5 mol·L-1 KHCO3 | 99 | 22 | CO | 0.58 | Ni-N x |
| FeN4Cl/NC[ | 0.5 mol·L-1 KHCO3 | 90.5 | 10.8 | CO | 0.49 | |
| Fe/NG-750[ | 0.1 mol·L-1 KHCO3 | 80 | 2.63 | CO | 0.5 | |
| Fe-NC-S[ | 0.5 mol·L-1 KHCO3 | 93 | 4 | CO | 0.29 | |
| Cu–N2/GN[ | 0.1 mol·L-1 KHCO3 | 81 | 2.1 | CO | 0.4 | |
| Cu SAs/GDY[ | 0.1 mol·L-1 KHCO3 | 66 | 24 | CH4 | 1.47 | |
| In SAs/NC[ | 0.5 mol·L-1 KHCO3 | 96 | 8.87 | HCOOH | 0.53 | |
| Pd-NC[ | 0.5 mol·L-1 NaHCO3 | 55 | 2.2 | CO | 0.4 | |
| Single-atom Sn δ+[ | 0.25 mol·L-1 KHCO3 | 74.3 | 11.7 | HCOOH | 1.24 | |
| (Cl, N)-Mn/G[ | 0.5 mol·L-1 KHCO3 | 97 | 10 | CO | 0.49 | |
| Co1-N4[ | 0.1 mol·L-1 KHCO3 | 82 | 15.8 | CO | 0.7 | |
| Zn–N–G[ | 0.5 mol·L-1 KHCO3 | 91 | 11.2 | CO | 0.39 | |
| Cu/Pc-C[ | 0.5 mol·L-1 KCl | 25 | 2.8 | C2H4 | 1.68 | |
| Dual Cu SAC[ | 0.1 mol·L-1 KHCO3 | 91 | >90 | C2+ | — |
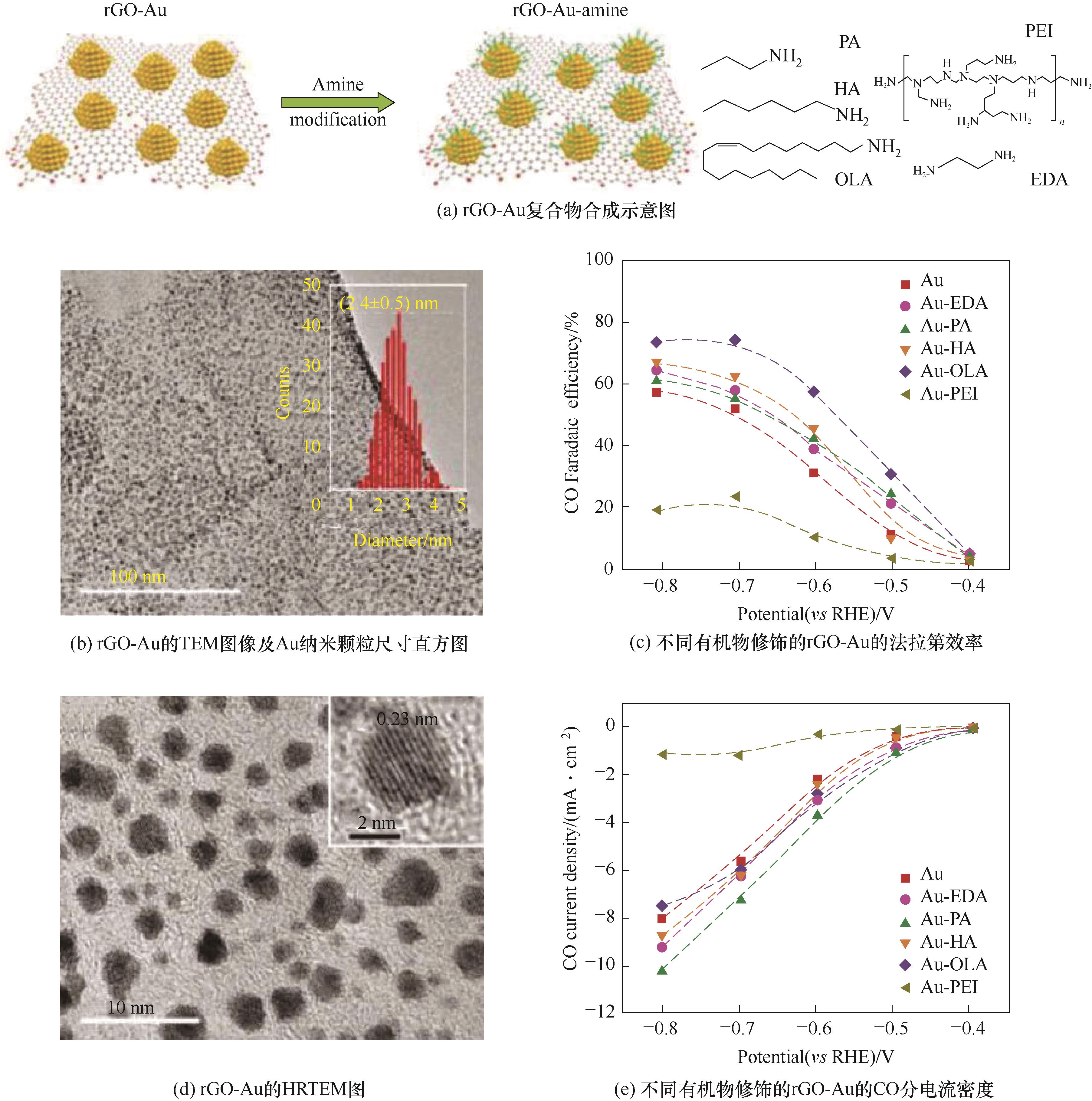
图8 胺改性的搭载于还原氧化石墨烯上的超微Au纳米颗粒催化剂[109]
Fig.8 Engineering surface amine modifiers of ultrasmall gold nanoparticles supported on reduced graphene oxide electrocatalyst[109]
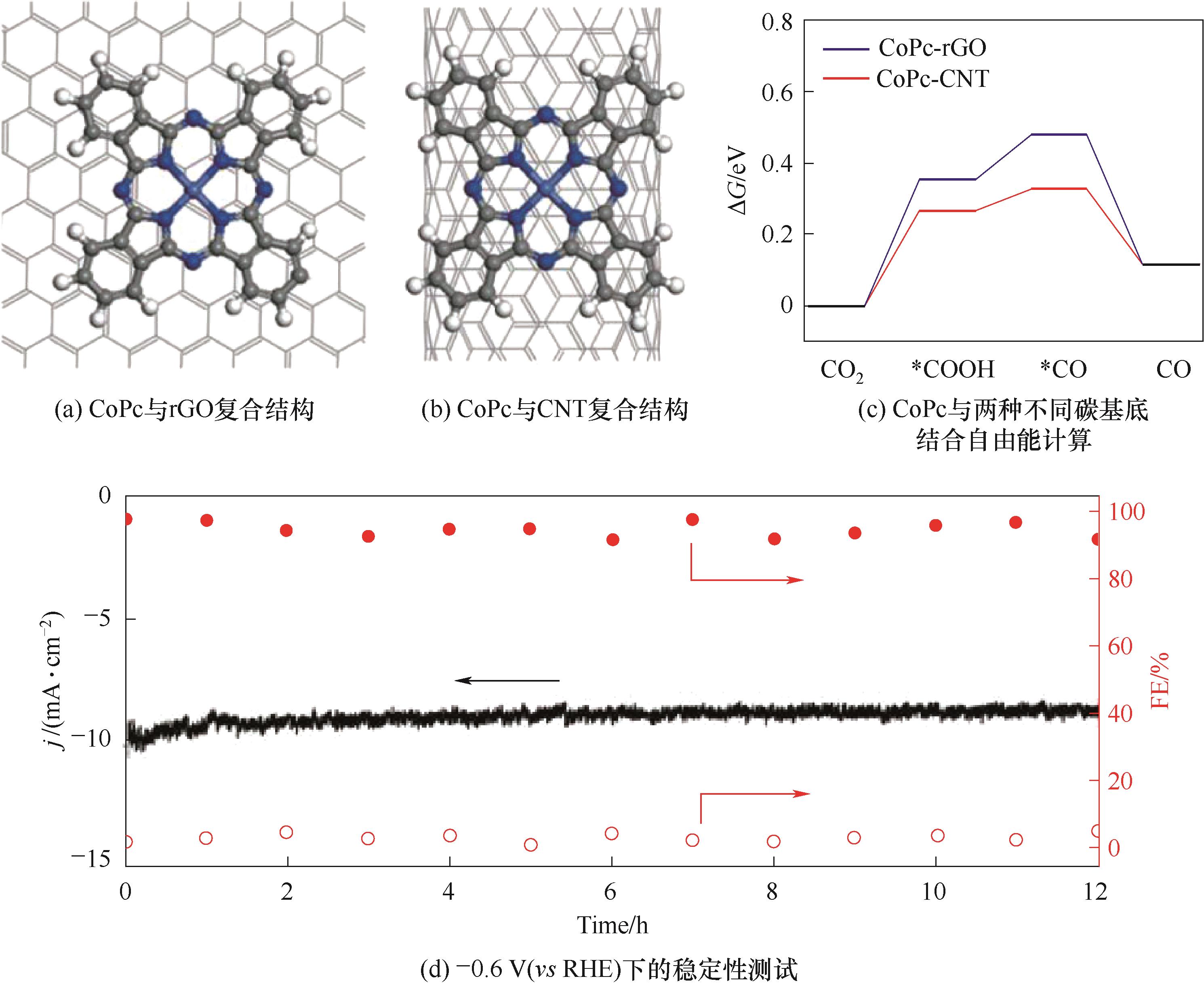
图10 rGO和CNTs搭载酞菁钴分子催化剂用于CO2RR的结构模型、理论计算及稳定性测试[133]
Fig.10 Structural modeling, theoretical calculations and stability test of rGO- and CNTs-loaded cobalt phthalocyanine molecular catalysts for CO2RR [133]
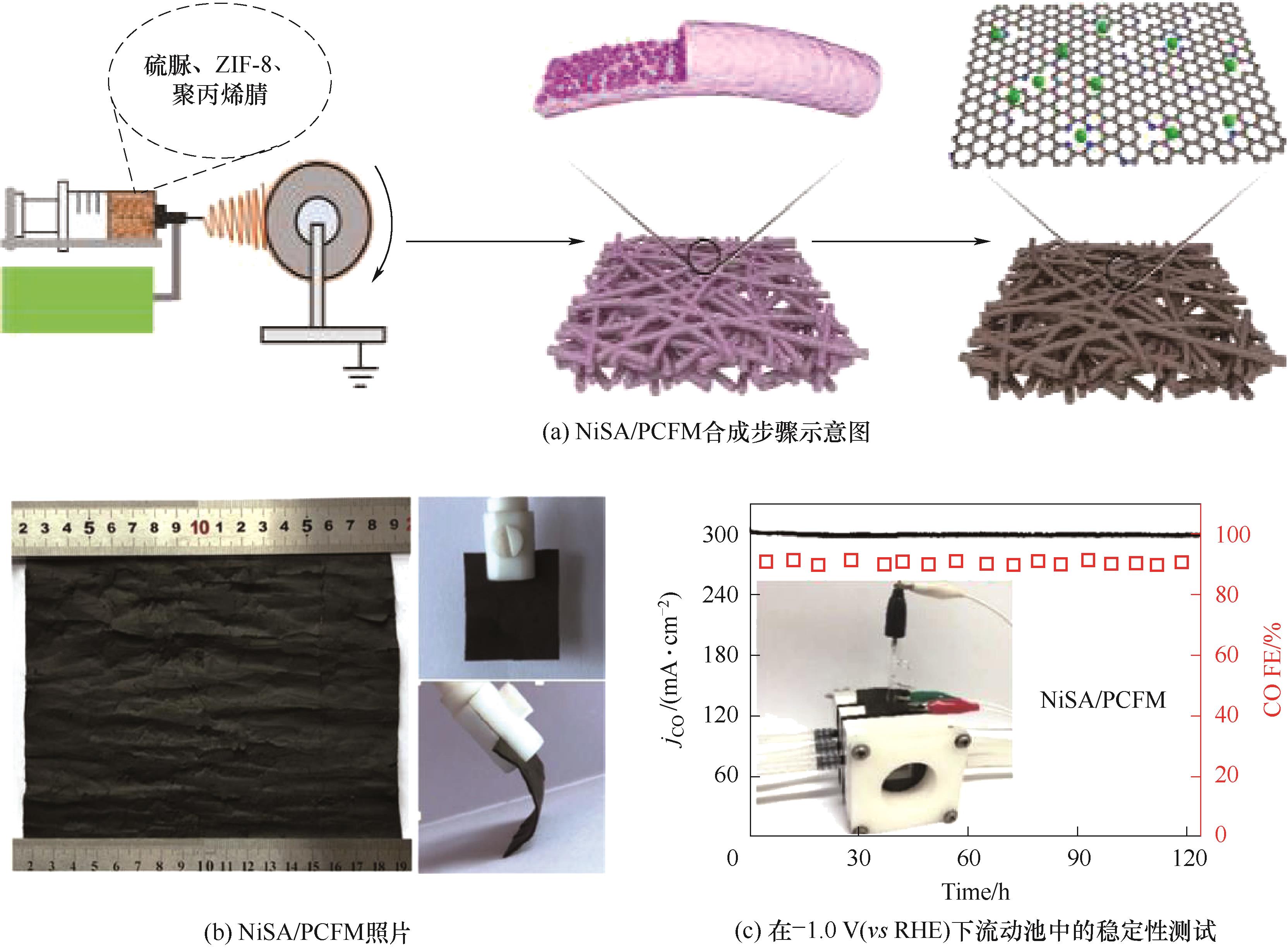
图11 搭载单原子Ni的碳纤维膜材料的制备过程、照片以及在流动池中的稳定性测试[16]
Fig.11 Preparation process, photos and stability test in a flow cell of carbon fiber membrane material loaded with single atom Ni[16]
| 1 | Shakun J D, Clark P U, He F, et al. Global warming preceded by increasing carbon dioxide concentrations during the last deglaciation[J]. Nature, 2012, 484(7392): 49-54. |
| 2 | 李鑫, 曾少娟, 彭奎霖, 等. CO2电催化还原制合成气研究进展及趋势[J]. 化工学报, 2023, 74(1): 313-329. |
| Li X, Zeng S J, Peng K L, et al. Research progress and tendency of CO2 electrocatalytic reduction to syngas[J]. CIESC Journal, 2023, 74(1): 313-329. | |
| 3 | Chen Y H, Li C W, Kanan M W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles[J]. Journal of the American Chemical Society, 2012, 134(49): 19969-19972. |
| 4 | Lv J J, Jouny M, Luc W, et al. A highly porous copper electrocatalyst for carbon dioxide reduction[J]. Advanced Materials, 2018, 30(49): 1803111. |
| 5 | Li F W, Chen L, Knowles G P, et al. Hierarchical mesoporous SnO2 nanosheets on carbon cloth: a robust and flexible electrocatalyst for CO2 reduction with high efficiency and selectivity[J]. Angewandte Chemie International Edition, 2017, 129(2): 520-524. |
| 6 | Ren S X, Joulié D, Salvatore D, et al. Molecular electrocatalysts can mediate fast, selective CO2 reduction in a flow cell[J]. Science, 2019, 365(6451): 367-369. |
| 7 | Yi J D, Si D H, Xie R K, et al. Conductive two-dimensional phthalocyanine-based metal-organic framework nanosheets for efficient electroreduction of CO2 [J]. Angewandte Chemie International Edition, 2021, 60(31): 17108-17114. |
| 8 | Xue D P, Xia H C, Yan W F, et al. Defect engineering on carbon-based catalysts for electrocatalytic CO2 reduction[J]. Nano-Micro Letters, 2021, 13(1): 5. |
| 9 | 董灵玉, 葛睿, 原亚飞, 等. 多孔炭基二氧化碳电催化材料研究进展[J]. 化工学报, 2020, 71(6): 2492-2509. |
| Dong L Y, Ge R, Yuan Y F, et al. Recent advances in porous carbon-based carbon dioxide electrocatalytic materials[J]. CIESC Journal, 2020, 71(6): 2492-2509. | |
| 10 | Ni J F, Li Y. Carbon nanomaterials in different dimensions for electrochemical energy storage[J]. Advanced Energy Materials, 2016, 6(17): 1600278. |
| 11 | Kumar S, Saeed G, Zhu L, et al. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: a review[J]. Chemical Engineering Journal, 2021, 403: 126352. |
| 12 | Zhao Y Y, Zhang Y L, Wang Y, et al. Versatile zero- to three-dimensional carbon for electrochemical energy storage[J]. Carbon Energy, 2021, 3(6): 895-915. |
| 13 | Zhao R Y, Wang Y D, Ji G P, et al. Partially nitrided Ni nanoclusters achieve energy-efficient electrocatalytic CO2 reduction to CO at ultralow overpotential[J]. Advanced Materials, 2023, 35(5): 2205262. |
