化工学报 ›› 2025, Vol. 76 ›› Issue (3): 1050-1063.DOI: 10.11949/0438-1157.20240891
收稿日期:2024-08-05
修回日期:2024-09-01
出版日期:2025-03-25
发布日期:2025-03-28
通讯作者:
王志远
作者简介:李中青(2000—),男,硕士研究生,llq10162457 @163.com
基金资助:
Zhongqing LI1, Zhiyuan WANG1( ), Xiaojian LUAN2, Sikai LIANG1, Kai WANG1
), Xiaojian LUAN2, Sikai LIANG1, Kai WANG1
Received:2024-08-05
Revised:2024-09-01
Online:2025-03-25
Published:2025-03-28
Contact:
Zhiyuan WANG
摘要:
利用电沉积-低氧分压方法在310S合金表面制备了MnO涂层,在石脑油热裂解条件下对涂层的抑制结焦性能进行了评价,系统考察了MnO x 组分在裂解-清焦周期过程中物相转变过程。采用X射线衍射、扫描电子显微镜、能量色散谱仪、X射线光电子能谱和拉曼光谱表征了涂层及热裂解焦炭的物相组成、表面形貌、元素化合价态和化学结构。结果表明,MnO涂层表面平整致密,与基体结合紧密,涂层厚度约为45 μm。当裂解时间为3 h和5 h时,MnO涂层的结焦抑制率分别为75.84%和74.22%。但涂层体相中MnO成分随着裂解/清焦循环次数增加发生改变,导致涂层剥落,抗结焦效率降低。裂解/清焦循环次数在3次以内,MnO涂层抗结焦效果稳定。本文研究结果有望为锰氧化物催化涂层的开发与应用提供指导。
中图分类号:
李中青, 王志远, 栾小建, 梁四凯, 王凯. 电沉积-低氧分压法制备MnO涂层及其抑制石脑油热裂解结焦性能研究[J]. 化工学报, 2025, 76(3): 1050-1063.
Zhongqing LI, Zhiyuan WANG, Xiaojian LUAN, Sikai LIANG, Kai WANG. Preparation of MnO coating based on electroplating-low oxygen partial pressure treatment and coking inhibition properties during thermal cracking of naphtha[J]. CIESC Journal, 2025, 76(3): 1050-1063.
| 成分 | 质量分数/% |
|---|---|
| C | ≤0.080 |
| Mn | ≤2.000 |
| P | ≤0.045 |
| S | ≤0.030 |
| Si | ≤1.500 |
| Cr | 24.000~28.000 |
| Ni | 19.000~22.000 |
| Fe | 其余 |
表1 310S合金化学成分
Table 1 Chemical composition of 310S alloy
| 成分 | 质量分数/% |
|---|---|
| C | ≤0.080 |
| Mn | ≤2.000 |
| P | ≤0.045 |
| S | ≤0.030 |
| Si | ≤1.500 |
| Cr | 24.000~28.000 |
| Ni | 19.000~22.000 |
| Fe | 其余 |
| 成分 | 质量分数/% |
|---|---|
| 正链烷烃 | 17.07 |
| 异链烷烃 | 53.47 |
| 环烷烃 | 27.78 |
| 烯烃 | 1.30 |
| 芳烃 | 0.37 |
表2 轻石脑油的物性参数
Table 2 Physical parameters of light naphtha
| 成分 | 质量分数/% |
|---|---|
| 正链烷烃 | 17.07 |
| 异链烷烃 | 53.47 |
| 环烷烃 | 27.78 |
| 烯烃 | 1.30 |
| 芳烃 | 0.37 |
| 参数 | 数值 |
|---|---|
| 裂解压力 | 0.1 MPa |
| 热裂解温度 | 850℃ |
| 石脑油流量 | 2 ml/min |
| 水流量 | 0.7 ml/min |
