化工学报 ›› 2021, Vol. 72 ›› Issue (3): 1382-1391.DOI: 10.11949/0438-1157.20200876
张芳芳1( ),韩敏1,赵娟1,凌丽霞1(
),韩敏1,赵娟1,凌丽霞1( ),章日光2,王宝俊2
),章日光2,王宝俊2
收稿日期:2020-07-03
修回日期:2020-09-03
出版日期:2021-03-05
发布日期:2021-03-05
通讯作者:
凌丽霞
作者简介:张芳芳(1993—),女,硕士研究生,基金资助:
ZHANG Fangfang1( ),HAN Min1,ZHAO Juan1,LING Lixia1(
),HAN Min1,ZHAO Juan1,LING Lixia1( ),ZHANG Riguang2,WANG Baojun2
),ZHANG Riguang2,WANG Baojun2
Received:2020-07-03
Revised:2020-09-03
Online:2021-03-05
Published:2021-03-05
Contact:
LING Lixia
摘要:
采用密度泛函理论(DFT)方法对单空缺石墨烯负载的Pd单原子(Pd/SVG)催化剂上H2还原NO的反应进行了研究,探究了Pd/SVG上NO还原生成N2和NH3的路径。在Pd/SVG上NO容易加氢形成HNO,需要的活化能为67.0 kJ·mol-1,显示了极高的催化活性。N2生成的有利路径为NO活化生成HNO后,HNO继续加氢生成中间体NH2O和NH2OH,然后NH2OH解离生成NH2和OH,生成的NH2中间体结合NO形成NH2NO,然后NH2NO异构化形成的NHNOH再经解离生成N2与H2O,这个过程中的决速步骤为NH2NO分子内氢转移生成NHNOH,能垒为144.3 kJ·mol-1。对于NH3的生成,从NO的活化到中间体NH2的形成与N2的形成过程相同,最后NH2加氢即可形成NH3,这个过程中的决速步骤为NH2O加氢生成NH2OH,能垒为86.4 kJ·mol-1。比较生成N2和NH3的决速步能垒可见,Pd/SVG催化剂上NO经H2还原更容易形成NH3。本研究为石墨烯负载型Pd基催化剂上H2还原NO的实验及工业应用提供理论参考。
中图分类号:
张芳芳, 韩敏, 赵娟, 凌丽霞, 章日光, 王宝俊. 单空缺石墨烯负载的Pd单原子催化剂上NO还原的密度泛函理论研究[J]. 化工学报, 2021, 72(3): 1382-1391.
ZHANG Fangfang, HAN Min, ZHAO Juan, LING Lixia, ZHANG Riguang, WANG Baojun. DFT study on reduction of NO over Pd atom anchored on single-vacancy graphene[J]. CIESC Journal, 2021, 72(3): 1382-1391.
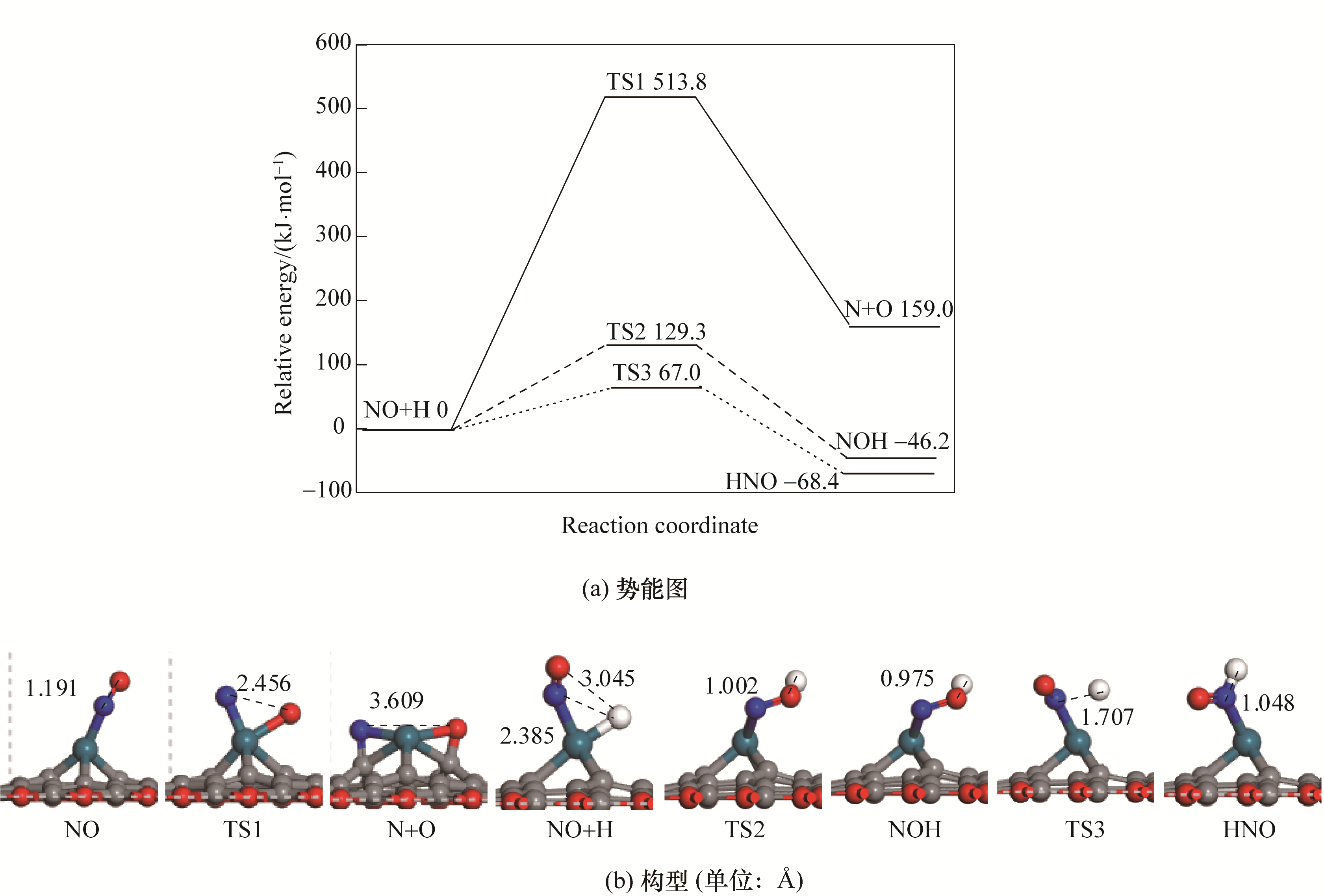
图2 Pd/SVG上NO的活化势能图和对应的反应物、过渡态以及产物的构型
Fig.2 Potential energy diagram of NO activation with corresponding configurations of initial states, transition states and final states
| Reaction | H | H′ | H″ | N | O | Q(H+N+O) |
|---|---|---|---|---|---|---|
NO N+O N+O | -0.254 | -0.451 | -0.705 | |||
NO+H NOH NOH | 0.298 | -0.164 | -0.306 | -0.172 | ||
NO+H HNO HNO | -0.023 | -0.012 | -0.15 | -0.185 | ||
HNO NH+O NH+O | 0.216 | -0.424 | -0.466 | -0.674 | ||
HNO+H NHOH NHOH | -0.138 | 0.196 | -0.011 | -0.298 | -0.251 | |