| 14 | Wang L L, Li X, Hao L D, et al. Integration of ultrafine CuO nanoparticles with two-dimensional MOFs for enhanced electrochemical CO2 reduction to ethylene[J]. Chinese Journal of Catalysis, 2022, 43(4): 1049-1057. |
| 15 | Hao J C, Zhuang Z C, Hao J C, et al. Interatomic electronegativity offset dictates selectivity when catalyzing the CO2 reduction reaction[J]. Advanced Energy Materials, 2022, 12(26): 2200579. |
| 16 | Yang H P, Lin Q, Zhang C, et al. Carbon dioxide electroreduction on single-atom nickel decorated carbon membranes with industry compatible current densities[J]. Nature Communications, 2020, 11: 593. |
| 17 | Zheng T T, Jiang K, Ta N, et al. Large-scale and highly selective CO2 electrocatalytic reduction on nickel single-atom catalyst[J]. Joule, 2019, 3(1): 265-278. |
| 18 | Wang W, Shang L, Chang G J, et al. Intrinsic carbon-defect-driven electrocatalytic reduction of carbon dioxide[J]. Advanced Materials, 2019, 31(19): 1808276. |
| 19 | Wang X Q, Chen Z, Zhao X Y, et al. Regulation of coordination number over single Co sites: triggering the efficient electroreduction of CO2 [J]. Angewandte Chemie International Edition, 2018, 57(7): 1944-1948. |
| 20 | Ju W, Bagger A, Hao G P, et al. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2 [J]. Nature Communications, 2017, 8(1): 1-9. |
| 21 | Wu J J, Liu M J, Sharma P P, et al. Incorporation of nitrogen defects for efficient reduction of CO2 via two-electron pathway on three-dimensional graphene foam[J]. Nano Letters, 2016, 16(1): 466-470. |
| 22 | Varela A S, Ranjbar Sahraie N, Steinberg J, et al. Metal-doped nitrogenated carbon as an efficient catalyst for direct CO2 electroreduction to CO and hydrocarbons[J]. Angewandte Chemie International Edition, 2015, 54(37): 10758-10762. |
| 23 | Zheng T T, Jiang K, Wang H T. Recent advances in electrochemical CO2-to-CO conversion on heterogeneous catalysts[J]. Advanced Materials, 2018, 30(48): 1802066. |
| 24 | Zhao K, Quan X. Carbon-based materials for electrochemical reduction of CO2 to C2+ oxygenates: recent progress and remaining challenges[J]. ACS Catalysis, 2021, 11(4): 2076-2097. |
| 25 | Zhang L, Zhao Z J, Gong J L. Nanostructured materials for heterogeneous electrocatalytic CO2 reduction and their related reaction mechanisms[J]. Angewandte Chemie International Edition, 2017, 56(38): 11326-11353. |
| 26 | Xiong W F, Li H F, Wang H M, et al. Hollow mesoporous carbon sphere loaded Ni-N4 single-atom: support structure study for CO2 electrocatalytic reduction catalyst[J]. Small, 2020, 16(41): 2003943. |
| 27 | Kortlever R, Shen J, Schouten K J P, et al. Catalysts and reaction pathways for the electrochemical reduction of carbon dioxide[J]. The Journal of Physical Chemistry Letters, 2015, 6(20): 4073-4082. |
| 28 | Zheng Y, Vasileff A, Zhou X L, et al. Understanding the roadmap for electrochemical reduction of CO2 to multi-carbon oxygenates and hydrocarbons on copper-based catalysts[J]. Journal of the American Chemical Society, 2019, 141(19): 7646-7659. |
| 29 | Wu J J, Sharifi T, Gao Y, et al. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value-added chemicals[J]. Advanced Materials, 2019, 31(13): 1804257. |
| 30 | Wu Q L, Yan X C, Jia Y, et al. Defective carbon-based materials: controllable synthesis and electrochemical applications[J]. EnergyChem, 2021, 3(5): 100059. |
| 31 | Li W B, Yu C, Tan X Y, et al. Recent advances in the electroreduction of carbon dioxide to formic acid over carbon-based materials[J]. New Carbon Materials, 2022, 37(2): 277-287. |
| 32 | Lv J J, Yin R N, Zhou L M, et al. Microenvironment engineering for the electrocatalytic CO2 reduction reaction[J]. Angewandte Chemie, 2022, 61(39): e202207252. |
| 33 | Tang T M, Wang Z L, Guan J Q. Optimizing the electrocatalytic selectivity of carbon dioxide reduction reaction by regulating the electronic structure of single-atom M-N-C materials[J]. Advanced Functional Materials, 2022, 32(19): 2111504. |
| 34 | Bagchi D, Roy S, Sarma S C, et al. Toward unifying the mechanistic concepts in electrochemical CO2 reduction from an integrated material design and catalytic perspective[J]. Advanced Functional Materials, 2022, 32(51): 2209023. |