| 水油比 | 0.5 |
表3 裂解实验参数
Table 3 Parameters of thermal cracking experiment
| 参数 | 数值 |
|---|---|
| 裂解压力 | 0.1 MPa |
| 热裂解温度 | 850℃ |
| 石脑油流量 | 2 ml/min |
| 水流量 | 0.7 ml/min |
| 水油比 | 0.5 |
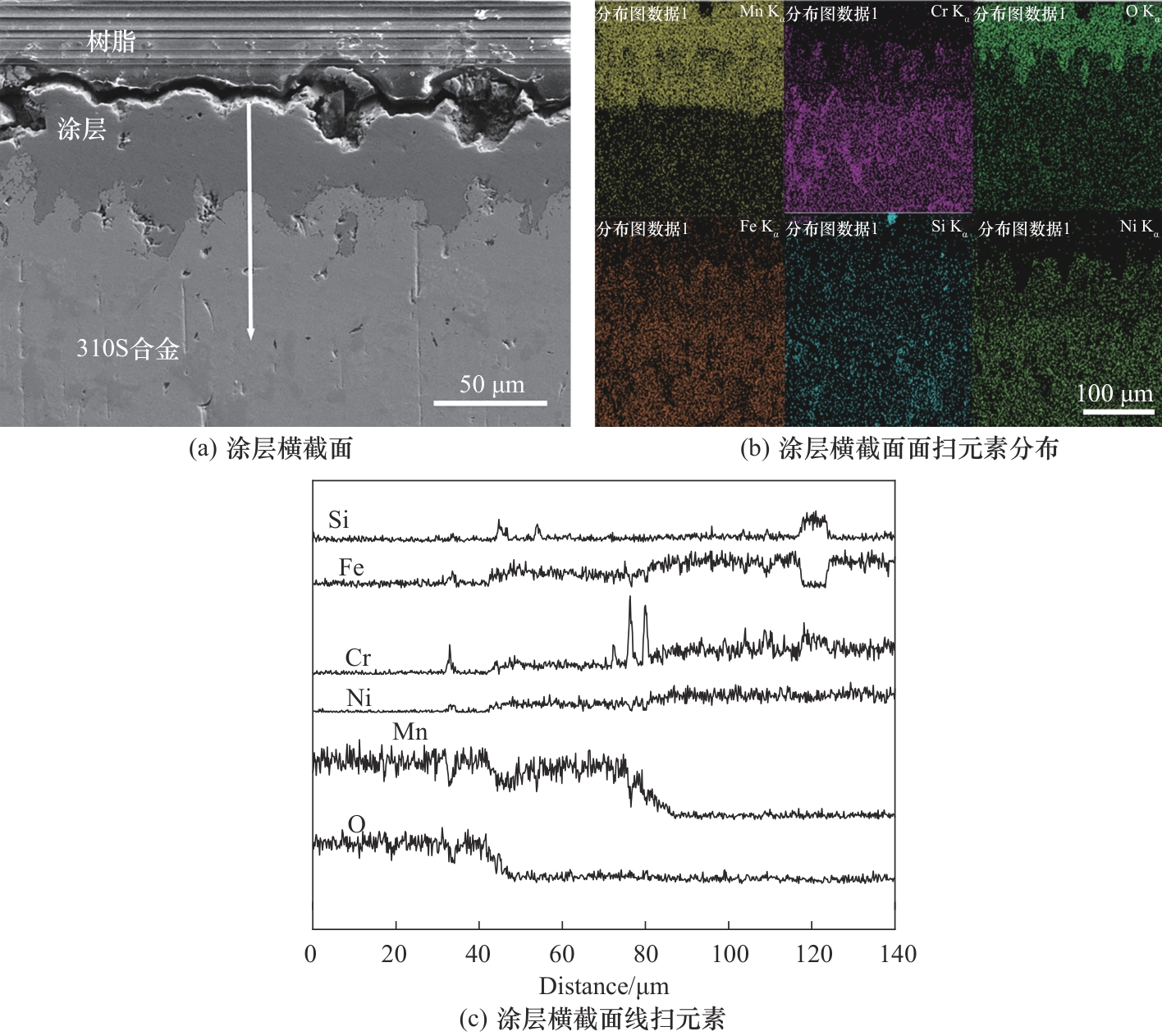
图5 Ar-H2O环境下热处理后涂层的截面形貌和元素分布情况
Fig.5 Cross-sectional morphologies and elemental distributions of the coating after thermal pretreatment in Ar-H2O environment
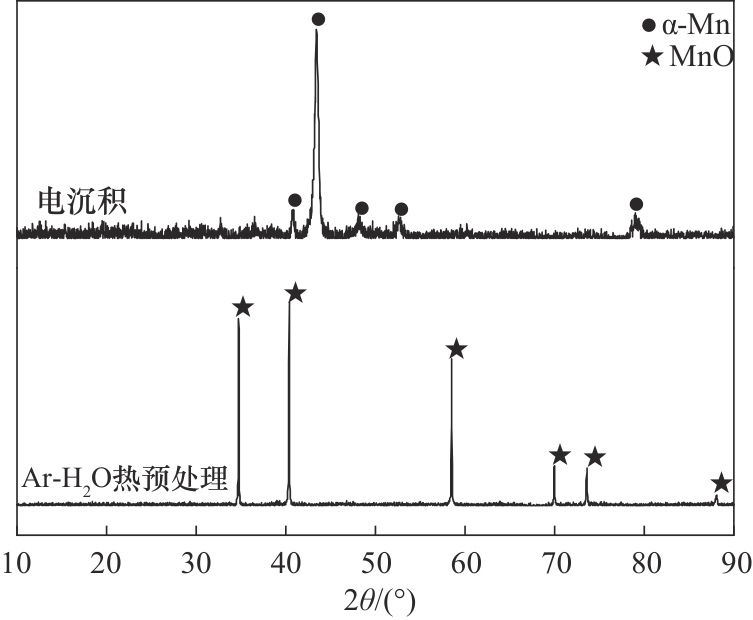
图6 经过电沉积和Ar-H2O环境中热处理后涂层的X射线衍射图
Fig.6 X-ray diffraction patterns of the coatings treated by electrodeposition and thermal heating in Ar-H2O environment
| 样品 | 3 h结焦速率/(10-3 mg/(mm2·h)) | 3 h结焦抑制率/% | 5 h结焦速率/(10-3 mg/(mm2·h)) | 5 h结焦抑制率/% |
|---|---|---|---|---|
| 空白氧化试样 | 19.10 | 0 | 15.00 | 0 |
| 涂层试样 | 4.62 | 75.84 | 4.33 | 74.22 |
表4 不同裂解时间下结焦实验结果
Table 4 Results of coking tests at different cracking time
| 样品 | 3 h结焦速率/(10-3 mg/(mm2·h)) | 3 h结焦抑制率/% | 5 h结焦速率/(10-3 mg/(mm2·h)) | 5 h结焦抑制率/% |
|---|---|---|---|---|
| 空白氧化试样 | 19.10 | 0 | 15.00 | 0 |
| 涂层试样 | 4.62 | 75.84 | 4.33 | 74.22 |
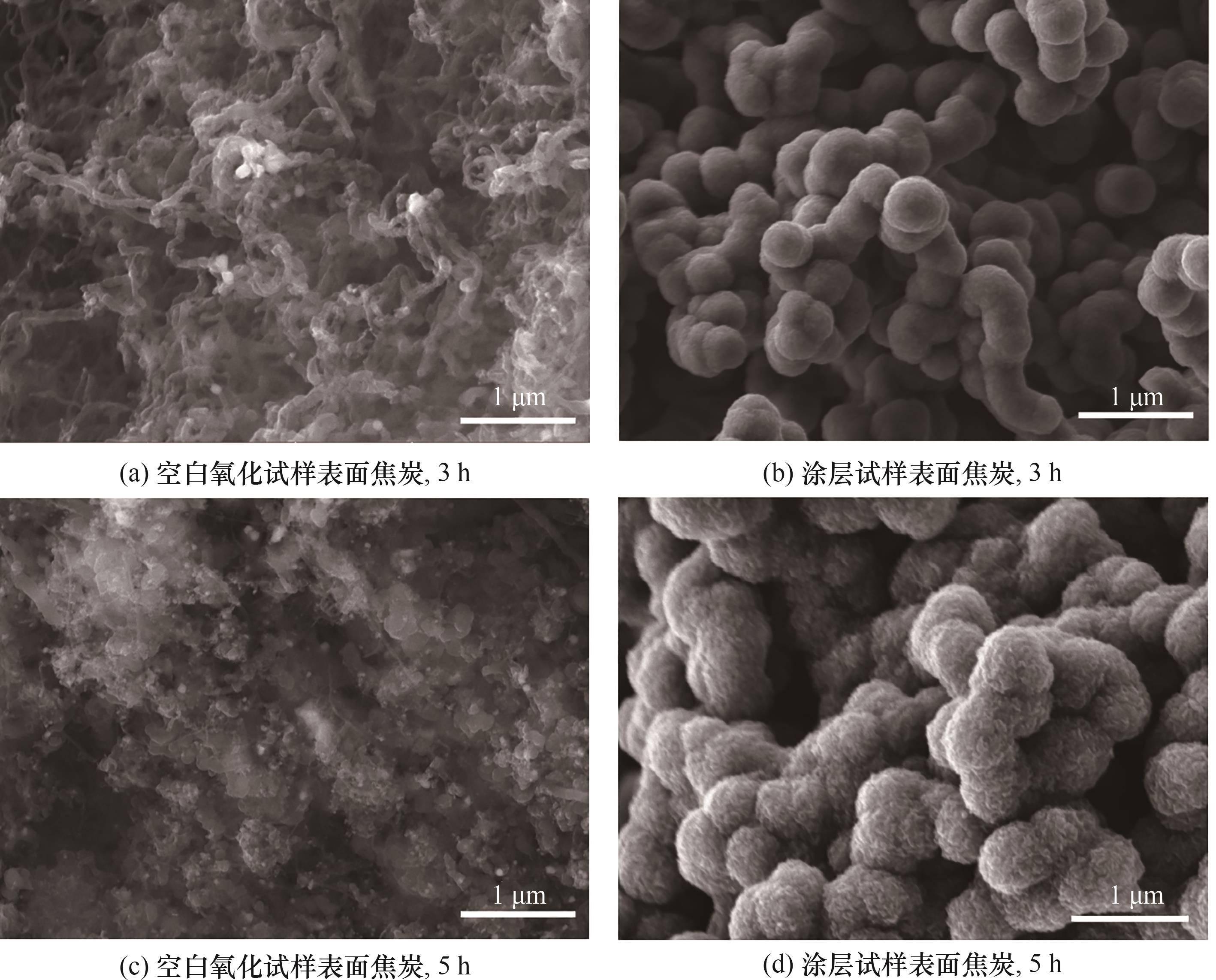
图9 裂解时间3 h和5 h条件下涂层及空白氧化试样表面焦炭沉积的SEM照片
Fig.9 SEM images of coke deposits at the surface of the coating and blank oxidation samples under the conditions of 3 h and 5 h of cracking time

图11 多次裂解/清焦循环后涂层及空白氧化试样表面焦炭沉积情况
Fig.11 Mass of coke deposits at the surface of the coating and blank oxidation samples after multiple cracking/decoking cycles
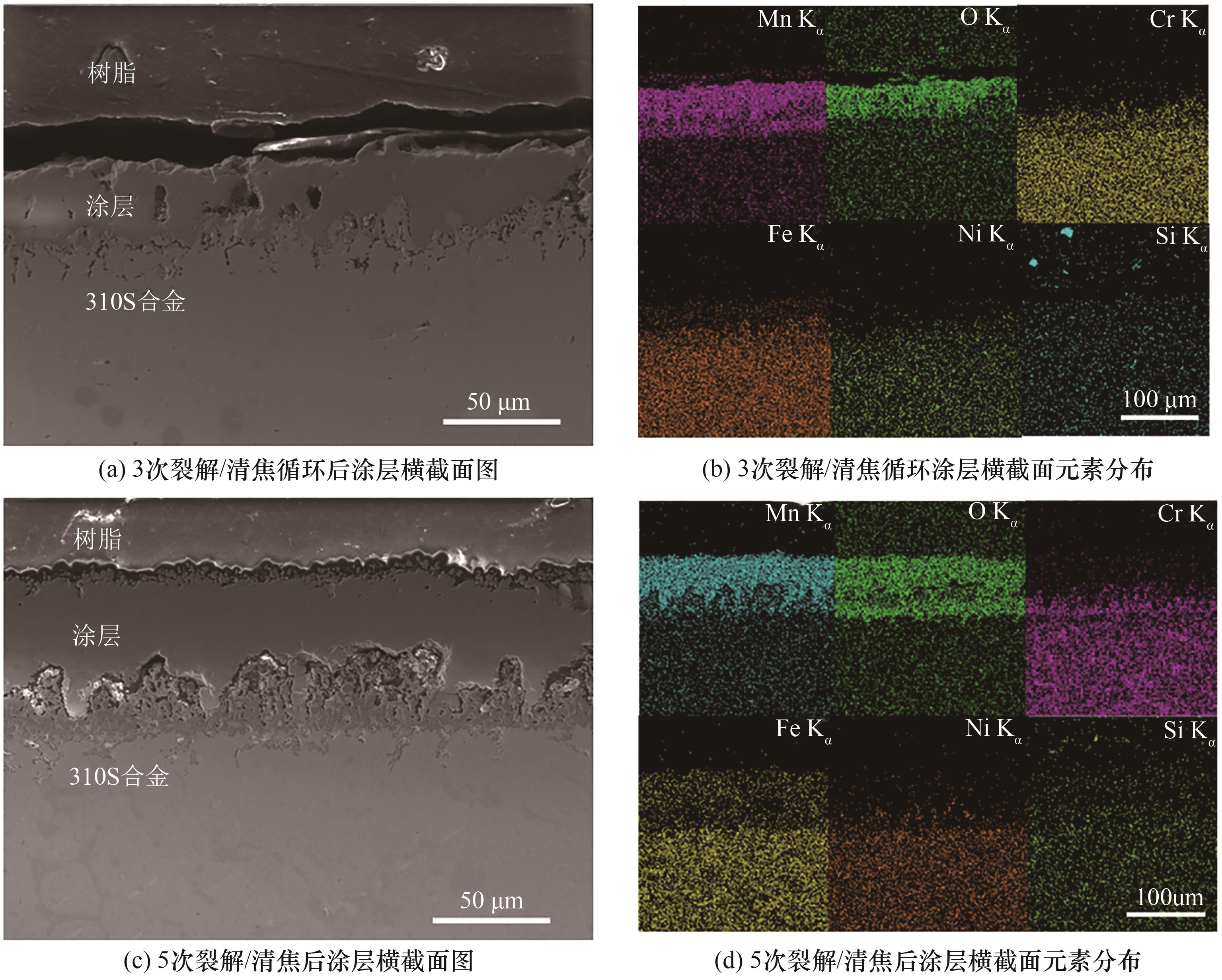
图14 不同次数裂解/清焦循环实验后涂层横截面形貌及元素分布
Fig.14 Cross-sectional morphologies and element distributions of the coatings after different times of cracking/decoking cycles
| 样品 | ΓD1/cm-1 | ΓD2/cm-1 | ΓD3/cm-1 | ΓD4/cm-1 | ΓG/cm-1 | ID1/IG | ID3/IG |
|---|---|---|---|---|---|---|---|
| 涂层试样 | 114.51 | 50.10 | 183.52 | 183.74 | 48.93 | 3.58 | 2.80 |
| 空白氧化试样 | 79.51 | 52.94 | 211.04 | 257.28 | 63.01 | 1.36 | 1.55 |
