化工学报 ›› 2024, Vol. 75 ›› Issue (6): 2157-2165.DOI: 10.11949/0438-1157.20231318
晁惠雨1( ), 白振敏2, 侯汉青1, 田立志3, 李洪3, 房晓权3, 石晓华1(
), 白振敏2, 侯汉青1, 田立志3, 李洪3, 房晓权3, 石晓华1( )
)
收稿日期:2023-12-11
修回日期:2024-04-08
出版日期:2024-06-25
发布日期:2024-07-03
通讯作者:
石晓华
作者简介:晁惠雨(1999—),女,硕士研究生,chaohuiyu@gs.zzu.edu.cn
Huiyu CHAO1( ), Zhenmin BAI2, Hanqing HOU1, Lizhi TIAN3, Hong LI3, Xiaoquan FANG3, Xiaohua SHI1(
), Zhenmin BAI2, Hanqing HOU1, Lizhi TIAN3, Hong LI3, Xiaoquan FANG3, Xiaohua SHI1( )
)
Received:2023-12-11
Revised:2024-04-08
Online:2024-06-25
Published:2024-07-03
Contact:
Xiaohua SHI
摘要:
以二元醇为循环剂液相法合成三聚氰酸是一个由醇解、消去和环加成反应构成的连串反应体系。采用基团贡献法计算了该反应体系中各组分热力学数据以及各步反应的焓变、Gibbs自由能变和平衡常数,依据热力学计算结果并结合已有文献实验数据分析反应路径的可行性及难易程度。结果表明:醇解和消去反应为吸热反应,升温有利于反应,但反应平衡常数小;环加成反应为放热反应且进行较为完全,适当强化条件下三步连串反应可顺利进行。计算及分析结果可为该体系的工业放大工艺过程开发提供理论指导。
中图分类号:
晁惠雨, 白振敏, 侯汉青, 田立志, 李洪, 房晓权, 石晓华. 液相法合成三聚氰酸体系热力学分析[J]. 化工学报, 2024, 75(6): 2157-2165.
Huiyu CHAO, Zhenmin BAI, Hanqing HOU, Lizhi TIAN, Hong LI, Xiaoquan FANG, Xiaohua SHI. Thermodynamics analysis on liquid-phase synthesis of cyanuric acid[J]. CIESC Journal, 2024, 75(6): 2157-2165.
| Point group | σext | Point group | σext |
|---|---|---|---|
C1, Ci, Cs C2, C2ν, C2h C3, C3ν, C3h C4, C4ν, C4h C6, C6ν, C6h D2, D2d, D2h D3, D3d, D3h | 1 2 3 4 6 4 6 | D4, D4d, D4h D6, D6d, D6h S6 C∞ν D∞ν T, Td Oh | 8 12 3 1 2 12 24 |
表1 不同点群对应外对称数[29-30]
Table 1 Symmetry number of various point groups[29-30]
| Point group | σext | Point group | σext |
|---|---|---|---|
C1, Ci, Cs C2, C2ν, C2h C3, C3ν, C3h C4, C4ν, C4h C6, C6ν, C6h D2, D2d, D2h D3, D3d, D3h | 1 2 3 4 6 4 6 | D4, D4d, D4h D6, D6d, D6h S6 C∞ν D∞ν T, Td Oh | 8 12 3 1 2 12 24 |
| Group | ni | Δ | ||||
|---|---|---|---|---|---|---|
| Urea | MPO | MPDC | HIBC | |||
| CO—(N)2* | 1 | 0 | 0 | 0 | -137.30 | 67.80 |
| N—(CO)(H)2 | 2 | 0 | 2 | 1 | -62.40 | 103.37 |
| C—(C)3(H) | 0 | 1 | 1 | 1 | -7.95 | -50.53 |
| C—(C)(H)3 | 0 | 1 | 1 | 1 | -42.20 | 127.32 |
| C—(O)(C)(H)2 | 0 | 2 | 0 | 2 | -30.14 | -46.05 |
| O—(C)(H) | 0 | 2 | 2 | 1 | -158.68 | 121.71 |
| O—(CO)(C) | 0 | 0 | 2 | 1 | -185.68 | 35.13 |
| CO—(N)(O)① | 0 | 0 | 2 | 1 | -137.30 | 67.80 |
| σ | 8 | 3 | 3 | 3 | — | — |
表2 Benson法基团贡献值(298.15 K)及各组分的对称数[20]
Table 2 Group contributions and symmetry number of components (Benson method, 298.15 K)[20]
| Group | ni | Δ | ||||
|---|---|---|---|---|---|---|
| Urea | MPO | MPDC | HIBC | |||
| CO—(N)2* | 1 | 0 | 0 | 0 | -137.30 | 67.80 |
| N—(CO)(H)2 | 2 | 0 | 2 | 1 | -62.40 | 103.37 |
| C—(C)3(H) | 0 | 1 | 1 | 1 | -7.95 | -50.53 |
| C—(C)(H)3 | 0 | 1 | 1 | 1 | -42.20 | 127.32 |
| C—(O)(C)(H)2 | 0 | 2 | 0 | 2 | -30.14 | -46.05 |
| O—(C)(H) | 0 | 2 | 2 | 1 | -158.68 | 121.71 |
| O—(CO)(C) | 0 | 0 | 2 | 1 | -185.68 | 35.13 |