| 35 | Han P, Yu X M, Yuan D, et al. Defective graphene for electrocatalytic CO2 reduction[J]. Journal of Colloid and Interface Science, 2019, 534: 332-337. |
| 36 | Banhart F, Kotakoski J, Krasheninnikov A V. Structural defects in graphene[J]. ACS Nano, 2011, 5(1): 26-41. |
| 37 | Lu J, Bao Y, Su C L, et al. Properties of strained structures and topological defects in graphene[J]. ACS Nano, 2013, 7(10): 8350-8357. |
| 38 | Yang F, Ma X Y, Cai W B, et al. Nature of oxygen-containing groups on carbon for high-efficiency electrocatalytic CO2 reduction reaction[J]. Journal of the American Chemical Society, 2019, 141(51): 20451-20459. |
| 39 | Tang C, Zhang Q. Nanocarbon for oxygen reduction electrocatalysis: dopants, edges, and defects[J]. Advanced Materials, 2017, 29(13): 1604103. |
| 40 | Li L G, Huang Y, Li Y G. Carbonaceous materials for electrochemical CO2 reduction[J]. EnergyChem, 2020, 2(1): 100024. |
| 41 | Li F W, Xue M Q, Knowles G P, et al. Porous nitrogen-doped carbon derived from biomass for electrocatalytic reduction of CO2 to CO[J]. Electrochimica Acta, 2017, 245: 561-568. |
| 42 | Shu Z Y, Ye G Y, Wang J, et al. Nitrogen-doped carbon with high graphitic-N exposure for electroreduction of CO2 to CO[J]. Ionics, 2021, 27(7): 3089-3098. |
| 43 | Zhi X, Jiao Y, Zheng Y, et al. Impact of interfacial electron transfer on electrochemical CO2 reduction on graphitic carbon nitride/doped graphene[J]. Small, 2019, 15(10): 1804224. |
| 44 | Li W, Seredych M, Rodríguez-castellón E, et al. Metal-free nanoporous carbon as a catalyst for electrochemical reduction of CO2 to CO and CH4 [J]. ChemSusChem, 2016, 9(6): 606-616. |
| 45 | Liu Y M, Zhang Y J, Cheng K, et al. Selective electrochemical reduction of carbon dioxide to ethanol on a boron- and nitrogen-co-doped nanodiamond[J]. Angewandte Chemie International Edition, 2017, 56(49): 15607-15611. |
| 46 | Liu T F, Ali S, Lian Z, et al. CO2 electoreduction reaction on heteroatom-doped carbon cathode materials[J]. Journal of Materials Chemistry A, 2017, 5(41): 21596-21603. |
| 47 | Sharma P P, Wu J J, Yadav R M, et al. Nitrogen-doped carbon nanotube arrays for high-efficiency electrochemical reduction of CO2: on the understanding of defects, defect density, and selectivity[J]. Angewandte Chemie International Edition, 2015, 54(46): 13701-13705. |
| 48 | Wu J J, Yadav R M, Liu M J, et al. Achieving highly efficient, selective, and stable CO2 reduction on nitrogen-doped carbon nanotubes[J]. ACS Nano, 2015, 9(5): 5364-5371. |
| 49 | Kumar B, Asadi M, Pisasale D, et al. Renewable and metal-free carbon nanofibre catalysts for carbon dioxide reduction[J]. Nature Communications, 2013, 4(1): 1-8. |
| 50 | Li J J, Zan W Y, Kang H X, et al. Graphitic-N highly doped graphene-like carbon: a superior metal-free catalyst for efficient reduction of CO2 [J]. Applied Catalysis B: Environmental, 2021, 298: 120510. |
| 51 | Shan J X, Sun X Q, Zheng S Y, et al. Graphitic N-dominated nitrogen-doped carbon nanotubes as efficient metal-free catalysts for hydrogenation of nitroarenes[J]. Carbon, 2019, 146: 60-69. |
| 52 | Song Y F, Chen W, Zhao C C, et al. Metal-free nitrogen-doped mesoporous carbon for electroreduction of CO2 to ethanol[J]. Angewandte Chemie International Edition, 2017, 56(36): 10840-10844. |
| 53 | Xie J F, Zhao X T, Wu M X, et al. Metal-free fluorine-doped carbon electrocatalyst for CO2 reduction outcompeting hydrogen evolution[J]. Angewandte Chemie International Edition, 2018, 57(31): 9640-9644. |
| 54 | Qiao B T, Wang A Q, Yang X F, et al. Single-atom catalysis of CO oxidation using Pt1/FeO x [J]. Nature Chemistry, 2011, 3(8): 634-641. |
| 55 | Li J, Liu J, Zhang T. Preface to the special issue of the international symposium on single-atom catalysis (ISSAC-2016)[J]. Chinese Journal of Catalysis, 2017, 38(9): 1431. |
| 56 | 吴诗德, 易峰, 平丹, 等. NH4Cl辅助热解制备镍-氮-碳纳米管催化剂及其电还原CO2性能[J]. 化工学报, 2022, 73(10): 4484-4497. |