HNO+H NH2O NH2O | 0.037 | 0.203 | -0.1 | -0.384 | -0.244 | |
NH2O NH2+O NH2+O | 0.203 | 0.198 | -0.516 | -0.512 | -0.627 | |
NH2O N+H2O N+H2O | 0.338 | 0.264 | -0.505 | -0.574 | -0.477 | |
NH2O+H NH2OH NH2OH | 0.093 | 0.229 | 0.244 | -0.127 | -0.461 | -0.022 |
表1 各基元反应过渡态结构中吸附物种各原子的Mulliken电荷以及整个吸附物种的电荷
Table 1 The Mulliken charge of each atom of the adsorbed species in the transition state structure of each elementary reaction and the charge of the entire adsorbed species
| Reaction | H | H′ | H″ | N | O | Q(H+N+O) |
|---|---|---|---|---|---|---|
NO N+O N+O | -0.254 | -0.451 | -0.705 | |||
NO+H NOH NOH | 0.298 | -0.164 | -0.306 | -0.172 | ||
NO+H HNO HNO | -0.023 | -0.012 | -0.15 | -0.185 | ||
HNO NH+O NH+O | 0.216 | -0.424 | -0.466 | -0.674 | ||
HNO+H NHOH NHOH | -0.138 | 0.196 | -0.011 | -0.298 | -0.251 | |
HNO+H NH2O NH2O | 0.037 | 0.203 | -0.1 | -0.384 | -0.244 | |
NH2O NH2+O NH2+O | 0.203 | 0.198 | -0.516 | -0.512 | -0.627 | |
NH2O N+H2O N+H2O | 0.338 | 0.264 | -0.505 | -0.574 | -0.477 | |
NH2O+H NH2OH NH2OH | 0.093 | 0.229 | 0.244 | -0.127 | -0.461 | -0.022 |
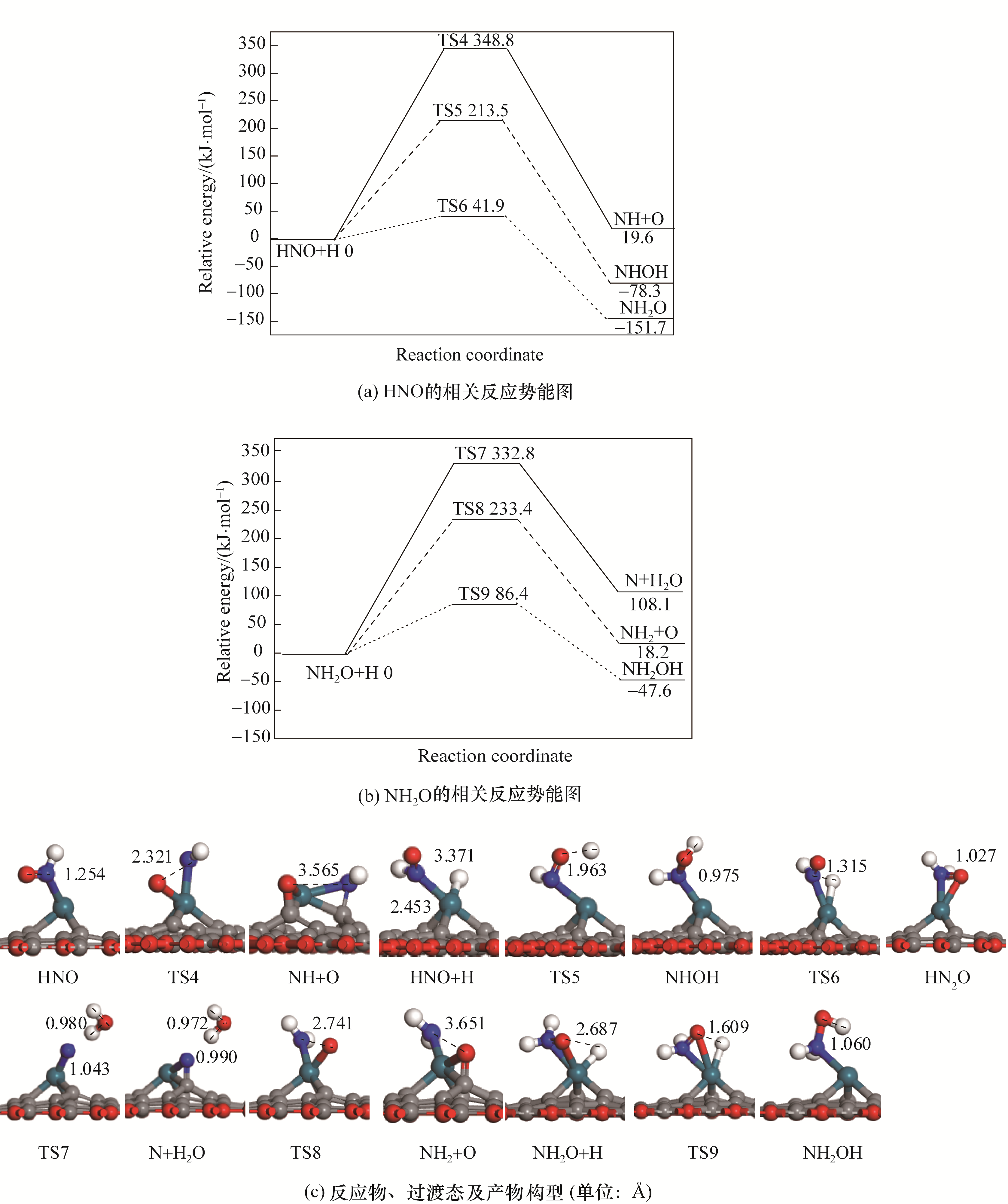
图3 Pd/SVG上NO还原的相关中间体解离加氢的势能图和对应的反应物、过渡态以及产物的构型
Fig.3 Potential energy diagram of related intermediates in the reduction of NO together with corresponding configurations of initial states, transition states and final states
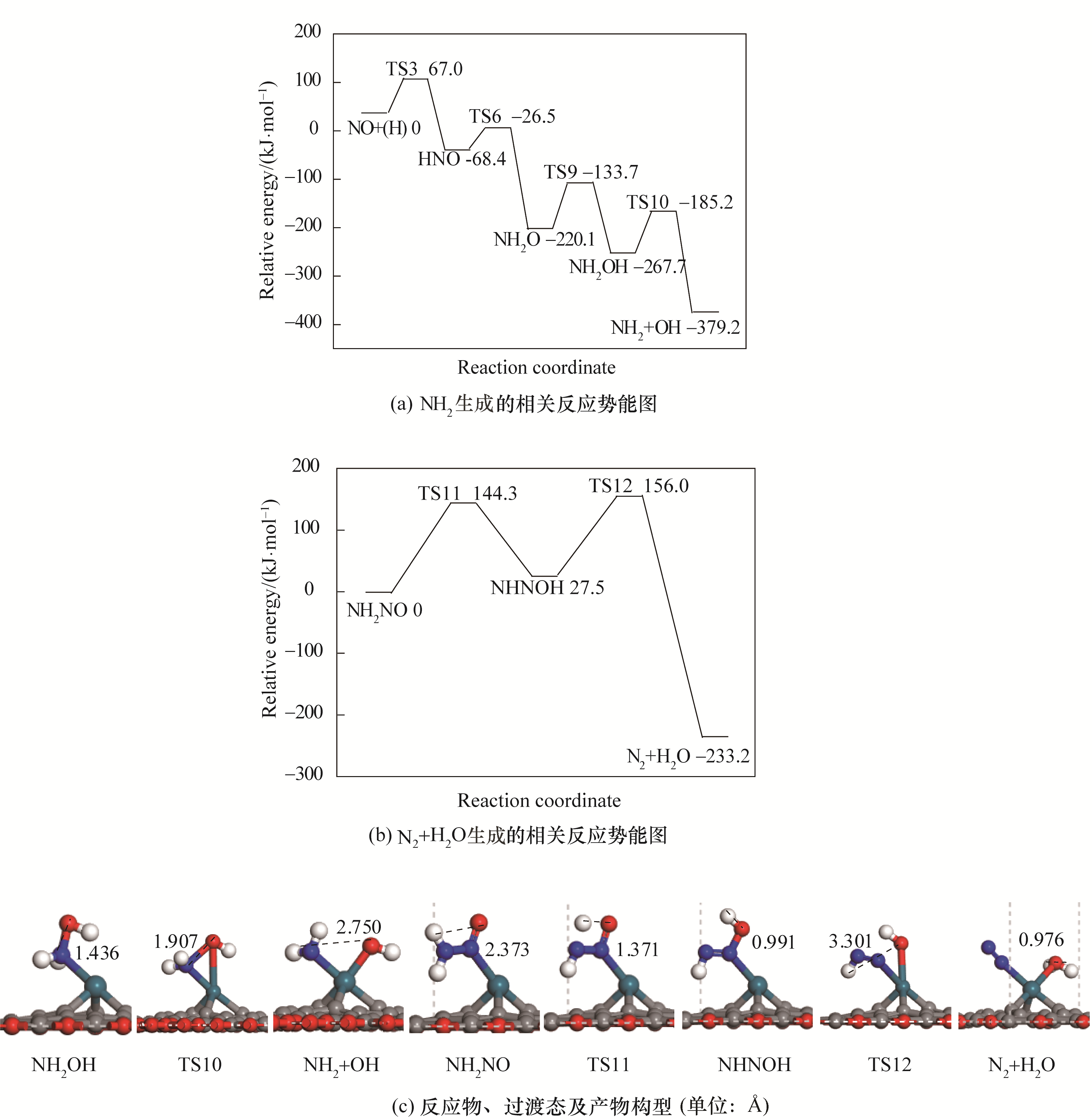
图4 N2与H2O生成势能图及对应的反应物、过渡态及产物构型
Fig.4 Potential energy diagram of correlated reactions of the formation of N2 and H2O together with corresponding configurations of initial states, transition states and final states
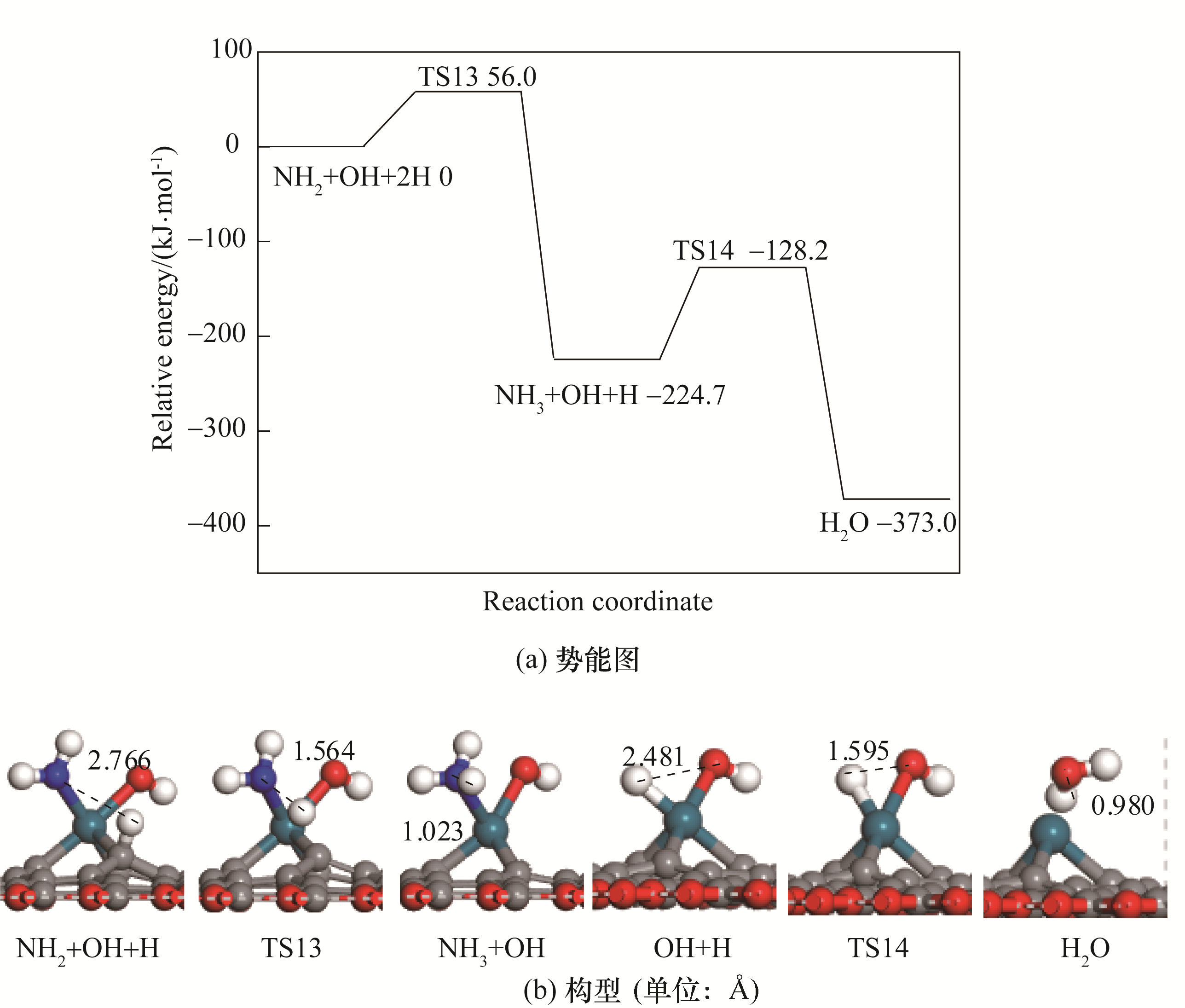
图5 NH3生成的势能图及对应的反应物、过渡态、产物的构型
Fig. 5 Potential energy diagram of correlated reactions of the formation of NH3 together with corresponding configurations of initial states, transition states and final states
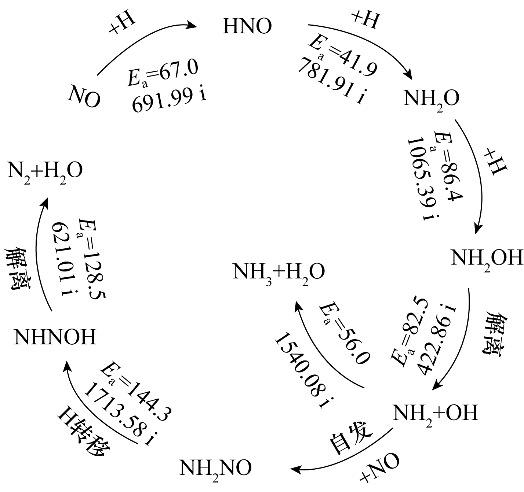