| CO—(N)(O)① | 0 | 0 | 2 | 1 | -137.30 | 67.80 |
| σ | 8 | 3 | 3 | 3 | — | — |
| Group | Δa/(J·mol-1·K-1) | Δb/(J·mol-1·K-1) | Δc/(J·mol-1·K-1) | Δd/(J·mol-1·K-1) |
|---|---|---|---|---|
| —NH2 | 26.90 | -4.12×10-2 | 1.64×10-4 | -9.76×10-8 |
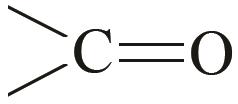 | 6.45 | 6.70×10-2 | -3.57×10-5 | 2.86×10-9 |
| —CH3 | 19.50 | -8.08×10-3 | 1.53×10-4 | -9.67×10-8 |
| —CH2— | -9.09×10-1 | 9.50×10-2 | -5.44×10-5 | 1.19×10-8 |
 | -2.03×10-1 | 2.04×10-1 | -2.65×10-4 | 1.20×10-7 |
| —COO—(ester) | 24.50 | 4.02×10-2 | 4.02×10-5 | -4.02×10-8 |
| —OH(alcohol) | 25.70 | -6.91×10-2 | 1.77×10-4 | -9.88×10-8 |
表3 cp 的基团贡献值[19]
Table 3 Group contributions of cp[19]
| Group | Δa/(J·mol-1·K-1) | Δb/(J·mol-1·K-1) | Δc/(J·mol-1·K-1) | Δd/(J·mol-1·K-1) |
|---|---|---|---|---|
| —NH2 | 26.90 | -4.12×10-2 | 1.64×10-4 | -9.76×10-8 |
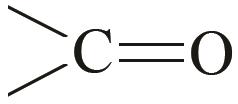 | 6.45 | 6.70×10-2 | -3.57×10-5 | 2.86×10-9 |
| —CH3 | 19.50 | -8.08×10-3 | 1.53×10-4 | -9.67×10-8 |
| —CH2— | -9.09×10-1 | 9.50×10-2 | -5.44×10-5 | 1.19×10-8 |
 | -2.03×10-1 | 2.04×10-1 | -2.65×10-4 | 1.20×10-7 |
| —COO—(ester) | 24.50 | 4.02×10-2 | 4.02×10-5 | -4.02×10-8 |
| —OH(alcohol) | 25.70 | -6.91×10-2 | 1.77×10-4 | -9.88×10-8 |
| T/K | cp,exp/(J·mol-1·K-1) | cp,cal/(J·mol-1·K-1) | Relative deviation/% |
|---|---|---|---|
| 300.477 | 142.2681 | 142.6466 | 0.2660 |
| 320.477 | 149.7612 | 151.3982 | 1.0931 |
| 340.477 | 157.4001 | 158.8823 | 0.9416 |
| 360.477 | 162.3271 | 165.3906 | 1.8872 |
| 380.477 | 169.1645 | 171.2149 | 1.2121 |
| 400.477 | 176.0664 | 176.6472 | 0.3298 |
| 420.477 | 182.4120 | 181.9793 | 0.2372 |
| 440.477 | 188.7937 | 187.5029 | 0.6837 |
| 460.477 | 195.7330 | 193.5100 | 1.1357 |
| 480.477 | 200.8936 | 200.2924 | 0.2993 |
| 500.477 | 206.2091 | 208.1418 | 0.9372 |
| 520.477 | 210.7179 | 217.3503 | 3.1475 |
| 540.477 | 223.7279 | 228.2095 | 1.0031 |
表4 三聚氰酸固体比热容拟合计算值与实验值
Table 4 Experimental and calculated values of CA(s)
| T/K | cp,exp/(J·mol-1·K-1) | cp,cal/(J·mol-1·K-1) | Relative deviation/% |
|---|---|---|---|
| 300.477 | 142.2681 | 142.6466 | 0.2660 |
| 320.477 | 149.7612 | 151.3982 | 1.0931 |
| 340.477 | 157.4001 | 158.8823 | 0.9416 |
| 360.477 | 162.3271 | 165.3906 | 1.8872 |
| 380.477 | 169.1645 | 171.2149 | 1.2121 |
| 400.477 | 176.0664 | 176.6472 | 0.3298 |
| 420.477 | 182.4120 | 181.9793 | 0.2372 |
| 440.477 | 188.7937 | 187.5029 | 0.6837 |
| 460.477 | 195.7330 | 193.5100 | 1.1357 |
| 480.477 | 200.8936 | 200.2924 | 0.2993 |
| 500.477 | 206.2091 | 208.1418 | 0.9372 |
| 520.477 | 210.7179 | 217.3503 | 3.1475 |
| 540.477 | 223.7279 | 228.2095 | 1.0031 |
| Component | Δ | cp /(J·mol-1·K-1) | |
|---|---|---|---|
