化工学报 ›› 2025, Vol. 76 ›› Issue (7): 3521-3530.DOI: 10.11949/0438-1157.20241433
陈佳祥1( ), 周伟1(
), 周伟1( ), 张学伟1, 王丽杰2, 黄玉明1, 于洋1, 孙苗婷1, 李宛静1, 袁骏舒1, 张宏博2, 孟晓晓1, 高继慧1, 赵广播1
), 张学伟1, 王丽杰2, 黄玉明1, 于洋1, 孙苗婷1, 李宛静1, 袁骏舒1, 张宏博2, 孟晓晓1, 高继慧1, 赵广播1
收稿日期:2024-12-11
修回日期:2025-03-28
出版日期:2025-07-25
发布日期:2025-08-13
通讯作者:
周伟
作者简介:陈佳祥(2001—),男,硕士研究生,23S102147@stu.hit.edu.cn
基金资助:
Jiaxiang CHEN1( ), Wei ZHOU1(
), Wei ZHOU1( ), Xuewei ZHANG1, Lijie WANG2, Yuming HUANG1, Yang YU1, Miaoting SUN1, Wanjing LI1, Junshu YUAN1, Hongbo ZHANG2, Xiaoxiao MENG1, Jihui GAO1, Guangbo ZHAO1
), Xuewei ZHANG1, Lijie WANG2, Yuming HUANG1, Yang YU1, Miaoting SUN1, Wanjing LI1, Junshu YUAN1, Hongbo ZHANG2, Xiaoxiao MENG1, Jihui GAO1, Guangbo ZHAO1
Received:2024-12-11
Revised:2025-03-28
Online:2025-07-25
Published:2025-08-13
Contact:
Wei ZHOU
摘要:
可再生能源电解水制氢是未来获取氢能的重要途径。为理解脉冲供电条件下质子交换膜电解水(PEMWE)体系的瞬态响应特性,结合电化学、气液两相流、固体和流体传热模块建立了二维PEMWE模型。数值模拟结果表明:施加脉冲方波电压可产生比恒电位更大的电流密度,在1.75 V、0.2 Hz、50%占空比条件下产氢速率为0.628 ml/(min·cm2);增大电压到2 V、频率降低为0.025 Hz时出现最大产氢速率1.59 ml/(min·cm2);不同电压匹配的最佳产氢频率不同。20%~90%占空比范围的仿真结果表明,50%和60%占空比的产氢速率比恒电位高,最佳占空比为50%。改变输入的脉冲电压波形,发现三角波的产氢速率最低,这可能与三角波的有效电解时间较短有关。
中图分类号:
陈佳祥, 周伟, 张学伟, 王丽杰, 黄玉明, 于洋, 孙苗婷, 李宛静, 袁骏舒, 张宏博, 孟晓晓, 高继慧, 赵广播. 脉冲电压下二维PEMWE模型的制氢特性仿真研究[J]. 化工学报, 2025, 76(7): 3521-3530.
Jiaxiang CHEN, Wei ZHOU, Xuewei ZHANG, Lijie WANG, Yuming HUANG, Yang YU, Miaoting SUN, Wanjing LI, Junshu YUAN, Hongbo ZHANG, Xiaoxiao MENG, Jihui GAO, Guangbo ZHAO. Simulation study on the hydrogen production performance of a two-dimensional PEMWE model under pulsed voltage[J]. CIESC Journal, 2025, 76(7): 3521-3530.
