化工学报 ›› 2025, Vol. 76 ›› Issue (6): 2667-2677.DOI: 10.11949/0438-1157.20241447
米晓天1( ), 刘宏臣1(
), 刘宏臣1( ), 王克军2, 唐文娜1, 徐永伟1, 杨梅3
), 王克军2, 唐文娜1, 徐永伟1, 杨梅3
收稿日期:2024-12-13
修回日期:2025-01-07
出版日期:2025-06-25
发布日期:2025-07-09
通讯作者:
刘宏臣
作者简介:米晓天(2000—),女,硕士,2279072974@qq.com
基金资助:
Xiaotian MI1( ), Hongchen LIU1(
), Hongchen LIU1( ), Kejun WANG2, Wenna TANG1, Yongwei XU1, Mei YANG3
), Kejun WANG2, Wenna TANG1, Yongwei XU1, Mei YANG3
Received:2024-12-13
Revised:2025-01-07
Online:2025-06-25
Published:2025-07-09
Contact:
Hongchen LIU
摘要:
利用高速摄像研究了微通道内两相吸收剂三乙烯四胺/二乙氨基乙醇(TETA/DEEA)的CO2吸收传质特性,系统考察了气液两相流量、吸收剂总胺浓度及总胺中TETA占比对初始气泡长度LB0、气泡体积减小量ΔVB、CO2吸收率φ和液侧体积传质系数kLa的影响。结果表明,LB0与ΔVB密切相关,均随液体流量、总胺浓度和总胺中TETA占比的增加,以及气体流量的降低而降低。CO2吸收率随气体流量的增加而降低,随总胺中TETA占比的增加呈现先增加后降低的趋势。实验测得的kLa值在0.88~13.65 s-1之间,比传统反应器高1~2个数量级,表明微通道反应器可显著强化TETA/DEEA两相吸收剂捕集CO2过程。此外,基于实验数据提出了kLa预测关联式,预测效果较好。
中图分类号:
米晓天, 刘宏臣, 王克军, 唐文娜, 徐永伟, 杨梅. 微通道内两相吸收剂TETA/DEEA吸收CO2过程的传质研究[J]. 化工学报, 2025, 76(6): 2667-2677.
Xiaotian MI, Hongchen LIU, Kejun WANG, Wenna TANG, Yongwei XU, Mei YANG. Mass transfer study of CO2 absorption by TETA/DEEA biphasic absorbent in the microchannel[J]. CIESC Journal, 2025, 76(6): 2667-2677.
| CTETA/(mol/L) | CDEEA/(mol/L) | 总胺浓度 CT/(mol/L) | 总胺中TETA 占比M | 密度 ρ/(kg·m-3) | 黏度 μ/(mPa·s) |
|---|---|---|---|---|---|
| 0.5 | 2.0 | 2.5 | 0.2 | 965 | 7.6 |
| 0.6 | 2.4 | 3.0 | 0.2 | 970 | 12.2 |
| 0.7 | 2.8 | 3.5 | 0.2 | 968 | 22.3 |
| 0.8 | 3.2 | 4.0 | 0.2 | 948 | 30.7 |
| 1.6 | 2.4 | 4.0 | 0.4 | 979 | 78.9 |
| 2.4 | 1.6 | 4.0 | 0.6 | 989 | 118.8 |
| 3.2 | 0.8 | 4.0 | 0.8 | 992 | 122.6 |