图6 N2与NH3生成的最优路径图及每一步基元反应的能垒(kJ·mol-1)和对应过渡态的虚频(cm-1)
Fig.6 The formation pathways of N2 and NH3 and the energy barrier (kJ·mol-1) for each elementary step and virtual frequency corresponding to the transition state(cm-1)
| 1 | Jabłońska M, Palkovits R. It is no laughing matter: nitrous oxide formation in diesel engines and advances in its abatement over rhodium-based catalysts[J]. Catalysis Science & Technology, 2016, 6: 7671-7687. |
| 2 | Tsukahara H, Ishida T, Mayumi M. Gas-phase oxidation of nitric oxide: chemical kinetics and rate constant[J]. Nitric Oxide, 1999, 3(3): 191-198. |
| 3 | Garda G R, Castellani N J. Isocyanate (NCO) evidence in the CO + NO reaction over palladium[J]. Applied Catalysis A: General, 2015, 494: 48-56. |
| 4 | Herman G S, Peden C H F, Schmieg S J, et al. A comparison of the NO-CO reaction over Rh(100), Rh(110) and Rh(111)[J]. Catalysis Letters, 1999, 62(2): 131-138. |
| 5 | Fink T, Dath J P, Bassett M R, et al. The mechanism of the “explosive” NO + CO reaction on Pt(100): experiments and mathematical modeling[J]. Surface Science, 1991, 245: 96-110. |
| 6 | Fujiwara K, Müller U, Pratsinis S E. Pd subnano-clusters on TiO2 for solar-light removal of NO[J]. ACS Catalysis, 2016, 6(3): 1887-1893. |
| 7 | Fujiwara K, Pratsinis S E. Atomically dispersed Pd on nanostructured TiO2 for NO removal by solar light[J]. AIChE Journal, 2017, 63(1): 139-146. |
| 8 | Chitpakdee C, Junkaew A, Maitarad P, et al. Understanding the role of Ru dopant on selective catalytic reduction of NO with NH3 over Ru-doped CeO2 catalyst[J]. Chemical Engineering Journal, 2019, 369: 124-133. |
| 9 | Wang J G, Liu C J. Density functional theory study of methane activation over PdO/HZSM-5[J]. Journal of Molecular Catalysis A: Chemical, 2006, 247(1/2):199-205. |