| Wu S D, Yi F, Ping D, et al. NH4Cl assisted preparation of Ni-N-CNTs catalyst and its performance for electrochemical CO2 reduction[J]. CIESC Journal, 2022, 73(10): 4484-4497. | |
| 57 | Bi W T, Li X G, You R, et al. Surface immobilization of transition metal ions on nitrogen-doped graphene realizing high-efficient and selective CO2 reduction[J]. Advanced Materials, 2018, 30(18): 1706617. |
| 58 | Jiang K, Siahrostami S, Zheng T T, et al. Isolated Ni single atoms in graphene nanosheets for high-performance CO2 reduction[J]. Energy & Environmental Science, 2018, 11(4): 893-903. |
| 59 | Yang H B, Hung S F, Liu S, et al. Atomically dispersed Ni(Ⅰ) as the active site for electrochemical CO2 reduction[J]. Nature Energy, 2018, 3(2): 140-147. |
| 60 | Zhao C M, Wang Y, Li Z J, et al. Solid-diffusion synthesis of single-atom catalysts directly from bulk metal for efficient CO2 reduction[J]. Joule, 2019, 3(2): 584-594. |
| 61 | Chen X, Ma D D, Chen B, et al. Metal-organic framework-derived mesoporous carbon nanoframes embedded with atomically dispersed Fe-N x active sites for efficient bifunctional oxygen and carbon dioxide electroreduction[J]. Applied Catalysis B: Environmental, 2020, 267: 118720. |
| 62 | Sun W J, Zhu J Y, Zhang M Y, et al. Recent advances and perspectives in cobalt-based heterogeneous catalysts for photocatalytic water splitting, CO2 reduction, and N2 fixation[J]. Chinese Journal of Catalysis, 2022, 43(9): 2273-2300. |
| 63 | Chen Z P, Mou K W, Yao S Y, et al. Zinc-coordinated nitrogen-codoped graphene as an efficient catalyst for selective electrochemical reduction of CO2 to CO[J]. ChemSusChem, 2018, 11(17): 2944-2952. |
| 64 | He Q, Lee J H, Liu D B, et al. Accelerating CO2 electroreduction to CO over Pd single-atom catalyst[J]. Advanced Functional Materials, 2020, 30(17): 2000407. |
| 65 | Zu X L, Li X D, Liu W, et al. Efficient and robust carbon dioxide electroreduction enabled by atomically dispersed Sn δ + sites[J]. Advanced Materials, 2019, 31(15): 1808135. |
| 66 | Creissen C E, Fontecave M. Keeping sight of copper in single-atom catalysts for electrochemical carbon dioxide reduction[J]. Nature Communications, 2022, 13(1): 1-4. |
| 67 | Karapinar D, Huan N T, Ranjbar Sahraie N, et al. Electroreduction of CO2 on single-site copper-nitrogen-doped carbon material: selective formation of ethanol and reversible restructuration of the metal sites[J]. Angewandte Chemie International Edition, 2019, 58(42): 15098-15103. |
| 68 | Zhang E H, Wang T, Yu K, et al. Bismuth single atoms resulting from transformation of metal-organic frameworks and their use as electrocatalysts for CO2 reduction[J]. Journal of the American Chemical Society, 2019, 141(42): 16569-16573. |
| 69 | Zhang B X, Zhang J L, Shi J B, et al. Manganese acting as a high-performance heterogeneous electrocatalyst in carbon dioxide reduction[J]. Nature Communications, 2019, 10(1): 1-8. |
| 70 | Shang H S, Wang T, Pei J J, et al. Design of a single-atom indium δ +-N4 interface for efficient electroreduction of CO2 to formate[J]. Angewandte Chemie International Edition, 2020, 59(50): 22465-22469. |
| 71 | Pan F P, Deng W, Justiniano C, et al. Identification of champion transition metals centers in metal and nitrogen-codoped carbon catalysts for CO2 reduction[J]. Applied Catalysis B: Environmental, 2018, 226: 463-472. |
| 72 | Pan F P, Zhang H G, Liu K X, et al. Unveiling active sites of CO2 reduction on nitrogen-coordinated and atomically dispersed iron and cobalt catalysts[J]. ACS Catalysis, 2018, 8(4): 3116-3122. |
| 73 | Gu J, Hsu C S, Bai L C, et al. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO[J]. Science, 2019, 364(6445): 1091-1094. |
| 74 | Hu C, Mu Y, Bai S L, et al. Polyvinyl pyrrolidone mediated fabrication of Fe, N-codoped porous carbon sheets for efficient electrocatalytic CO2 reduction[J]. Carbon, 2019, 153: 609-616. |
| 75 | Pan Y, Lin R, Chen Y J, et al. Design of single-atom Co-N5 catalytic site: a robust electrocatalyst for CO2 reduction with nearly 100% CO selectivity and remarkable stability[J]. Journal of the American Chemical Society, 2018, 140(12): 4218-4221. |