表1 TETA/DEEA两相吸收剂的物性参数
Table 1 Physical properties of the TETA/DEEA biphasic solvents
| CTETA/(mol/L) | CDEEA/(mol/L) | 总胺浓度 CT/(mol/L) | 总胺中TETA 占比M | 密度 ρ/(kg·m-3) | 黏度 μ/(mPa·s) |
|---|---|---|---|---|---|
| 0.5 | 2.0 | 2.5 | 0.2 | 965 | 7.6 |
| 0.6 | 2.4 | 3.0 | 0.2 | 970 | 12.2 |
| 0.7 | 2.8 | 3.5 | 0.2 | 968 | 22.3 |
| 0.8 | 3.2 | 4.0 | 0.2 | 948 | 30.7 |
| 1.6 | 2.4 | 4.0 | 0.4 | 979 | 78.9 |
| 2.4 | 1.6 | 4.0 | 0.6 | 989 | 118.8 |
| 3.2 | 0.8 | 4.0 | 0.8 | 992 | 122.6 |
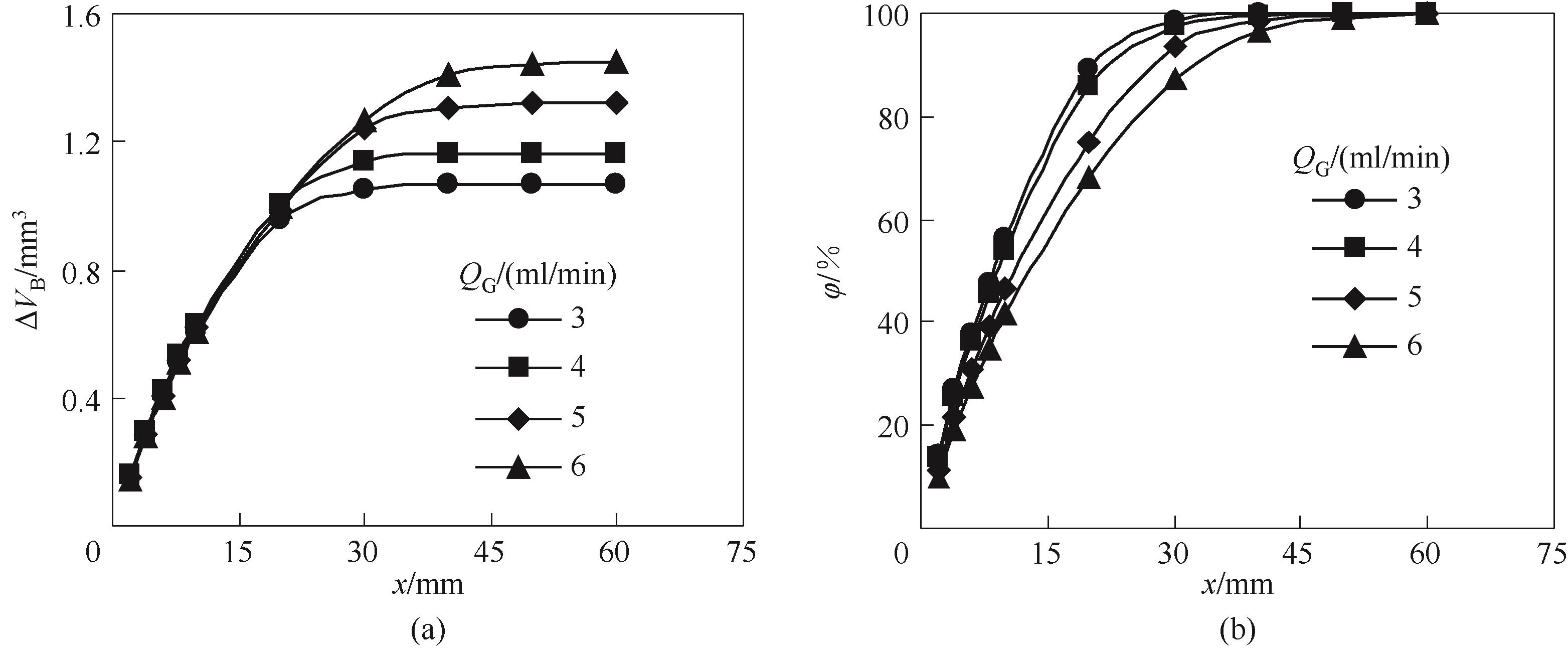
图6 气体流量对气泡体积减小量(a)和CO2吸收率(b)沿微通道演变规律的影响
Fig.6 Effect of gas flow rates on the evolution of bubble volume reduction (a) and CO2 absorption percent (b) along the microchannel (QL=3 ml/min, CT=4 mol/L, M=0.2)
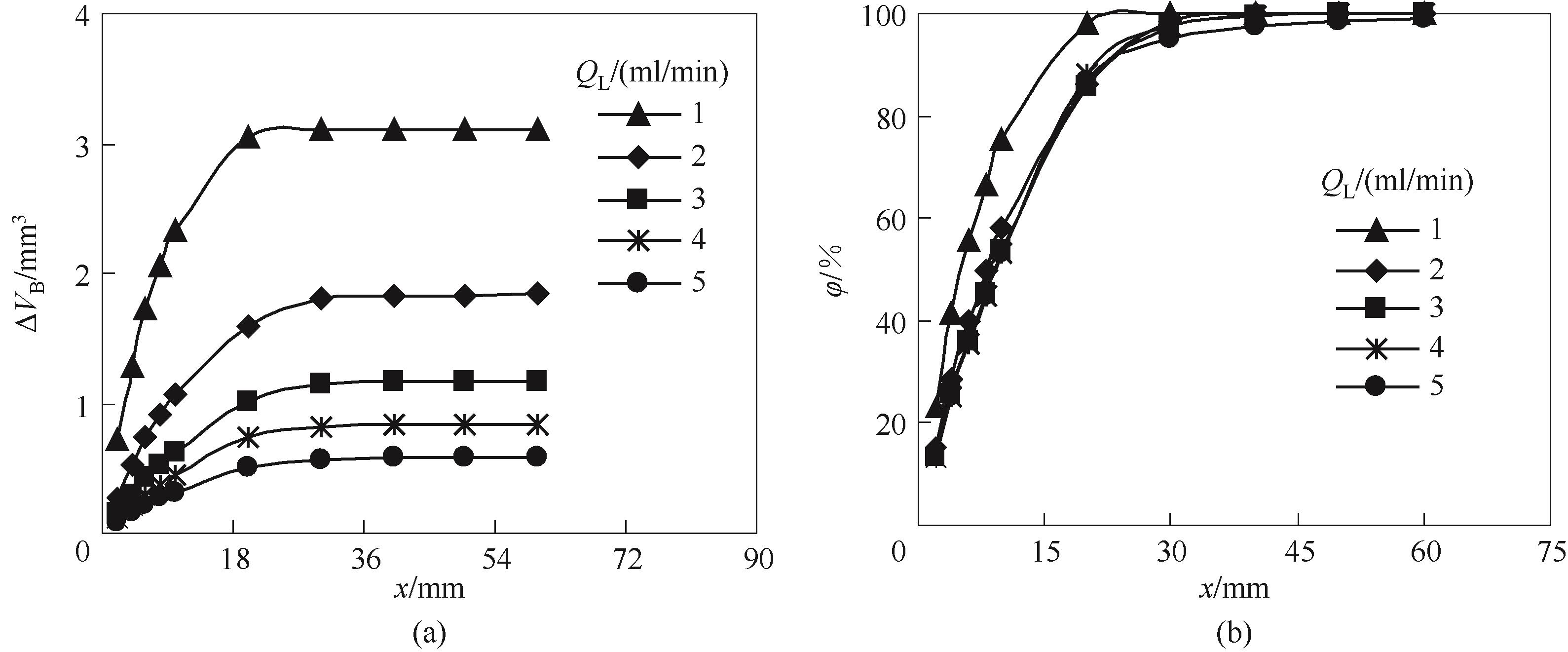
图7 液体流量对气泡体积减小量(a)和CO2吸收率(b)沿微通道演变规律的影响
Fig.7 Effect of liquid flow rates on the evolution of bubble volume reduction (a) and CO2 absorption percent (b) along the microchannel (QG=4 ml/min, CT=4 mol/L, M=0.2)
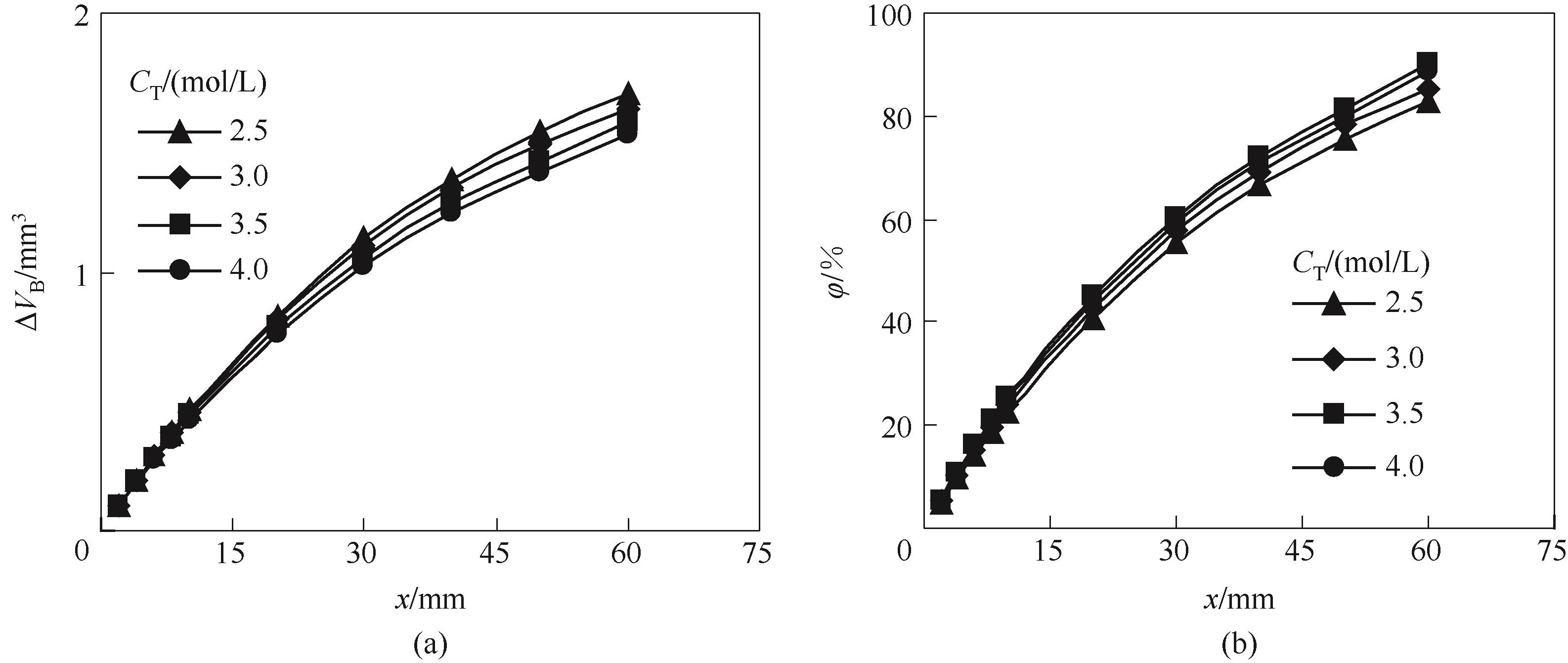
图8 吸收剂总胺浓度CT对气泡体积减小量(a)和CO2吸收率(b)沿微通道演变规律的影响
Fig.8 Effect of absorbent concentrations on the evolution of bubble volume reduction (a) and CO2 absorption percent (b) along the microchannel (M=0.2, QL=5 ml/min, QG=20 mL/min)
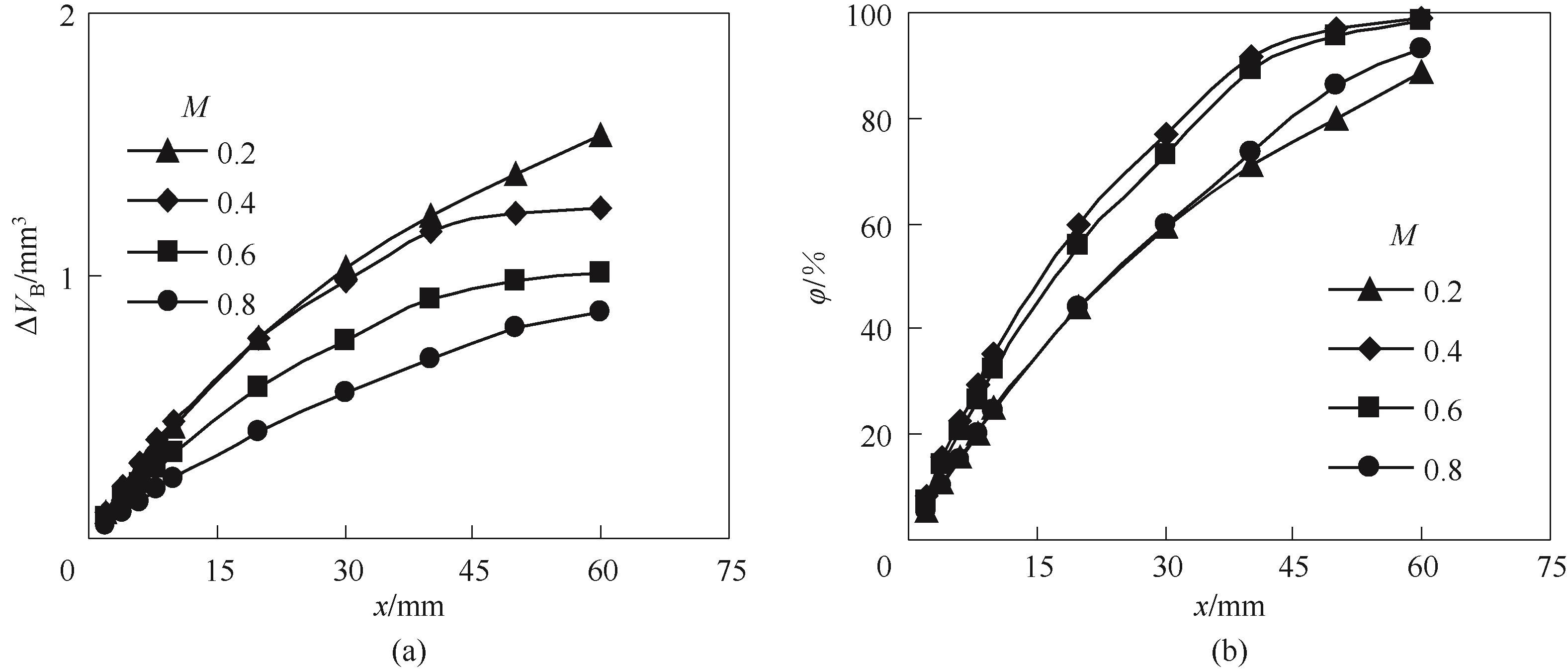
图9 总胺中TETA占比对气泡体积减小量(a)和CO2吸收率(b)沿微通道演变规律的影响
Fig.9 Effect of TETA proportion on the evolution of bubble volume reduction (a) and CO2 absorption percent (b) along the microchannel (CT=4 mol/L, QL=5 ml/min, QG=20 mL/min)