| Urea(g) | -262.10 | 257.25 | 1.366×10-8T3-9.87×10-5T2+0.1946T+22.32 |
| MPO(g) | -435.33 | 393.14 | 5.55×10-8T3-2.58×10-4T2+0.4577T+30.95 |
| MPDC(g) | -937.87 | 723.68 | -2.55×10-8T3-2.03×10-4T2+0.5939T+82.35 |
| HIBC(g) | -561.83 | 483.49 | 1.68×10-8T3-2.31×10-4T2+0.5258T+56.65 |
| CA(s) | -703.50 | 142.20 | 6.08×10-6T3-7.43×10-3T2+3.2924T-340.76 |
| NH3(g) | -46.19 | 192.51 | 6.69×10-8T3+9.90×10-5T2+0.0260T+27.55 |
| HNCO(g) | -101.67 | 238.22 | 4.49×10-8T3-1.13×10-4T2+0.11756T+18.86 |
表5 各物质基本热力学数据(298.15 K)
Table 5 Thermodynamic data of components(298.15 K)
| Component | Δ | cp /(J·mol-1·K-1) | |
|---|---|---|---|
| Urea(g) | -262.10 | 257.25 | 1.366×10-8T3-9.87×10-5T2+0.1946T+22.32 |
| MPO(g) | -435.33 | 393.14 | 5.55×10-8T3-2.58×10-4T2+0.4577T+30.95 |
| MPDC(g) | -937.87 | 723.68 | -2.55×10-8T3-2.03×10-4T2+0.5939T+82.35 |
| HIBC(g) | -561.83 | 483.49 | 1.68×10-8T3-2.31×10-4T2+0.5258T+56.65 |
| CA(s) | -703.50 | 142.20 | 6.08×10-6T3-7.43×10-3T2+3.2924T-340.76 |
| NH3(g) | -46.19 | 192.51 | 6.69×10-8T3+9.90×10-5T2+0.0260T+27.55 |
| HNCO(g) | -101.67 | 238.22 | 4.49×10-8T3-1.13×10-4T2+0.11756T+18.86 |
| Group | Δ (kJ·mol-1) | Δ (kJ·mol-1) | Δi / (kJ·mol-1) | ΔTbi /K | ΔTci /K |
|---|---|---|---|---|---|
| —CH3 | -17.520 | 70.037 | 2.373 | 23.58 | 0.0141 |
| —CH2— | 190.833 | -27.338 | 2.226 | 22.88 | 0.0189 |
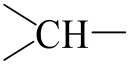 | 194.137 | 118.629 | 1.691 | 21.74 | 0.0164 |
| —NH2 | 286.164 | 410.002 | 10.788 | 73.23 | 0.0243 |
 | 644.875 | -428.108 | — | 76.75 | 0.0380 |
| —COO— | 363.706 | 1315.350 | 9.633 | 81.10 | 0.0481 |
| —OH | — | — | 16.826 | 92.88 | 0.0741 |
表6 ΔvHb的基团贡献值[19]
Table 6 Group contributions of ΔvHb[19]
| Group | Δ (kJ·mol-1) | Δ (kJ·mol-1) | Δi / (kJ·mol-1) | ΔTbi /K | ΔTci /K |
|---|---|---|---|---|---|
| —CH3 | -17.520 | 70.037 | 2.373 | 23.58 | 0.0141 |
| —CH2— | 190.833 | -27.338 | 2.226 | 22.88 | 0.0189 |
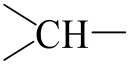 | 194.137 | 118.629 | 1.691 | 21.74 | 0.0164 |
| —NH2 | 286.164 | 410.002 | 10.788 | 73.23 | 0.0243 |
 | 644.875 | -428.108 | — | 76.75 | 0.0380 |
| —COO— | 363.706 | 1315.350 | 9.633 | 81.10 | 0.0481 |
| —OH | — | — | 16.826 | 92.88 | 0.0741 |
| Component | Tb/K | Tc/K | ΔvHb/(kJ·mol-1) | ΔvH/(kJ·mol-1) |
|---|---|---|---|---|
| Urea | 421.21 | 638.13 | 42.19 | 64.61×(1-T/638.13)0.395 |
| MPO | 474.84 | 636.47 | 57.47 | 102.76×(1-T/636.47)0.424 |
| MPDC | 597.74 | 803.17 | 53.75 | 90.37×(1-T/803.17)0.381 |
| HIBC | 536.29 | 719.71 | 61.06 | 107.54×(1-T/721.06)0.414 |
表7 各组分的Tb、Tc、ΔvHb值及ΔvH表达式
Table 7 Tb, Tc, ΔvHb and ΔvH of components
| Component | Tb/K | Tc/K | ΔvHb/(kJ·mol-1) | ΔvH/(kJ·mol-1) |
|---|---|---|---|---|
| Urea | 421.21 | 638.13 | 42.19 | 64.61×(1-T/638.13)0.395 |