| 76 | Zheng W Z, Yang J, Chen H Q, et al. Atomically defined undercoordinated active sites for highly efficient CO2 electroreduction[J]. Advanced Functional Materials, 2020, 30(4): 1907658. |
| 77 | Rong X, Wang H J, Lu X L, et al. Controlled synthesis of a vacancy-defect single-atom catalyst for boosting CO2 electroreduction[J]. Angewandte Chemie International Edition, 2020, 59(5): 1961-1965. |
| 78 | Li Z, Wu R, Xiao S H, et al. Axial chlorine coordinated iron-nitrogen-carbon single-atom catalysts for efficient electrochemical CO2 reduction[J]. Chemical Engineering Journal, 2022, 430: 132882. |
| 79 | Jia C, Tan X, Zhao Y, et al. Sulfur-dopant-promoted electroreduction of CO2 over coordinatively unsaturated Ni-N2 moieties[J]. Angewandte Chemie International Edition, 2021, 60(43): 23342-23348. |
| 80 | Gao H X, Liu K, Luo T, et al. CO2 reduction reaction pathways on single-atom Co sites: impacts of local coordination environment[J]. Chinese Journal of Catalysis, 2022, 43(3): 832-838. |
| 81 | Chen S Y, Li X Q, Kao C W, et al. Unveiling proton-feeding effect in sulfur-doped Fe-N-C single-atom catalyst for enhanced CO2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 61(32): e202206233. |
| 82 | Chen Y Q, Yao Y J, Xia Y J, et al. Advanced Ni-N x -C single-site catalysts for CO2 electroreduction to CO based on hierarchical carbon nanocages and S-doping[J]. Nano Research, 2020, 13(10): 2777-2783. |
| 83 | Cao S F, Wei S X, Wei X F, et al. Can N, S cocoordination promote single atom catalyst performance in CO2RR? Fe-N2S2 porphyrin versus Fe-N4 porphyrin[J]. Small, 2021, 17(29): 2100949. |
| 84 | Yan C C, Li H B, Ye Y F, et al. Coordinatively unsaturated nickel-nitrogen sites towards selective and high-rate CO2 electroreduction[J]. Energy & Environmental Science, 2018, 11(5): 1204-1210. |
| 85 | Ren W H, Tan X, Yang W F, et al. Isolated diatomic Ni-Fe metal-nitrogen sites for synergistic electroreduction of CO2 [J]. Angewandte Chemie International Edition, 2019, 58(21): 6972-6976. |
| 86 | Li Y, Shan W T, Zachman M J, et al. Atomically dispersed dual-metal site catalysts for enhanced CO2 reduction: mechanistic insight into active site structures[J]. Angewandte Chemie International Edition, 2022, 61(28): e202205632. |
| 87 | Li S, Guan A X, Yang C, et al. Dual-atomic Cu sites for electrocatalytic CO reduction to C2+ products[J]. ACS Materials Letters, 2021, 3(12): 1729-1737. |
| 88 | Ren W H, Tan X, Jia C, et al. Electronic regulation of nickel single atom by confined nickel nanoparticles for energy-efficient CO2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 61(26): e202203335. |
| 89 | 黄小雄, 马英杰, 智林杰. 超薄氮掺杂碳纳米片负载单原子镍用于高效电催化还原二氧化碳[J]. 物理化学学报, 2022, 38(2): 112-120. |
| Huang X X, Ma Y J, Zhi L J. Ultra thin nitrogen doped carbon nanosheets loaded with single atom nickel for efficient electrocatalytic reduction of carbon dioxide[J]. Journal of Physical Chemistry, 2022, 38 (2): 112-120. | |
| 90 | Fan Q, Hou P F, Choi C H, et al. Activation of Ni particles into single Ni-N atoms for efficient electrochemical reduction of CO2 [J]. Advanced Energy Materials, 2020, 10(5): 1903068. |
| 91 | Cheng Y, Zhao S Y, Johannessen B, et al. Atomically dispersed transition metals on carbon nanotubes with ultrahigh loading for selective electrochemical carbon dioxide reduction[J]. Advanced Materials, 2018, 30(13): 1706287. |
| 92 | Li X G, Bi W T, Chen M L, et al. Exclusive Ni-N4 sites realize near-unity CO selectivity for electrochemical CO2 reduction[J]. Journal of the American Chemical Society, 2017, 139(42): 14889-14892. |
| 93 | Zhang C H, Yang S Z, Wu J J, et al. Electrochemical CO2 reduction with atomic iron-dispersed on nitrogen-doped graphene[J]. Advanced Energy Materials, 2018, 8(19): 1703487. |
| 94 | Li X N, Zeng Y Q, Tung C W, et al. Unveiling the in situ generation of a monovalent Fe(Ⅰ) site in the single-Fe-atom catalyst for electrochemical CO2 reduction[J]. ACS Catalysis, 2021, 11(12): 7292-7301. |