| [1] | Hepburn C, Adlen E, Beddington J, et al. The technological and economic prospects for CO2 utilization and removal[J]. Nature, 2019, 575(7781): 87-97. |
| [2] | Bui M, Adjiman C S, Bardow A, et al. Carbon capture and storage (CCS): the way forward[J]. Energy & Environmental Science, 2018, 11(5): 1062-1176. |
| [3] | Liang Z W, Fu K Y, Idem R, et al. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents[J]. Chinese Journal of Chemical Engineering, 2016, 24(2): 278-288. |
| [4] | Veawab A, Tontiwachwuthikul P, Chakma A. Corrosion behavior of carbon steel in the CO2 absorption process using aqueous amine solutions[J]. Industrial & Engineering Chemistry Research, 1999, 38(10): 3917-3924. |
| [5] | Borhani T N, Wang M H. Role of solvents in CO2 capture processes: the review of selection and design methods[J]. Renewable and Sustainable Energy Reviews, 2019, 114: 109299. |
| [6] | Jing G H, Qian Y H, Zhou X B, et al. Designing and screening of multi-amino-functionalized ionic liquid solution for CO2 capture by quantum chemical simulation[J]. ACS Sustainable Chemistry & Engineering, 2018, 6(1): 1182-1191. |
| [7] | Sun C, Wen S J, Zhao J K, et al. Mechanism and kinetics study of CO2 absorption into blends of N-methyldiethanolamine and 1-hydroxyethyl-3-methylimidazolium glycine aqueous solution[J]. Energy & Fuels, 2017, 31(11): 12425-12433. |
| [8] | Heldebrant D J, Koech P K, Glezakou V A, et al. Water-lean solvents for post-combustion CO2 capture: fundamentals, uncertainties, opportunities, and outlook[J]. Chemical Reviews, 2017, 117(14): 9594-9624. |