| MPO | 474.84 | 636.47 | 57.47 | 102.76×(1-T/636.47)0.424 |
| MPDC | 597.74 | 803.17 | 53.75 | 90.37×(1-T/803.17)0.381 |
| HIBC | 536.29 | 719.71 | 61.06 | 107.54×(1-T/721.06)0.414 |
| Component | Parameter | Calculation | Literature | Relative deviation/% |
|---|---|---|---|---|
| Urea(g) | Δ | -262.10 | -262.10 | 0 |
| 257.25 | 257.25 | 0 | ||
| ΔvHb/(kJ·mol-1) | 42.19 | 42.19 | 0 | |
| Tb/K | 421.21 | 421.21 | 0 | |
| Tc/K | 638.13 | 638.13 | 0 | |
| HNCO(g) | cp (298.15 K)/(J·mol-1·K-1) | 45.043 | 45.056 | 0.028 |
| cp (400 K)/(J·mol-1·K-1) | 50.729 | 50.678 | 0.101 | |
| cp (500 K)/(J·mol-1·K-1) | 55.007 | 55.003 | 0.008 | |
| cp (600 K)/(J·mol-1·K-1) | 58.410 | 58.414 | 0.007 | |
| cp (700 K)/(J·mol-1·K-1) | 61.228 | 61.183 | 0.074 |
表8 部分热力学数据的估算值和文献值[22, 26]对比
Table 8 Comparison of calculated thermodynamic data with literature[22, 26]
| Component | Parameter | Calculation | Literature | Relative deviation/% |
|---|---|---|---|---|
| Urea(g) | Δ | -262.10 | -262.10 | 0 |
| 257.25 | 257.25 | 0 | ||
| ΔvHb/(kJ·mol-1) | 42.19 | 42.19 | 0 | |
| Tb/K | 421.21 | 421.21 | 0 | |
| Tc/K | 638.13 | 638.13 | 0 | |
| HNCO(g) | cp (298.15 K)/(J·mol-1·K-1) | 45.043 | 45.056 | 0.028 |
| cp (400 K)/(J·mol-1·K-1) | 50.729 | 50.678 | 0.101 | |
| cp (500 K)/(J·mol-1·K-1) | 55.007 | 55.003 | 0.008 | |
| cp (600 K)/(J·mol-1·K-1) | 58.410 | 58.414 | 0.007 | |
| cp (700 K)/(J·mol-1·K-1) | 61.228 | 61.183 | 0.074 |
| Parameter | T/K | Components | ||||||
|---|---|---|---|---|---|---|---|---|
| Urea(l) | MPO(l) | MPDC(l) | HIBC(l) | CA(s) | NH3(g) | HNCO(g) | ||
Δf (kJ·mol-1) | 483.15 | -283.55 | -459.50 | -899.95 | -687.75 | -671.47 | -35.59 | -92.41 |
| 493.15 | -281.63 | -455.92 | -895.98 | -683.95 | -669.44 | -34.87 | -91.87 | |
| 503.15 | -279.66 | -452.25 | -891.95 | -680.09 | -667.37 | -34.14 | -91.32 | |
| 513.25 | -277.64 | -448.49 | -887.87 | -676.16 | -665.25 | -33.39 | -90.76 | |
| 523.15 | -275.56 | -444.62 | -883.74 | -672.17 | -663.09 | -32.62 | -90.21 | |
| 483.15 | 297.21 | 477.36 | 696.40 | 591.76 | 224.69 | 219.70 | 262.20 | |
| 493.15 | 299.17 | 481.45 | 702.98 | 597.09 | 228.85 | 221.17 | 263.32 | |
| 503.15 | 301.11 | 485.51 | 709.50 | 602.38 | 233.01 | 222.64 | 264.43 | |
| 513.25 | 303.03 | 489.53 | 715.97 | 607.63 | 237.18 | 224.12 | 265.51 | |
| 523.15 | 304.94 | 493.5 2 | 722.38 | 612.84 | 241.25 | 225.60 | 266.58 | |
表9 不同温度下各组分相应状态的ΔfHm⊝(T)、Sm⊝(T)
Table 9 ΔfHm⊝ (T), Sm⊝(T) of the corresponding states of components at different temperatures
| Parameter | T/K | Components | ||||||
|---|---|---|---|---|---|---|---|---|
| Urea(l) | MPO(l) | MPDC(l) | HIBC(l) | CA(s) | NH3(g) | HNCO(g) | ||
Δf (kJ·mol-1) | 483.15 | -283.55 | -459.50 | -899.95 | -687.75 | -671.47 | -35.59 | -92.41 |