| 95 | Shi G D, Xie Y L, Du L L, et al. Constructing Cu-C bonds in a graphdiyne-regulated Cu single-atom electrocatalyst for CO2 reduction to CH4 [J]. Angewandte Chemie International Edition, 2022, 61(23): e202203569. |
| 96 | Geng Z G, Cao Y J, Chen W X, et al. Regulating the coordination environment of Co single atoms for achieving efficient electrocatalytic activity in CO2 reduction[J]. Applied Catalysis B: Environmental, 2019, 240: 234-240. |
| 97 | Kusama S, Saito T, Hashiba H, et al. Crystalline copper(Ⅱ) phthalocyanine catalysts for electrochemical reduction of carbon dioxide in aqueous media[J]. ACS Catalysis, 2017, 7(12): 8382-8385. |
| 98 | Wang X Y, Zhao Q D, Yang B, et al. Emerging nanostructured carbon-based non-precious metal electrocatalysts for selective electrochemical CO2 reduction to CO[J]. Journal of Materials Chemistry A, 2019, 7(44): 25191-25202. |
| 99 | Wu J, Sharifi T, Gao Y, et al. Emerging carbon-based heterogeneous catalysts for electrochemical reduction of carbon dioxide into value‐added chemicals[J]. Advanced Materials, 2019, 31(13): 1804257. |
| 100 | 于丰收, 张鲁华. Cu基纳米材料电催化还原CO2的结构-性能关系[J]. 化工学报, 2021, 72(4): 1815-1824. |
| Yu F S, Zhang L H. Structure-performance relationship of Cu-based nanocatalyst for electrochemical CO2 reduction[J]. CIESC Journal, 2021, 72(4): 1815-1824. | |
| 101 | Reske R, Mistry H, Behafarid F, et al. Particle size effects in the catalytic electroreduction of CO2 on Cu nanoparticles[J]. Journal of the American Chemical Society, 2014, 136(19): 6978-6986. |
| 102 | Dinh C T, Burdyny T, Kibria M G, et al. CO2 electroreduction to ethylene via hydroxide-mediated copper catalysis at an abrupt interface[J]. Science, 2018, 360(6390): 783-787. |
| 103 | Peng L W, Wang Y X, Masood I, et al. Self-growing Cu/Sn bimetallic electrocatalysts on nitrogen-doped porous carbon cloth with 3D-hierarchical honeycomb structure for highly active carbon dioxide reduction[J]. Applied Catalysis B: Environmental, 2020, 264: 118447. |
| 104 | Xiong H T, Zou H Y, Rong W F, et al. A single-step strategy for general construction of metal sub-nanoclusters on graphdiyne[J]. 2D Materials, 2022, 9(1): 014002. |
| 105 | Fu J J, Wang Y, Liu J, et al. Low overpotential for electrochemically reducing CO2 to CO on nitrogen-doped graphene quantum dots-wrapped single-crystalline gold nanoparticles[J]. ACS Energy Letters, 2018, 3(4): 946-951. |
| 106 | 穆春辉, 张艺馨, 寇伟, 等. 镍氮掺杂有序大孔/介孔碳负载银纳米颗粒用于高效电催化CO2还原[J]. 化学学报, 2021, 79(7): 925-931. |
| Mu C H, Zhang Y X, Kou W, et al. Nickel nitrogen doped ordered macroporous/mesoporous carbon loaded silver nanoparticles for efficient electrocatalytic CO2 reduction[J]. Journal of Chemistry, 2021, 79 (7): 925-931. | |
| 107 | Fan T T, Zhang J G, Zhang Y P, et al. Unraveling the interfacial polarization effect between Pd and polymeric carbon nitride toward efficient CO2 electroreduction to CO[J]. ACS Applied Materials & Interfaces, 2022, 14(10): 12314-12322. |
| 108 | Wang X W, Wu D, Dai C Z, et al. Novel folic acid complex derived nitrogen and nickel co-doped carbon nanotubes with embedded Ni nanoparticles as efficient electrocatalysts for CO2 reduction[J]. Journal of Materials Chemistry A, 2020, 8(10): 5105-5114. |
| 109 | Zhao Y, Wang C Y, Liu Y Q, et al. Engineering surface amine modifiers of ultrasmall gold nanoparticles supported on reduced graphene oxide for improved electrochemical CO2 reduction[J]. Advanced Energy Materials, 2018, 8(25): 1801400. |
| 110 | Jin L, Liu B, Wang P, et al. Ultrasmall Au nanocatalysts supported on nitrided carbon for electrocatalytic CO2 reduction: the role of the carbon support in high selectivity[J]. Nanoscale, 2018, 10(30): 14678-14686. |
| 111 | Wang M, Xie Q, Chen H M, et al. Surface regulated Ni nanoparticles on N-doped mesoporous carbon as an efficient electrocatalyst for CO2 reduction[J]. Chinese Journal of Catalysis, 2021, 42(12): 2306-2312. |
| 112 | Ning H, Wang X S, Wang W H, et al. Cubic Cu2O on nitrogen-doped carbon shells for electrocatalytic CO2 reduction to C2H4 [J]. Carbon, 2019, 146: 218-223. |