| [9] | Raksajati A, Ho M T, Wiley D E. Comparison of solvent development options for capture of CO2 from flue gases[J]. Industrial & Engineering Chemistry Research, 2018, 57(19): 6746-6758. |
| [10] | Wang X F, Akhmedov N G, Hopkinson D, et al. Phase change amino acid salt separates into CO2-rich and CO2-lean phases upon interacting with CO2 [J]. Applied Energy, 2016, 161: 41-47. |
| [11] | Zhang X W, Zhang R, Liu H L, et al. Evaluating CO2 desorption performance in CO2-loaded aqueous tri-solvent blend amines with and without solid acid catalysts[J]. Applied Energy, 2018, 218: 417-429. |
| [12] | Zhang W D, Jin X H, Tu W W, et al. Development of MEA-based CO2 phase change absorbent[J]. Applied Energy, 2017, 195: 316-323. |
| [13] | Zhuang Q, Clements B, Dai J Y, et al. Ten years of research on phase separation absorbents for carbon capture: achievements and next steps[J]. International Journal of Greenhouse Gas Control, 2016, 52: 449-460. |
| [14] | Raynal L, Bouillon P A, Gomez A, et al. From MEA to demixing solvents and future steps, a roadmap for lowering the cost of post-combustion carbon capture[J]. Chemical Engineering Journal, 2011, 171(3): 742-752. |
| [15] | Yang F S, Jin X H, Fang J W, et al. Development of CO2 phase change absorbents by means of the cosolvent effect[J]. Green Chemistry, 2018, 20(10): 2328-2336. |
| [16] | Wang L D, Liu S S, Wang R J, et al. Regulating phase separation behavior of a DEEA-TETA biphasic solvent using sulfolane for energy-saving CO2 capture[J]. Environmental Science & Technology, 2019, 53(21): 12873-12881. |