| 493.15 | -281.63 | -455.92 | -895.98 | -683.95 | -669.44 | -34.87 | -91.87 | |
| 503.15 | -279.66 | -452.25 | -891.95 | -680.09 | -667.37 | -34.14 | -91.32 | |
| 513.25 | -277.64 | -448.49 | -887.87 | -676.16 | -665.25 | -33.39 | -90.76 | |
| 523.15 | -275.56 | -444.62 | -883.74 | -672.17 | -663.09 | -32.62 | -90.21 | |
| 483.15 | 297.21 | 477.36 | 696.40 | 591.76 | 224.69 | 219.70 | 262.20 | |
| 493.15 | 299.17 | 481.45 | 702.98 | 597.09 | 228.85 | 221.17 | 263.32 | |
| 503.15 | 301.11 | 485.51 | 709.50 | 602.38 | 233.01 | 222.64 | 264.43 | |
| 513.25 | 303.03 | 489.53 | 715.97 | 607.63 | 237.18 | 224.12 | 265.51 | |
| 523.15 | 304.94 | 493.5 2 | 722.38 | 612.84 | 241.25 | 225.60 | 266.58 | |
| 反应 | T/K | ΔrH/(kJ·mol-1) | ΔrS/(J·mol-1·K-1) | ΔrG/(kJ·mol-1) | lnKӨ | KӨ |
|---|---|---|---|---|---|---|
| 483.15 | 55.46 | 64.02 | 24.53 | -6.11 | 2.23×10-3 | |
| 493.15 | 53.46 | 65.52 | 21.14 | -5.16 | 5.76×10-3 | |
| (1) | 503.15 | 51.35 | 67.06 | 17.61 | -4.21 | 1.48×10-2 |
| 513.25 | 49.12 | 68.61 | 13.92 | -3.26 | 3.83×10-2 | |
| 523.15 | 46.75 | 70.19 | 10.04 | -2.31 | 9.95×10-2 | |
| 483.15 | 119.79 | 157.55 | 43.67 | -10.87 | 1.90×10-5 | |
| 493.15 | 120.16 | 157.42 | 42.53 | -10.37 | 3.13×10-5 | |
| (2) | 503.15 | 120.55 | 157.30 | 41.40 | -9.90 | 5.03×10-5 |
| 513.25 | 120.95 | 157.17 | 40.30 | -9.44 | 7.91×10-5 | |
| 523.15 | 121.36 | 157.03 | 39.21 | -9.01 | 1.22×10-4 | |
| 483.15 | -394.23 | -561.90 | -122.75 | 30.46 | 1.87×1013 | |
| 493.15 | -393.84 | -561.09 | -117.14 | 28.48 | 2.56×1012 | |
| (3) | 503.15 | -393.41 | -560.24 | -111.53 | 26.58 | 3.79×1011 |
| 513.25 | -392.96 | -559.34 | -105.93 | 24.75 | 6.07×1010 | |
| 523.15 | -392.47 | -558.39 | -100.34 | 23.00 | 1.05×1010 |
表10 不同温度下各反应ΔrH、ΔrS、ΔrG及KӨ值
Table 10 ΔrH, ΔrS, ΔrGand KӨ of reactions at different temperatures
| 反应 | T/K | ΔrH/(kJ·mol-1) | ΔrS/(J·mol-1·K-1) | ΔrG/(kJ·mol-1) | lnKӨ | KӨ |
|---|---|---|---|---|---|---|
| 483.15 | 55.46 | 64.02 | 24.53 | -6.11 | 2.23×10-3 | |
| 493.15 | 53.46 | 65.52 | 21.14 | -5.16 | 5.76×10-3 | |
| (1) | 503.15 | 51.35 | 67.06 | 17.61 | -4.21 | 1.48×10-2 |
| 513.25 | 49.12 | 68.61 | 13.92 | -3.26 | 3.83×10-2 | |
| 523.15 | 46.75 | 70.19 | 10.04 | -2.31 | 9.95×10-2 | |
| 483.15 | 119.79 | 157.55 | 43.67 | -10.87 | 1.90×10-5 | |
| 493.15 | 120.16 | 157.42 | 42.53 | -10.37 | 3.13×10-5 | |
| (2) | 503.15 | 120.55 | 157.30 | 41.40 | -9.90 | 5.03×10-5 |
| 513.25 | 120.95 | 157.17 | 40.30 | -9.44 | 7.91×10-5 | |
| 523.15 | 121.36 | 157.03 | 39.21 | -9.01 | 1.22×10-4 | |
| 483.15 | -394.23 | -561.90 | -122.75 | 30.46 | 1.87×1013 | |
| 493.15 | -393.84 | -561.09 | -117.14 | 28.48 | 2.56×1012 | |