| 113 | Irfan Malik M, Malaibari Z O, Atieh M, et al. Electrochemical reduction of CO2 to methanol over MWCNTs impregnated with Cu2O[J]. Chemical Engineering Science, 2016, 152: 468-477. |
| 114 | Mou K W, Chen Z P, Yao S Y, et al. Enhanced electrochemical reduction of carbon dioxide to formate with in-situ grown indium-based catalysts in an aqueous electrolyte[J]. Electrochimica Acta, 2018, 289: 65-71. |
| 115 | Zhai J R, Kang Q L, Liu Q Y, et al. In-situ generation of In2O3 nanoparticles inside In[Co(CN)6] quasi-metal-organic-framework nanocubes for efficient electroreduction of CO2 to formate[J]. Journal of Colloid and Interface Science, 2022, 608: 1942-1950. |
| 116 | Cheng Y Y, Hou P F, Pan H, et al. Selective electrocatalytic reduction of carbon dioxide to oxalate by lead tin oxides with low overpotential[J]. Applied Catalysis B: Environmental, 2020, 272: 118954. |
| 117 | Zhang Y H, Ye D D, Zhu X, et al. Performance of CO2 electrochemical reduction with surface modified self-growing SnO2 on carbon cloth electrode prepared by hydrothermal method[J]. Chinese Science Bulletin, 2021, 66(26): 3488-3496. |
| 118 | Miao Z P, Hu P, Nie C Y, et al. ZrO2 nanoparticles anchored on nitrogen-doped carbon nanosheets as efficient catalyst for electrochemical CO2 reduction[J]. Journal of Energy Chemistry, 2019, 38: 114-118. |
| 119 | Wang X Y, Feng S H, Lu W C, et al. A new strategy for accelerating dynamic proton transfer of electrochemical CO2 reduction at high current densities[J]. Advanced Functional Materials, 2021, 31(50): 2104243. |
| 120 | Qin B H, Li Y H, Wang H J, et al. Efficient electrochemical reduction of CO2 into CO promoted by sulfur vacancies[J]. Nano Energy, 2019, 60: 43-51. |
| 121 | Liu S Q, Shahini E, Gao M R, et al. Bi2O3 nanosheets grown on carbon nanofiber with inherent hydrophobicity for high-performance CO2 electroreduction in a wide potential window[J]. ACS Nano, 2021, 15(11): 17757-17768. |
| 122 | Cheng Y Y, Hou J, Kang P. Integrated capture and electroreduction of flue gas CO2 to formate using amine functionalized SnO x nanoparticles[J]. ACS Energy Letters, 2021, 6(9): 3352-3358. |
| 123 | Li H Q, Xiao N, Wang Y W, et al. Promoting the electroreduction of CO2 with oxygen vacancies on a plasma-activated SnO x /carbon foam monolithic electrode[J]. Journal of Materials Chemistry A, 2020, 8(4): 1779-1786. |
| 124 | Huo S J, Weng Z, Wu Z S, et al. Coupled metal/oxide catalysts with tunable product selectivity for electrocatalytic CO2 reduction[J]. ACS Applied Materials & Interfaces, 2017, 9(34): 28519-28526. |
| 125 | 宁汇, 王文行, 毛勤虎, 等. 1-辛基-3-甲基咪唑功能化石墨片负载氧化亚铜催化二氧化碳电还原制乙烯[J]. 物理化学学报, 2018, 34(8): 938-944. |
| Ning H, Wang W X, Mao Q H, et al. Electroreduction of carbon dioxide to ethylene catalyzed by copper(Ⅰ) oxide supported on 1-octyl-3-methylimidazole functional fossil ink[J]. Journal of Physical Chemistry, 2018, 34 (8): 938-944. | |
| 126 | Zhou Y J, Qi H H, Wu J, et al. Amino modified carbon dots with electron sink effect increase interface charge transfer rate of Cu-based electrocatalyst to enhance the CO2 conversion selectivity to C2H4 [J]. Advanced Functional Materials, 2022, 32(22): 2113335. |
| 127 | Sun X F, Lu L, Zhu Q G, et al. MoP nanoparticles supported on indium-doped porous carbon: outstanding catalysts for highly efficient CO2 electroreduction[J]. Angewandte Chemie International Edition, 2018, 57(9): 2427-2431. |
| 128 | Weng Z, Jiang J B, Wu Y S, et al. Electrochemical CO2 reduction to hydrocarbons on a heterogeneous molecular Cu catalyst in aqueous solution[J]. Journal of the American Chemical Society, 2016, 138(26): 8076-8079. |
| 129 | Elouarzaki K, Kannan V, Jose V, et al. Recent trends, benchmarking, and challenges of electrochemical reduction of CO2 by molecular catalysts[J]. Advanced Energy Materials, 2019, 9(24): 1900090. |
| 130 | Margarit C G, Asimow N G, Costentin C, et al. Tertiary amine-assisted electroreduction of carbon dioxide to formate catalyzed by iron tetraphenylporphyrin[J]. ACS Energy Letters, 2020, 5(1): 72-78. |