| [17] | Zhang S H, Shen Y, Shao P J, et al. Kinetics, thermodynamics, and mechanism of a novel biphasic solvent for CO2 capture from flue gas[J]. Environmental Science & Technology, 2018, 52(6): 3660-3668. |
| [18] | Wang L D, Yu S H, Li Q W, et al. Performance of sulfolane/DETA hybrids for CO2 absorption: phase splitting behavior, kinetics and thermodynamics[J]. Applied Energy, 2018, 228: 568-576. |
| [19] | Ye Q, Wang X L, Lu Y Q. Screening and evaluation of novel biphasic solvents for energy-efficient post-combustion CO2 capture[J]. International Journal of Greenhouse Gas Control, 2015, 39: 205-214. |
| [20] | Zhang S H, Shen Y, Wang L D, et al. Phase change solvents for post-combustion CO2 capture: principle, advances, and challenges[J]. Applied Energy, 2019, 239: 876-897. |
| [21] | Aghel B, Sahraie S, Heidaryan E, et al. Experimental study of carbon dioxide absorption by mixed aqueous solutions of methyl diethanolamine (MDEA) and piperazine (PZ) in a microreactor[J]. Process Safety and Environmental Protection, 2019, 131: 152-159. |
| [22] | 宋仕容, 刘宏臣, 米晓天, 等. 同轴微通道内管结构对液滴生成的影响规律研究[J]. 化工学报, 2024, 75(2): 566-574. |
| Song S R, Liu H C, Mi X T, et al. Experimental investigation of droplet formation in coaxial microchannels with different geometries of inner channel[J]. CIESC Journal, 2024, 75(2): 566-574. | |
| [23] | Yao C Q, Zhao Y C, Ma H Y, et al. Two-phase flow and mass transfer in microchannels: a review from local mechanism to global models[J]. Chemical Engineering Science, 2021, 229: 116017. |
| [24] | Chen G W, Yue J, Yuan Q. Gas-liquid microreaction technology: recent developments and future challenges[J]. Chinese Journal of Chemical Engineering, 2008, 16(5): 663-669. |