| (3) | 503.15 | -393.41 | -560.24 | -111.53 | 26.58 | 3.79×1011 |
| 513.25 | -392.96 | -559.34 | -105.93 | 24.75 | 6.07×1010 | |
| 523.15 | -392.47 | -558.39 | -100.34 | 23.00 | 1.05×1010 |
| 1 | Prabhaharan M, Prabakaran A R, Srinivasan S, et al. Density functional theory studies on molecular structure, vibrational spectra and electronic properties of cyanuric acid[J]. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 2015, 138: 711-722. |
| 2 | Montgomery W, Crowhurst J C, Zaug J M, et al. The chemistry of cyanuric acid (H3C3N3O3) under high pressure and high temperature[J]. The Journal of Physical Chemistry B, 2008, 112(9): 2644-2648. |
| 3 | 王付燕. 二氧化碳化学封存产物三聚氰酸的合成及应用研究[D]. 济南: 山东大学, 2015. |
| Wang F Y. Study on the synthesis of carbon dioxide chemical sequestration product cyanuric acid and its application[D]. Jinan: Shandong University, 2015. | |
| 4 | 陈焕章, 杨路, 冯雪. 氰尿酸合成工艺研究进展[J]. 应用化工, 2016, 45(5): 953-957. |
| Chen H Z, Yang L, Feng X. Research process in cyanuric acid synthesis[J]. Applied Chemical Industry, 2016, 45(5): 953-957. | |
| 5 | 朱止阳, 张少利, 张蒙恩, 等. 尿素下游技术产品发展[J]. 河南化工, 2020, 37(10): 3-5. |
| Zhu Z Y, Zhang S L, Zhang M E, et al. Technical development of urea downstream products[J]. Henan Chemical Industry, 2020, 37(10): 3-5. | |
| 6 | Blotny G. Recent applications of 2,4,6-trichloro-1,3,5-triazine and its derivatives in organic synthesis[J]. Tetrahedron, 2006, 62(41): 9507-9522. |
| 7 | Pop F, Socaci C, Terec A, et al. Cryptands and bismacrocycles with cyanuric and isocyanuric units: synthesis and structural investigations[J]. Tetrahedron, 2012, 68(41): 8581-8588. |
| 8 | 张宁, 董志鹏, 张洁, 等. 三聚氰酸制备方法综述[J]. 河北科技大学学报, 2021, 42(3): 271-279. |
| Zhang N, Dong Z P, Zhang J, et al. Review of preparation methods of pyrouric caid[J]. Journal of Hebei University of Science and Technology, 2021, 42(3): 271-279. | |
| 9 | 颜玉桂, 张伟伟, 蔡可迎. 混合溶剂法合成氰尿酸的研究[J]. 广州化工, 2012, 40(20): 57-58, 108. |
| Yan Y G, Zhang W W, Cai K Y. Study on synthesis of cyanuric acid in mixed solvents[J]. Guangzhou Chemical Industry, 2012, 40(20): 57-58, 108. | |
| 10 | 朱文明. 固相和液相法合成氰尿酸的对比研究[J]. 精细化工中间体, 2004, 34(6): 45-46. |
| Zhu W M. The contrast of solid and liquid hromatography to synthesize cyanuric acid[J]. Fine Chemical Intermediates, 2004, 34(6): 45-46. | |
| 11 | She D M, Yu H L, Huang Q L, et al. Liquid-phase synthesis of cyanuric acid from urea[J]. Molecules, 2010, 15(3): 1898-1902. |
| 12 | 邱玉娥, 张存兰, 刘爱珍. 高纯度氰尿酸合成工艺研究[J]. 天津化工, 2005, 19(2): 35-36. |
| Qiu Y E, Zhang C L, Liu A Z. Research on the process of synthsing high-purity cyanuric acid[J]. Tianjin Chemical Industry, 2005, 19(2): 35-36. | |
| 13 | 门吉帅, 王颖, 邵长银, 等. 离子液体法合成氰尿酸绿色新工艺研究[J]. 化学试剂, 2021, 43(3): 390-394. |