表5 热裂解结焦拉曼光谱的分峰拟合结果
Table 5 Fitting results of Raman spectra of coke deposits
| 样品 | ΓD1/cm-1 | ΓD2/cm-1 | ΓD3/cm-1 | ΓD4/cm-1 | ΓG/cm-1 | ID1/IG | ID3/IG |
|---|---|---|---|---|---|---|---|
| 涂层试样 | 114.51 | 50.10 | 183.52 | 183.74 | 48.93 | 3.58 | 2.80 |
| 空白氧化试样 | 79.51 | 52.94 | 211.04 | 257.28 | 63.01 | 1.36 | 1.55 |
| 1 | Bukhovko M P, Yang L, Li L W, et al. Gasification of radical coke with steam and steam-hydrogen mixtures over manganese-chromium oxides[J]. Industrial & Engineering Chemistry Research, 2020, 59(23): 10813-10822. |
| 2 | Amghizar I, Vandewalle L A, van Geem K M, et al. New trends in olefin production[J]. Engineering, 2017, 3(2): 171-178. |
| 3 | Xiao S F, Wang J L, Huang C P, et al. Failure analysis of convection section tube in an ethylene cracking furnace due to metal dusting[J]. Engineering Failure Analysis, 2023, 154: 107642. |
| 4 | Pourabdollah K, Khoshbin R, Moghaddam M A, et al. Predictive modeling of coke formation in ethylbenzene cracking on 304 H austenitic steel surface using response surface methodology (RSM)[J]. Chemical Engineering Research and Design, 2024, 202: 191-207. |
| 5 | Kucora I, Paunjoric P, Tolmac J, et al. Coke formation in pyrolysis furnaces in the petrochemical industry[J]. Petroleum Science and Technology, 2017, 35(3): 213-221. |
| 6 | 屈笑雨, 刘京雷, 徐宏, 等. 25Cr35NiNb合金表面Al-Si-Cr涂层抑制结焦性能[J]. 化工学报, 2015, 66(3): 1059-1065. |
| Qu X Y, Liu J L, Xu H, et al. Anti-coking characteristics of Al-Si-Cr coating on 25Cr35NiNb alloy[J]. CIESC Journal, 2015, 66(3): 1059-1065. | |
| 7 | Symoens S H, Olahova N, Muñoz Gandarillas A E, et al. State-of-the-art of coke formation during steam cracking: anti-coking surface technologies[J]. Industrial & Engineering Chemistry Research, 2018, 57(48): 16117-16136. |