| [25] | Kashid M N, Renken A, Kiwi-Minsker L. Gas-liquid and liquid-liquid mass transfer in microstructured reactors[J]. Chemical Engineering Science, 2011, 66(17): 3876-3897. |
| [26] | Chu C Y, Zhang F B, Zhu C Y, et al. Mass transfer characteristics of CO2 absorption into 1-butyl-3-methylimidazolium tetrafluoroborate aqueous solution in microchannel[J]. International Journal of Heat and Mass Transfer, 2019, 128: 1064-1071. |
| [27] | Xu Z B, Wang T T, Wu J M, et al. Mass transfer characteristics of CO2 and blended aqueous solutions of [C2OHmim] [Lys]/MDEA in a microchannel[J]. Industrial & Engineering Chemistry Research, 2023, 62(22): 8926-8938. |
| [28] | Guo R W, Zhu C Y, Yin Y R, et al. Mass transfer characteristics of CO2 absorption into 2-amino-2-methyl-1-propanol non-aqueous solution in a microchannel[J]. Journal of Industrial and Engineering Chemistry, 2019, 75: 194-201. |
| [29] | Shaterabadi F, Rashidi H. Experimental and modeling study of CO2 capture by phase change blend of triethylenetetramine-ethanol solvent[J]. Energy, 2024, 307: 132809. |
| [30] | Ye J X, Jiang C K, Chen H, et al. Novel biphasic solvent with tunable phase separation for CO2 capture: role of water content in mechanism, kinetics, and energy penalty[J]. Environmental Science & Technology, 2019, 53(8): 4470-4479. |
| [31] | Yao C Q, Dong Z Y, Zhao Y C, et al. An online method to measure mass transfer of slug flow in a microchannel[J]. Chemical Engineering Science, 2014, 112: 15-24. |