| 10 | Yu M, Wang Z Y. Understanding catalytic reactions over zeolites: a density functional theory study of selective catalytic reduction of NOx by NH3 over Cu-SAPO-34[J]. ACS Catalysis, 2016, 6: 7882-7891. |
| 11 | Chen P R, Khetan A, Jabłońska M, et al. Local dynamics of copper active sites in zeolite catalysts for selective catalytic reduction of NOx with NH3[J]. Applied Catalysis B: Environmental, 2018, 237: 263-272. |
| 12 | Begum P, Gogoi P, Mishra B K, et al. Theoretical insight of nitric oxide adsorption on neutral and charged Pdn (n = 1—5) clusters[J]. International Journal of Quantum Chemistry, 2015, 115(13): 837-845. |
| 13 | Loffreda D, Simon D, Sautet P. Structure sensitivity for NO dissociation on palladium and rhodium surfaces[J]. Journal of Catalysis, 2003, 213(2): 211-225. |
| 14 | Gao Y, Zhang L M, Kong C C, et al. NO adsorption and dissociation on palladium clusters: the importance of charged state and metal doping[J]. Chemical Physics Letters, 2016, 658: 7-11. |
| 15 | Pei G X, Liu X Y, Yang X, et al. Performance of Cu-alloyed Pd single-atom catalyst for semihydrogenation of acetylene under simulated front-end conditions[J]. ACS Catalysis, 2017, 7(2): 1491-1500. |
| 16 | Shi Y, Zhao C, Wei H, et al. Single-atom catalysis in mesoporous photovoltaics: the principle of utility maximization[J]. Advanced Materials, 2014, 26(48): 8147-8153. |
| 17 | Paredis K, Ono L K, Behafarid F, et al. Evolution of the structure and chemical state of Pd nanoparticles during the in situ catalytic reduction of NO with H2[J]. Journal of the American Chemical Society, 2011, 133(34): 13455-13464. |
| 18 | Yin Z, Li C, Su Y, et al. Investigation of reaction mechanisms of NO with CO on Pd1/MgO and Pd4/MgO catalysts[J]. Chemical Physics, 2012, 395: 108-114. |
| 19 | Ding W C, Gu X K, Su H Y, et al. Single Pd atom embedded in CeO2(111) for NO reduction with CO: a first-principles study[J]. The Journal of Physical Chemistry C, 2014, 118(23): 12216-12223. |