| 8 | Kwon H T, Bukhovko M P, Mahamulkar S, et al. Sol-gel derived CeO2/α-Al2O3 bilayer thin film as an anti-coking barrier and its catalytic coke oxidation performance[J]. AIChE Journal, 2018, 64(11): 4019-4026. |
| 9 | 王志远, 丁旭东, 王博研, 等. 硫化物和硫/磷化合物的添加方式对石脑油热裂解结焦影响的研究[J]. 化工学报, 2020, 71(11): 5320-5336. |
| Wang Z Y, Ding X D, Wang B Y, et al. Addition methods of sulfur and sulfur/phosphorus-based compounds on coking behavior during thermal cracking of naphtha[J]. CIESC Journal, 2020, 71(11): 5320-5336. | |
| 10 | Panjapornpon C, Rochpuang C, Bardeeniz S, et al. Machine learning approach with a posteriori-based feature to predict service life of a thermal cracking furnace with coking deposition[J]. Results in Engineering, 2024, 22: 102349. |
| 11 | Guan Y T, Zhang Y J, Zhang Z L, et al. Band gap regulation of LaFeO3 via doping Sr for efficient conversion of coke and steam[J]. Ceramics International, 2024, 50(12): 21526-21537. |
| 12 | Xiong H H, Liu J L, Zhang Y J, et al. Anti-coking performance of Al/Si/Cr/Ce ceramic coating during naphtha steam cracking applied on Cr25Ni35Nb alloy[J]. Chemical Engineering Research and Design, 2023, 194: 756-767. |
| 13 | Bao B B, Liu J L, Xu H, et al. Insight into a high temperature selective oxidation of HP40 alloy under a H2-H2O environment[J]. RSC Advances, 2017, 7(14): 8589-8597. |
| 14 | Bukhovko M P, Yang L, Li L W, et al. Anticoking performance of electrodeposited Mn/MnO surface coating on Fe-Ni-Cr alloy during steam cracking[J]. ACS Engineering Au, 2021, 1(1): 73-84. |
| 15 | 梁贻景, 马岩, 卢展烽, 等. La1- x Sr x MnO3钙钛矿涂层的抗结焦性能[J]. 化工进展, 2023, 42(4): 1769-1778. |
| Liang Y J, Ma Y, Lu Z F, et al. Experimental investigation on the anti-coking performance of La1- x Sr x MnO3 perovskite coating[J]. Chemical Industry and Engineering Progress, 2023, 42(4): 1769-1778. | |
| 16 | 栾小建, 徐宏, 王志远, 等. SiO2/S涂层和硫磷抑制剂的抑制结焦性能研究[J]. 石油炼制与化工, 2011, 42(5): 75-80. |