| 131 | Zhu M H, Chen J C, Guo R, et al. Cobalt phthalocyanine coordinated to pyridine-functionalized carbon nanotubes with enhanced CO2 electroreduction[J]. Applied Catalysis B: Environmental, 2019, 251: 112-118. |
| 132 | Choi J, Wagner P, Gambhir S, et al. Steric modification of a cobalt phthalocyanine/graphene catalyst to give enhanced and stable electrochemical CO2 reduction to CO[J]. ACS Energy Letters, 2019, 4(3): 666-672. |
| 133 | Tian P F, Zhang B, Chen J C, et al. Curvature-induced electronic tuning of molecular catalysts for CO2 reduction[J]. Catalysis Science & Technology, 2021, 11(7): 2491-2496. |
| 134 | Kim C, Eom T, Jee M S, et al. Insight into electrochemical CO2 reduction on surface-molecule-mediated Ag nanoparticles[J]. ACS Catalysis, 2017, 7(1): 779-785. |
| 135 | Zhang S, Kang P, Ubnoske S, et al. Polyethylenimine-enhanced electrocatalytic reduction of CO2 to formate at nitrogen-doped carbon nanomaterials[J]. Journal of the American Chemical Society, 2014, 136(22): 7845-7848. |
| 136 | Zhu M H, Chen J C, Huang L B, et al. Covalently grafting cobalt porphyrin onto carbon nanotubes for efficient CO2 electroreduction[J]. Angewandte Chemie International Edition, 2019, 58(20): 6595-6599. |
| 137 | Reddu V, Sun L B, Duo S, et al. Heterogeneous carbon dioxide reduction reaction by cobalt complexes of 4′,4‴-disubstituted derivatives of quinquepyridine immobilized on carbon black[J]. Electrochimica Acta, 2021, 380: 138224. |
| 138 | Wu Y S, Jiang Z, Lu X, et al. Domino electroreduction of CO2 to methanol on a molecular catalyst[J]. Nature, 2019, 575(7784): 639-642. |
| 139 | Chen C J, Sun X F, Yan X P, et al. Boosting CO2 electroreduction on N,P-co-doped carbon aerogels[J]. Angewandte Chemie International Edition, 2020, 59(27): 11123-11129. |
| 140 | Yang H P, Wu Y, Lin Q, et al. Composition tailoring via N and S co-doping and structure tuning by constructing hierarchical pores: metal-free catalysts for high-performance electrochemical reduction of CO2 [J]. Angewandte Chemie International Edition, 2018, 57(47): 15476-15480. |
| 141 | Yang H P, Lin Q, Wu Y, et al. Highly efficient utilization of single atoms via constructing 3D and free-standing electrodes for CO2 reduction with ultrahigh current density[J]. Nano Energy, 2020, 70: 104454. |
| 142 | Yang H P, Wu Y, Li G D, et al. Scalable production of efficient single-atom copper decorated carbon membranes for CO2 electroreduction to methanol[J]. Journal of the American Chemical Society, 2019, 141(32): 12717-12723. |
| 143 | Xie L Y, Yu X Y, Wang S Y, et al. A multiscale strategy to construct cobalt nanoparticles confined within hierarchical carbon nanofibers for efficient CO2 electroreduction[J]. Small, 2022, 18(1): 2104958. |
| [1] | 宋悦, 张启成, 彭文朝, 李阳, 张凤宝, 范晓彬. MoS2基单原子催化剂的合成及其在电催化中的应用[J]. 化工学报, 2023, 74(2): 535-545. |
| [2] | 张浩, 王子悦, 程钰洁, 何晓辉, 纪红兵. 单原子催化剂规模化制备的研究进展[J]. 化工学报, 2023, 74(1): 276-289. |
| [3] | 陈健鑫, 朱瑞杰, 盛楠, 朱春宇, 饶中浩. 纤维素基生物质多孔炭的制备及其超级电容器性能研究[J]. 化工学报, 2022, 73(9): 4194-4206. |
| [4] | 温怡静, 张博, 陈晓霏, 赵思洋, 周欣, 黄艳, 李忠. 多孔炭吸附剂的乙烯-乙烷选择性反转机制[J]. 化工学报, 2021, 72(9): 4768-4774. |
| [5] | 张芳芳, 韩敏, 赵娟, 凌丽霞, 章日光, 王宝俊. 单空缺石墨烯负载的Pd单原子催化剂上NO还原的密度泛函理论研究[J]. 化工学报, 2021, 72(3): 1382-1391. |
| [6] | 董灵玉, 葛睿, 原亚飞, 唐宋元, 郝广平, 陆安慧. 多孔炭基二氧化碳电催化材料研究进展[J]. 化工学报, 2020, 71(6): 2492-2509. |
| [7] | 王晓波,赵青山,程智年,张浩然,胡涵,王路海,吴明铂. 高性能碳基储能材料的设计、合成与应用[J]. 化工学报, 2020, 71(6): 2660-2677. |
| [8] | 张亚婷, 张博超, 张建兰, 李可可, 党永强, 段瑛峰. “自下而上”化学合成纳米石墨烯的研究进展[J]. 化工学报, 2020, 71(6): 2628-2642. |
| [9] | 王尧,唐艺芸. 氧电极金属单原子催化剂的研究进展[J]. 化工学报, 2020, 71(10): 4409-4428. |
| [10] | 周宇, 王宇新. 杂原子掺杂碳基氧还原反应电催化剂研究进展[J]. 化工学报, 2017, 68(2): 519-534. |
| [11] | 王恩民, 李文翠, 雷成, 陆安慧. 碱式碳酸镁催化酚醛聚合制备多孔炭及其CO2吸附性能[J]. 化工学报, 2015, 66(7): 2565-2572. |
| [12] | 杨杰, 浦群, 包永忠. 基于偏氯乙烯嵌段共聚物的多级多孔炭的制备[J]. 化工学报, 2014, 65(1): 358-364. |
| [13] | 吴启强, 包永忠. 偏氯乙烯共聚物/纳米水滑石复合材料及多孔炭的制备与表征 [J]. 化工学报, 2011, 62(4): 1130-1135. |
| [14] | 柳召永,郑经堂,王艳飞,赵玉翠. 溶胶凝胶法N,N-二甲基甲酰胺对多孔炭孔隙结构的影响 [J]. CIESC Journal, 2007, 26(9): 1316-. |
| [15] | 柳召永,郑经堂,王艳飞,赵玉翠. 溶胶凝胶法多孔炭材料的制备及其表征 [J]. CIESC Journal, 2007, 26(8): 1145-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号