| [32] | Yao C Q, Zhao Y C, Ye C B, et al. Characteristics of slug flow with inertial effects in a rectangular microchannel[J]. Chemical Engineering Science, 2013, 95: 246-256. |
| [33] | 尧超群, 陈光文, 袁权. 微通道内气-液两相传质过程行为及其应用[J]. 化工学报, 2019, 70(10): 3635-3644. |
| Yao C Q, Chen G W, Yuan Q. Mass transfer characteristics of gas-liquid two-phase flow in microchannels and applications[J]. CIESC Journal, 2019, 70(10): 3635-3644. | |
| [34] | Yao C Q, Zhao Y C, Zheng J, et al. The effect of liquid viscosity and modeling of mass transfer in gas-liquid slug flow in a rectangular microchannel[J]. AIChE Journal, 2020, 66(5): e16934. |
| [35] | Powell R E, Roseveare W E, Eyring H. Diffusion, thermal conductivity, and viscous flow of liquids[J]. Industrial & Engineering Chemistry, 1941, 33(4): 430-435. |
| [36] | Yin Y R, Fu T T, Zhu C Y, et al. Dynamics and mass transfer characteristics of CO2 absorption into MEA/[Bmim] [BF4] aqueous solutions in a microchannel[J]. Separation and Purification Technology, 2019, 210: 541-552. |
| [37] | Kies F K, Benadda B, Otterbein M. Experimental study on mass transfer of a co-current gas-liquid contactor performing under high gas velocities[J]. Chemical Engineering and Processing: Process Intensification, 2004, 43(11): 1389-1395. |
| [38] | Yue J, Chen G W, Yuan Q, et al. Hydrodynamics and mass transfer characteristics in gas-liquid flow through a rectangular microchannel[J]. Chemical Engineering Science, 2007, 62(7): 2096-2108. |