| 20 | Esrafili M D, Saeidi N. Catalytic reduction of NO by CO molecules over Ni-doped graphene: a DFT investigation[J]. New Journal of Chemistry, 2017, 41: 13149-13155. |
| 21 | 金燕, 杨倩, 赵文斌, 等. 石墨烯化学气相沉积法可控制备的催化反应体系研究[J]. 化工学报, 2020, 71(6): 2564-2585. |
| Jin Y, Yang Q, Zhao W B, et al. Catalytic reaction system for controllable synthesis of graphene with chemical vapor deposition[J]. CIESC Journal, 2020, 71(6): 2564-2585. | |
| 22 | Jia T T, Lu C H, Zhang Y F, et al. A comparative study of CO catalytic oxidation on Pd-anchored graphene oxide and Pd-embedded vacancy graphene[J]. Journal of Nanoparticle Research, 2014, 16(2): 1-11. |
| 23 | Zhao J X, Chen Y, Fu H G. Si-embedded graphene: an efficient and metal-free catalyst for CO oxidation by N2O or O2[J]. Theoretical Chemistry Accounts, 2012, 131(6): 1-11. |
| 24 | Yang W, Gao Z, Liu X. Directly catalytic reduction of NO without NH3 by single atom iron catalyst: a DFT calculation[J]. Fuel, 2019, 243: 262-270. |
| 25 | Esrafili M D. Exploring reaction mechanisms for the reduction of NO molecules over Al- or Si-anchored graphene oxide: a metal-free approach[J]. ChemistrySelect, 2018, 3: 12072-12079. |
| 26 | Esrafili M D, Dinparast L. A DFT study on the catalytic hydrogenation of CO2 to formic acid over Ti-doped graphene nanoflake[J]. Chemical Physics Letters, 2017, 682: 49-54. |
| 27 | Liu Z B, Li S F, Yang J, et al. Ultrafast construction of oxygen-containing scaffold over graphite for trapping Ni2+ into single atom catalysts[J]. ACS Nano, 2020, 14(9): 11622-11669. |
| 28 | Han Y, Wang Y G, Chen W, et al. Hollow N-doped carbon spheres with isolated cobalt single atomic sites: superior electrocatalysts for oxygen reduction[J]. Journal of the American Chemical Society, 2017, 139(48): 17269-17272. |