| Luan X J, Xu H, Wang Z Y, et al. Research on the coking inhibition performance of SiO2/S coating and sulfur/phosphorus containing coking inhibitor[J]. Petroleum Processing and Petrochemicals, 2011, 42(5): 75-80. | |
| 17 | Wang B, Gong X L, Zhang Z D, et al. Investigation on carburization during the repeated coking and decoking process[J]. Industrial & Engineering Chemistry Research, 2020, 59(29): 13051-13059. |
| 18 | Ali S A, Ahmad T. Treasure trove for efficient hydrogen evolution through water splitting using diverse perovskite photocatalysts[J]. Materials Today Chemistry, 2023, 29: 101387. |
| 19 | Halder S, Kumar R A, Maity R, et al. A tailored direct-to-indirect band structure transition in double perovskite oxides influences its photocatalysis efficiency[J]. Ceramics International, 2023, 49(5): 8634-8645. |
| 20 | Wang B, Wang S X, Liu B, et al. Oxide film prepared by selective oxidation of stainless steel and anti-coking behavior during n-hexane thermal cracking[J]. Surface and Coatings Technology, 2019, 378: 124952. |
| 21 | Jampaiah D, Velisoju V K, Devaiah D, et al. Flower-like Mn3O4/CeO2 microspheres as an efficient catalyst for diesel soot and CO oxidation: synergistic effects for enhanced catalytic performance[J]. Applied Surface Science, 2019, 473: 209-221. |
| 22 | Miao S, Chen S, Zeng J, et al. Synergistic effects between Mn and Co species in CO2 hydrogenation over xCo/MnO catalysts[J]. Fuel, 2024, 362: 130853. |
| 23 | Touahra F, Sehailia M, Halliche D, et al. (MnO/Mn3O4)-NiAl nanoparticles as smart carbon resistant catalysts for the production of syngas by means of CO2 reforming of methane: advocating the role of concurrent carbothermic redox looping in the elimination of coke[J]. International Journal of Hydrogen Energy, 2016, 41(46): 21140-21156. |