| Men J S, Wang Y, Shao C Y, et al. Green technology of cyanuric acid synthesis by ionic liquid[J]. Chemical Reagents, 2021, 43(3): 390-394. | |
| 14 | 石晓华, 姚彦辉, 任庆辉, 等. 一种三聚氰酸的制备方法: 111925337B[P]. 2023-03-31. |
| Shi X H, Yao Y H, Ren Q H, et al. Preparation method of cyanuric acid: 111925337B[P]. 2023-03-31. | |
| 15 | Ren Q H, Qian L F, Yao Y H, et al. A safe and effective strategy for the synthesis of cyanuric acid using the solvent method[J]. Russian Journal of Applied Chemistry, 2022, 95(10): 1618-1626. |
| 16 | 诺曼 E B. 化学反应器的设计、优化和放大[M]. 朱开宏, 李伟, 张元兴, 译. 北京: 中国石化出版社, 2004. |
| Nauman E B. Chemical Reactor Design, Optimization, and Scaleup[M]. Zhu K H, Li W, Zhang Y Y, trans. Beijing: China Petrochemical Press, 2004. | |
| 17 | 杨幸川, 位根磊, 徐丽, 等. 己二酸二甲酯加氢反应热力学分析及动力学研究[J].化工学报, 2021, 72(5): 2465-2473. |
| Yang X C, Wei G L, Xu L, et al. Thermodynamic analysis and kinetic study on hydrogenation of dimethyl adipate[J]. CIESC Journal, 2021, 72(5): 2465-2473. | |
| 18 | 张继龙, 赵志仝, 乔燕, 等. 酯交换制油酸甲酯的基团贡献法热力学分析[J]. 化工学报, 2012, 63(6): 1684-1690. |
| Zhang J L, Zhao Z T, Qiao Y, et al. Thermodynamic analysis on preparation of methyl oleate via transesterification by group contribution method[J]. CIESC Journal, 2012, 63(6): 1684-1690. | |
| 19 | 马沛生. 化工数据[M]. 北京: 中国石化出版社, 2003. |
| Ma P S. Chemical Data[M]. Beijing: China Petrochemical Press, 2003. | |
| 20 | 董新法, 方利国, 陈砺. 物性估算原理及计算机计算[M]. 北京: 化学工业出版社, 2006. |
| Dong X F, Fang L G, Chen L. Principle of Physical Property Estimation and Computer Calculation[M]. Beijing: Chemical Industry Press, 2006. | |
| 21 | De Wit H G M, De Kruif C G, Van Miltenburg J C. Thermodynamic properties of molecular organic crystals containing nitrogen, oxygen, and sulfur(Ⅱ): Molar heat capacities of eight compounds by adiabatic calorimetry[J]. The Journal of Chemical Thermodynamics, 1983, 15(9): 891-902. |
| 22 | Chase M W. NIST-JANAF Thermochemical Tables[M]. 4th ed. New York: American Institute of Physics and the American Chemical Society, 1998. |
| 23 | 朱自强, 吴有庭. 化工热力学[M]. 3版. 北京: 化学工业出版社, 2010. |
| Zhu Z Q, Wu Y T. Chemical Engineering Thermodynamics[M]. 3rd ed. Beijing: Chemical Industry Press, 2010. | |
| 24 | 王琳琳, 陈建云, 梁杰珍, 等. 枞酸与甲醇酯化反应的基团贡献法热力学分析[J]. 化工学报, 2013, 64(6): 1900-1906. |
| Wang L L, Chen J Y, Liang J Z, et al. Thermodynamic analysis for esterification of abietic acid with methanol by group-contribution method[J]. CIESC Journal, 2013, 64(6): 1900-1906. | |
| 25 | 付丽丽, 将登高. 棕榈酸异丙酯合成反应的热力学分析[J]. 高校化学工程学报, 2016, 30(2): 398-403. |
| Fu L L, Jiang D G. Thermodynamic analysis of isopropyl palmitate synthesis[J]. Journal of Chemical Engineering of Chinese Universities, 2016, 30(2): 398-403. | |