| 29 | Luo D, Zhang G, Liu J, et al. Evaluation criteria for reduced graphene oxide[J]. The Journal of Physical Chemistry C, 2011, 115(23): 11327-11335. |
| 30 | Shen J, Hu Y, Shi M, et al. Fast and facile preparation of graphene oxide and reduced graphene oxide nanoplatelets[J]. Chemistry of Materials, 2009, 21(15): 3514-3520. |
| 31 | Gómez-Navarro C, Meyer J C, Sundaram R S, et al. Atomic structure of reduced graphene oxide[J]. Nano Letters, 2010, 10(4): 1144-1148. |
| 32 | Erickson K, Erni R, Lee Z, et al. Determination of the local chemical structure of graphene oxide and reduced graphene oxide[J]. Advanced Materials, 2010, 22(40): 4467-4472. |
| 33 | Hu Y, Griffiths K, Norton P R. Surface science studies of selective catalytic reduction of NO: progress in the last ten years[J]. Surface Science, 2009, 603(10/11/12): 1740-1750. |
| 34 | 曹蕃, 苏胜, 向军, 等. SCR反应过程中NO/NH3在γ-Al2O3表面吸附特性[J]. 化工学报, 2014, 65(10): 4056-4062. |
| Cao F, Su S, Xiang J, et al. NO/NH3 adsorption properties on γ-Al2O3(110) surface during SCR process[J]. CIESC Journal, 2014, 65(10): 4056-4062. | |
| 35 | Wen H, Huai L, Jin X, et al. Mechanism of nitric oxide reduction by hydrogen on Ni(110) and Ir/Ni(110): first principles and microkinetic modeling[J]. The Journal of Physical Chemistry C, 2019, 123: 4825-4836. |
| 36 | Carlsson J M, Scheffler M. Structural, electronic, and chemical properties of nanoporous carbon[J]. Physical Review Letters, 2006, 96(4): 046806. |
| 37 | Lu Y, Zhou M, Zhang C, et al. Metal-embedded graphene: a possible catalyst with high activity[J]. The Journal of Physical Chemistry C, 2009, 113(47): 20156-20160. |
| 38 | Ling L, Feng X, Cao Y, et al. The catalytic CO oxidative coupling to dimethyl oxalate on Pd clusters anchored on defected graphene: a theoretical study[J]. Molecular Catalysis, 2018, 453: 100-112. |