| 24 | Müller D, Knoll C, Artner W, et al. Combining in situ X-ray diffraction with thermogravimetry and differential scanning calorimetry—an investigation of Co3O4, MnO2 and PbO2 for thermochemical energy storage[J]. Solar Energy, 2017, 153: 11-24. |
| 25 | Petric A, Ling H. Electrical conductivity and thermal expansion of spinels at elevated temperatures[J]. Journal of the American Ceramic Society, 2007, 90(5): 1515-1520. |
| 26 | Zhang G N, Xu Y H, Wu X Y, et al. Ultrathin ZnO coating layer to boost the electrochemical reaction kinetics of MnO cathode for advanced aqueous zinc-ion batteries[J]. Solid State Sciences, 2023, 146: 107371. |
| 27 | Oquab D, Xu N, Monceau D, et al. Subsurface microstructural changes in a cast heat resisting alloy caused by high temperature corrosion[J]. Corrosion Science, 2010, 52(1): 255-262. |
| 28 | Zhang Z B, Albright L F. Pretreatments of coils to minimize coke formation in ethylene furnaces[J]. Industrial & Engineering Chemistry Research, 2010, 49(4): 1991-1994. |
| 29 | Lu J M, Dreisinger D, Glück T. Manganese electrodeposition—A literature review[J]. Hydrometallurgy, 2014, 141: 105-116. |
| 30 | Sulcius A, Griskonis E, Kantminiene K, et al. Influence of different electrolysis parameters on electrodeposition of γ- and α-Mn from pure electrolytes—A review with special reference to Russian language literature[J]. Hydrometallurgy, 2013, 137: 33-37. |
| 31 | Xiao L, Wang S Y, Wang Y F, et al. High-capacity and self-stabilized manganese carbonate microspheres as anode material for lithium-ion batteries[J]. ACS Applied Materials & Interfaces, 2016, 8(38): 25369-25378. |
| 32 | Zhang J, Lin J, Zeng Y B, et al. Morphological and structural evolution of MnO@C anode and its application in lithium-ion capacitors[J]. ACS Applied Energy Materials, 2019, 2(11): 8345-8358. |
| 33 | Tang W X, Yao M S, Deng Y Z, et al. Decoration of one-dimensional MnO2 with Co3O4 nanoparticles: a heterogeneous interface for remarkably promoting catalytic oxidation activity[J]. Chemical Engineering Journal, 2016, 306: 709-718. |