| [39] | Li C F, Zhu C Y, Ma Y G, et al. Experimental study on volumetric mass transfer coefficient of CO2 absorption into MEA aqueous solution in a rectangular microchannel reactor[J]. International Journal of Heat and Mass Transfer, 2014, 78: 1055-1059. |
| [1] | 段浩磊, 陈浩远, 梁坤峰, 王林, 陈彬, 曹勇, 张晨光, 李硕鹏, 朱登宇, 何亚茹, 杨大鹏. 纯电动车热管理系统低GWP工质替代方案性能分析与综合评价[J]. 化工学报, 2025, 76(S1): 54-61. |
| [2] | 任现超, 谷雅秀, 段少斌, 贾文竹, 李汉林. 翅片式椭圆套管蒸发式冷凝器传热传质性能实验研究[J]. 化工学报, 2025, 76(S1): 75-83. |
| [3] | 王俊鹏, 冯佳琪, 张恩搏, 白博峰. 曲折式与阵列式迷宫阀芯结构内流动与空化特性研究[J]. 化工学报, 2025, 76(S1): 93-105. |
| [4] | 苏伟, 赵大海, 金旭, 刘忠彦, 李静, 张小松. 吸湿液滴与混合润湿性表面协同抑霜特性研究[J]. 化工学报, 2025, 76(S1): 140-151. |
| [5] | 燕子腾, 詹飞龙, 丁国良. 空调用套管式分流器结构设计及分流效果验证[J]. 化工学报, 2025, 76(S1): 152-159. |
| [6] | 赵子祥, 段钟弟, 孙浩然, 薛鸿祥. 大温差两相流动诱导水锤冲击的数值模型[J]. 化工学报, 2025, 76(S1): 170-180. |
| [7] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [8] | 何婷, 黄舒阳, 黄坤, 陈利琼. 基于余热利用的天然气化学吸收脱碳-高温热泵耦合流程研究[J]. 化工学报, 2025, 76(S1): 297-308. |
| [9] | 马爱华, 赵帅, 王林, 常明慧. 太阳能吸收制冷循环动态特性仿真方法研究[J]. 化工学报, 2025, 76(S1): 318-325. |
| [10] | 黄琮琪, 邵双全. 液冷数据中心余热驱动的压缩-吸收式制冷系统特性研究[J]. 化工学报, 2025, 76(S1): 326-335. |
| [11] | 何昌秋, 田加猛, 陈义齐, 朱宇琛, 刘鑫, 王海, 王贞涛, 王军锋, 周致富, 陈斌. 电场-宏观结构表面协同强化薄液膜沸腾传热特性[J]. 化工学报, 2025, 76(6): 2589-2602. |
| [12] | 马瑞洁, 黄子轩, 关雪倩, 陈光进, 刘蓓. ZIF-8/DMPU浆液分离C2H6/ CH4混合气研究[J]. 化工学报, 2025, 76(5): 2262-2269. |
| [13] | 陈建兵, 常昊, 高明, 邢兵, 张磊, 刘奇磊. 基于反应模板与分子动力学的胺基相变吸收剂分相预测方法[J]. 化工学报, 2025, 76(5): 2387-2396. |
| [14] | 王光磊, 刘晓玲, 徐震, 李琳. 面向压缩空气储能的气-水直接接触换热特性[J]. 化工学报, 2025, 76(4): 1595-1603. |
| [15] | 吴罗长, 杨泽宇, 颜建国, 朱旭涛, 陈阳, 王子辰. 微小方形通道内近超临界压力二氧化碳流动换热特性实验研究[J]. 化工学报, 2025, 76(4): 1583-1594. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号