| 26 | 崔雪霞, 王桂荣, 赵茜, 等. 尿素法合成甲苯二氨基甲酸苯酯反应体系的热力学分析[J]. 化工学报, 2015, 66(9): 3367-3376. |
| Cui X X, Wang G R, Zhao Q, et al. Thermodynamic analysis on synthesis of diphenyl toluene dicarbamate via urea route[J]. CIESC Journal, 2015, 66(9): 3367-3376. | |
| 27 | 王毅, 王盟, 安华良, 等. 尿素法合成二苯甲烷二氨基甲酸甲酯反应热力学分析及实验研究[J]. 精细石油化工, 2016, 33(2): 71-76. |
| Wang Y, Wang M, An H L, et al. Thermodynamic analysis and experimental study on the synthesis of methylene diphenyl dicarbamate via urea route[J]. Speciality Petrochemicals, 2016, 33(2): 71-76. | |
| 28 | 科顿 F A. 群论在化学中的应用[M]. 刘春万, 游效单, 赖伍江, 译. 福州: 福建科学技术出版社, 1999. |
| Cotton F A. Chemical Applications of Group Theory[M]. Liu W C, You X D, Lai W J, trans. Fuzhou: Fujian Science & Technology Publishing House, 1999. | |
| 29 | Benson S W. Thermochemical Kinetics: Methods for the Estimation of Thermochemical Data and Rate Parameters[M]. 2nd ed. New York: Wiley, 1976. |
| 30 | 李惠萍. 2-甲基-1,3-丙二醇的合成及其工程基础研究[D]. 郑州:郑州大学, 2007. |
| Li H P. Study on the synthesis and engineering basis of 2-methyl-1,3-propanediol[D]. Zhengzhou: Zhengzhou University, 2007. | |
| 31 | 孙大雷, 洪展鹏, 叶嘉辉, 等. CO2、NH3和醇“一步法”合成氨基甲酸酯的热力学分析[J]. 化学世界, 2016, 57(3): 153-158, 164. |
| Sun D L, Hong Z P, Ye J H, et al. Thermodynamic analysis of synthesis of carbamates from carbon dioxide, ammonia and alcohols[J]. Chemical World, 2016, 57(3): 153-158, 164. |
| [1] | 杨艳, 郭亚丽, 于硕文, 潘泊年, 沈胜强. 液氨喷射泵热力性能的计算分析[J]. 化工学报, 2024, 75(6): 2134-2142. |
| [2] | 李子扬, 郑楠, 方嘉宾, 魏进家. 再压缩S-CO2布雷顿循环性能分析及多目标优化[J]. 化工学报, 2024, 75(6): 2143-2156. |
| [3] | 李新泽, 张双星, 杨洪海, 杜文静. 基于电池冷却用新型脉动热管性能的实验研究[J]. 化工学报, 2024, 75(6): 2222-2232. |
| [4] | 徐欣欣, 冀芸丽, 武鲜凤, 安霞, 吴旭. 水滑石衍生CuMgFe-LDO催化剂协同净化氮氧化物和甲醇[J]. 化工学报, 2024, 75(5): 1890-1902. |
| [5] | 武颖韬, 费立涵, 孔祥东, 王帜, 汤成龙, 黄佐华. 咪唑二氰胺离子液体掺混糠醇的自燃及推进性能[J]. 化工学报, 2024, 75(5): 2017-2025. |
| [6] | 刘东飞, 张帆, 刘铮, 卢滇楠. 机器学习势及其在分子模拟中的应用综述[J]. 化工学报, 2024, 75(4): 1241-1255. |
| [7] | 张政, 汪妩琼, 张雅静, 王康军, 吉远辉. 理论计算在药物制剂设计中的研究进展[J]. 化工学报, 2024, 75(4): 1429-1438. |
| [8] | 吴希, 孙博, 刘银东, 齐传磊, 陈凯毅, 王路海, 许崇, 李永峰. 钠离子电池沥青基碳负极材料制备技术研究进展[J]. 化工学报, 2024, 75(4): 1270-1283. |
| [9] | 张劲, 郭志斌, 罗来明, 卢善富, 相艳. 5 kW重整甲醇高温质子交换膜燃料电池系统设计与性能[J]. 化工学报, 2024, 75(4): 1697-1704. |
| [10] | 陈好奇, 史博会, 彭琪, 康琦, 宋尚飞, 姚海元, 陈海宏, 吴海浩, 宫敬. 基于稳定性分析的含酸/醇烃水体系相平衡计算[J]. 化工学报, 2024, 75(3): 789-800. |
| [11] | 周辛梓, 李增辉, 孟现阳, 吴江涛. 低温下高纯空气黏度实验研究[J]. 化工学报, 2024, 75(3): 782-788. |
| [12] | 李浩文, 兰昊, 郑幼丹, 孙勇辉, 杨子昕, 宋谦石, 汪小憨. 热通道内典型碳氢燃料的热解结焦行为[J]. 化工学报, 2024, 75(2): 626-636. |
| [13] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [14] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [15] | 张化福, 童莉葛, 张振涛, 杨俊玲, 王立, 张俊浩. 机械蒸汽压缩蒸发技术研究现状与发展趋势[J]. 化工学报, 2023, 74(S1): 8-24. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号