| 34 | Guo Y Y, Zheng L M, Lan J L, et al. MnO nanoparticles encapsulated in carbon nanofibers with sufficient buffer space for high-performance lithium-ion batteries[J]. Electrochimica Acta, 2018, 269: 624-631. |
| 35 | Stokłosa A. Point defects diagrams for pure and doped manganese oxide Mn1- δ O in the temperature range of 1173-1830 K[J]. Materials Chemistry and Physics, 2012, 134(2/3): 1136-1145. |
| 36 | Guan Y T, Zhang Y J, Zhang Z L, et al. Alkali metal and alkali earth metal-modified La-Fe-based perovskite catalyzed coke combustion[J]. Molecular Catalysis, 2024, 558: 114012. |
| 37 | Berbenni V, Marini A. Thermoanalytical (TGA-DSC) and high temperature X-ray diffraction (HT-XRD) study of the thermal decomposition processes in Li2CO3-MnO mixtures[J]. Journal of Analytical and Applied Pyrolysis, 2002, 64(1): 43-58. |
| 38 | Liu B B, Zhang Y B, Wang J, et al. A further investigation on the MnO2-Fe2O3 system roasted under CO-CO2 atmosphere[J]. Advanced Powder Technology, 2019, 30(2): 302-310. |
| 39 | Ilton E S, Post J E, Heaney P J, et al. XPS determination of Mn oxidation states in Mn (hydr)oxides[J]. Applied Surface Science, 2016, 366: 475-485. |
| 40 | Pawlyta M, Rouzaud J N, Duber S. Raman microspectroscopy characterization of carbon blacks: spectral analysis and structural information[J]. Carbon, 2015, 84: 479-490. |
| 41 | Morga R, Jelonek I, Kruszewska K, et al. Relationships between quality of coals, resulting cokes, and micro-Raman spectral characteristics of these cokes[J]. International Journal of Coal Geology, 2015, 144: 130-137. |
| 42 | Xie B S, Han H Z, Luo W. Pyrolysis coking performance of supercritical n-decane in additively manufacturing channel[J]. International Journal of Heat and Mass Transfer, 2024, 229: 125743. |