| 39 | Fan L L, Ling L X, Wang B J, et al. The adsorption of mercury species and catalytic oxidation of Hg0 on the metal-loaded activated carbon[J]. Applied Catalysis A: General, 2016, 520: 13-23. |
| 40 | Esrafili M D, Nurazar R, Vessally E. Application of Si-doped graphene as a metal-free catalyst for decomposition of formic acid: a theoretical study[J]. International Journal of Quantum Chemistry, 2015, 115(17): 1153-1160. |
| 41 | Ling L, Cao Y, Zhao Z, et al. Density functional theory calculations and analysis for the reduction of NO by H2 on Pd6/TiO2[J]. Computational Materials Science, 2018, 149: 182-190. |
| 42 | Farberow C A, Dumesic J A, Mavrikakis M. Density functional theory calculations and analysis of reaction pathways for reduction of nitric oxide by hydrogen on Pt(111)[J]. ACS Catalysis, 2014, 4(10): 3307-3319. |
| 43 | Chen Y, Liu Y J, Wang H X, et al. Silicon-doped graphene: an effective and metal-free catalyst for NO reduction to N2O?[J]. ACS Applied Materials & Interfaces, 2013, 5(13): 5994-6000. |
| 44 | Wang Y Y, Zhang D J, Yu Z Y, et al. Mechanism of N2O formation during NO reduction on the Au(111) Surface[J]. The Journal of Physical Chemistry C, 2010, 114: 2711-2716. |
| 45 | Ling L, Zhao Z, Feng X. Insight into the reduction of NO by H2 on the stepped Pd(211) surface[J]. The Journal of Physical Chemistry C, 2017, 121: 16399-16414. |
| 46 | Huai L Y, He C Z, Wang H, et al. NO dissociation and reduction by H2 on Pd(111): a first-principles study[J]. Journal of Catalysis, 2015, 322: 73-83. |
| 47 | Bryliakova A A, Matveev A V, Tapilin V M, et al. NO + H2 reaction over Pd(110): TPD, TPR and DFT study[J]. Molecular Catalysis, 2018, 448: 53-62. |
| 48 | Huai L, Su T, Wen H, et al. NO reduction by H2 on the Rh(111) and Rh(221) surfaces: a mechanistic and kinetic study[J]. The Journal of Physical Chemistry C, 2016, 120: 5410-5419. |