| 43 | Rantitsch G, Bhattacharyya A, Günbati A, et al. Microstructural evolution of metallurgical coke: evidence from Raman spectroscopy[J]. International Journal of Coal Geology, 2020, 227: 103546. |
| 44 | Sheng C D. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity[J]. Fuel, 2007, 86(15): 2316-2324. |
| [1] | 徐芳, 张锐, 崔达, 王擎. ReaxFF-MD揭示木质素热解反应机制的分子动力学研究[J]. 化工学报, 2025, 76(3): 1253-1263. |
| [2] | 姚国家, 王志, 苏昂, 冯东阁, 唐宏, 孙灵芳. 空气系数对煤粉预热解燃烧特性的影响分析[J]. 化工学报, 2025, 76(3): 1243-1252. |
| [3] | 杨猛, 丁晓倩, 余涛, 刘畅, 汤成龙, 黄佐华. 甲烷/氧化亚氮绿色推进剂自着火特性实验及动力学[J]. 化工学报, 2025, 76(3): 1221-1229. |
| [4] | 吴学红, 韦新, 侯加文, 吕财, 刘勇, 刘鹤, 常志娟. 热解法制备碳纳米管及其在散热涂层中的应用研究[J]. 化工学报, 2024, 75(9): 3360-3368. |
| [5] | 黄正梁, 冯铭瑞, 宋琦, 任聪静, 杨遥, 孙婧元, 王靖岱, 阳永荣. 预混进料对废树脂流化裂解反应中颗粒团聚的抑制作用[J]. 化工学报, 2024, 75(9): 3094-3102. |
| [6] | 王舒英, 左涛, 石志伟, 范小明, 张卫新. 阳离子交换树脂基介孔石墨化碳合成与储钠性能[J]. 化工学报, 2024, 75(9): 3338-3347. |
| [7] | 丁湧, 李文建, 陈昭宇, 曹立辉, 刘轩铭, 任强强, 胡松, 向军. 废旧晶体硅光伏组件EVA有氧热解动力学与产物特性[J]. 化工学报, 2024, 75(9): 3310-3319. |
| [8] | 李沛奇, 陈雪娇, 武博翔, 蒋榕培, 杨超, 刘朝晖. 高参数石油基和煤基火箭煤油射线法密度测量实验研究[J]. 化工学报, 2024, 75(7): 2422-2432. |
| [9] | 姚宏哲, 黄飞宇, 杨松, 钟梅, 代正华. 重质油高温快速热解自动反应网络的动力学建模[J]. 化工学报, 2024, 75(7): 2644-2655. |
| [10] | 晁惠雨, 白振敏, 侯汉青, 田立志, 李洪, 房晓权, 石晓华. 液相法合成三聚氰酸体系热力学分析[J]. 化工学报, 2024, 75(6): 2157-2165. |
| [11] | 吴希, 孙博, 刘银东, 齐传磊, 陈凯毅, 王路海, 许崇, 李永峰. 钠离子电池沥青基碳负极材料制备技术研究进展[J]. 化工学报, 2024, 75(4): 1270-1283. |
| [12] | 李浩文, 兰昊, 郑幼丹, 孙勇辉, 杨子昕, 宋谦石, 汪小憨. 热通道内典型碳氢燃料的热解结焦行为[J]. 化工学报, 2024, 75(2): 626-636. |
| [13] | 王茂先, 孙启典, 付哲, 华放, 纪晔, 程易. 分子水平动力学模型和机器学习方法相结合研究废弃塑料热解[J]. 化工学报, 2024, 75(11): 4320-4332. |
| [14] | 颜诗宇, 高姣姣, 杨太顺, 谢尚志, 杨艳娟, 徐晶. 钌基催化剂配位环境对聚乙烯氢解性能的影响[J]. 化工学报, 2024, 75(10): 3588-3599. |
| [15] | 吴雷, 刘姣, 李长聪, 周军, 叶干, 刘田田, 朱瑞玉, 张秋利, 宋永辉. 低阶粉煤催化微波热解制备含碳纳米管的高附加值改性兰炭末[J]. 化工学报, 2023, 74(9): 3956-3967. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号