| 49 | Yang M, Yuan H, Wang H. Insights into the selective catalytic reduction of NO by NH3 over Mn3O4(110): a DFT study coupled with microkinetic analysis[J]. Science China Chemistry, 2018, 61: 457-467. |
| 50 | Zheng H, Song W, Zhou Y. Mechanistic study of selective catalytic reduction of NOx with NH3 over Mn-TiO2: a combination of experimental and DFT Study[J]. The Journal of Physical Chemistry C, 2017, 121(36): 19859-19871. |
| [1] | 赵佳佳, 田世祥, 李鹏, 谢洪高. SiO2-H2O纳米流体强化煤尘润湿性的微观机理研究[J]. 化工学报, 2023, 74(9): 3931-3945. |
| [2] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [3] | 吕龙义, 及文博, 韩沐达, 李伟光, 高文芳, 刘晓阳, 孙丽, 王鹏飞, 任芝军, 张光明. 铁基导电材料强化厌氧去除卤代有机污染物:研究进展及未来展望[J]. 化工学报, 2023, 74(8): 3193-3202. |
| [4] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| [5] | 王杰, 丘晓琳, 赵烨, 刘鑫洋, 韩忠强, 许雍, 蒋文瀚. 聚电解质静电沉积改性PHBV抗氧化膜的制备与性能研究[J]. 化工学报, 2023, 74(7): 3068-3078. |
| [6] | 朱理想, 罗默也, 张晓东, 龙涛, 余冉. 醌指纹法指示三氯乙烯污染土功能微生物活性应用研究[J]. 化工学报, 2023, 74(6): 2647-2654. |
| [7] | 张兰河, 赖青燚, 王铁铮, 关潇卓, 张明爽, 程欣, 徐小惠, 贾艳萍. H2O2对SBR脱氮效率和污泥性能的影响[J]. 化工学报, 2023, 74(5): 2186-2196. |
| [8] | 徐文超, 孙志高, 李翠敏, 李娟, 黄海峰. 静态条件下表面活性剂E-1310对HCFC-141b水合物生成的影响[J]. 化工学报, 2023, 74(5): 2179-2185. |
| [9] | 葛运通, 王玮, 李楷, 肖帆, 于志鹏, 宫敬. 多相分散体系中微油滴与改性二氧化硅表面间作用力的AFM研究[J]. 化工学报, 2023, 74(4): 1651-1659. |
| [10] | 王锋, 陈钰, 裴鸿艳, 刘东东, 张静, 张立新. 1,2,4-𫫇二唑类衍生物的设计、合成及抗菌活性[J]. 化工学报, 2023, 74(3): 1390-1398. |
| [11] | 王倩, 李神勇, 康帅, 庞薇, 郝龙龙, 秦身钧. 粉煤灰分质高效利用预处理技术的研究进展[J]. 化工学报, 2023, 74(3): 1010-1032. |
| [12] | 张娜, 潘鹤林, 牛波, 张亚运, 龙东辉. 酚醛树脂热裂解反应机理的密度泛函理论研究[J]. 化工学报, 2023, 74(2): 843-860. |
| [13] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| [14] | 唐茹意, 潘罕骞, 郑侠俊, 张广欣, 汪星平, 崔希利, 邢华斌. Z型全氟聚醚的结构表征[J]. 化工学报, 2023, 74(1): 479-486. |
| [15] | 廖艺, 牛亚宾, 潘艳秋, 俞路. 复配表面活性剂对油水界面行为和性质影响的模拟研究[J]. 化工学报, 2022, 73(9): 4003-4014. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号