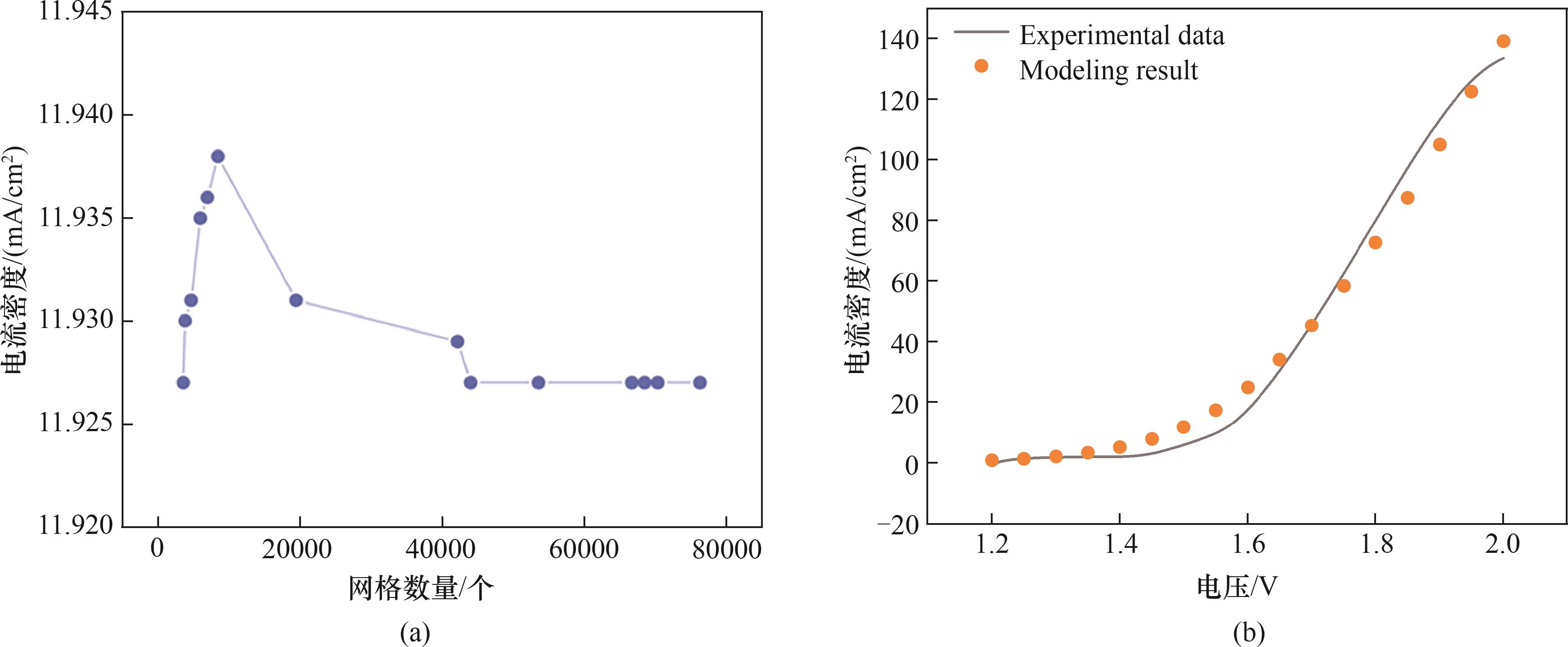
图2 网格无关性和模型验证:(a)网格独立性检验;(b)模拟和实验结果的极化曲线
Fig.2 Grid-independence and model validation: (a) grid-independence test; (b) polarization curves for simulated and experimental results
| 方程 | 表面 | 表达 |
|---|---|---|
| 电荷守恒 | ACL CCL | 电势:Ecell 电接地:0 V |
| 质量和动量守恒 | ACH的入口 CCH的出口 | 入口速度:0.2 m/s 出口压力:1 atm |
| 能量守恒 | ACH的入口 CCH的出口 | 恒温入口:293 K 流出:-nq=0 |
| 相传输守恒 | ACH的入口 CCH的出口 | 气体体积分数:0 自然对流:20℃ |
表1 主要守恒方程的边界条件
Table 1 Boundary conditions for the main conservation equations
| 方程 | 表面 | 表达 |
|---|---|---|
| 电荷守恒 | ACL CCL | 电势:Ecell 电接地:0 V |
| 质量和动量守恒 | ACH的入口 CCH的出口 | 入口速度:0.2 m/s 出口压力:1 atm |
| 能量守恒 | ACH的入口 CCH的出口 | 恒温入口:293 K 流出:-nq=0 |
| 相传输守恒 | ACH的入口 CCH的出口 | 气体体积分数:0 自然对流:20℃ |
| 参数 | 数值 |
|---|---|
| 阳极/阴极传递系数 | 0.027/0.5 |
| 膜的比定压热容[ | 1090 |
| 阳极/阴极反应活化能[ | 72997/16000 |
| 孔隙率(APTL/CLs/CPTL) | 0.65/0.25/0.78 |
| 阳极/阴极参考交换电流密度/(A/m2) | 0.227/10000 |
| 膜的导热率[ | 0.21 |
| 渗透率[ | |
| 出口压力/Pa | |
| 入口速度/(m/s) | 0.2 |
| 膜的密度[ | 1980 |
| 电导率[ | 2000/1250/1000/1000 |
| 对流传热系数[ | 25 |
| 接触角[ | 80/120/100 |
| 进水温度/K | 293.15 |
表2 PEMWE的模拟参数和物理参数
Table 2 Simulation and physical parameters of PEMWE
| 参数 | 数值 |
|---|---|
| 阳极/阴极传递系数 | 0.027/0.5 |
| 膜的比定压热容[ | 1090 |
| 阳极/阴极反应活化能[ | 72997/16000 |
| 孔隙率(APTL/CLs/CPTL) | 0.65/0.25/0.78 |
| 阳极/阴极参考交换电流密度/(A/m2) | 0.227/10000 |
| 膜的导热率[ | 0.21 |
| 渗透率[ | |
| 出口压力/Pa | |
| 入口速度/(m/s) | 0.2 |
| 膜的密度[ | 1980 |
| 电导率[ | 2000/1250/1000/1000 |
| 对流传热系数[ | 25 |
| 接触角[ | 80/120/100 |
| 进水温度/K | 293.15 |
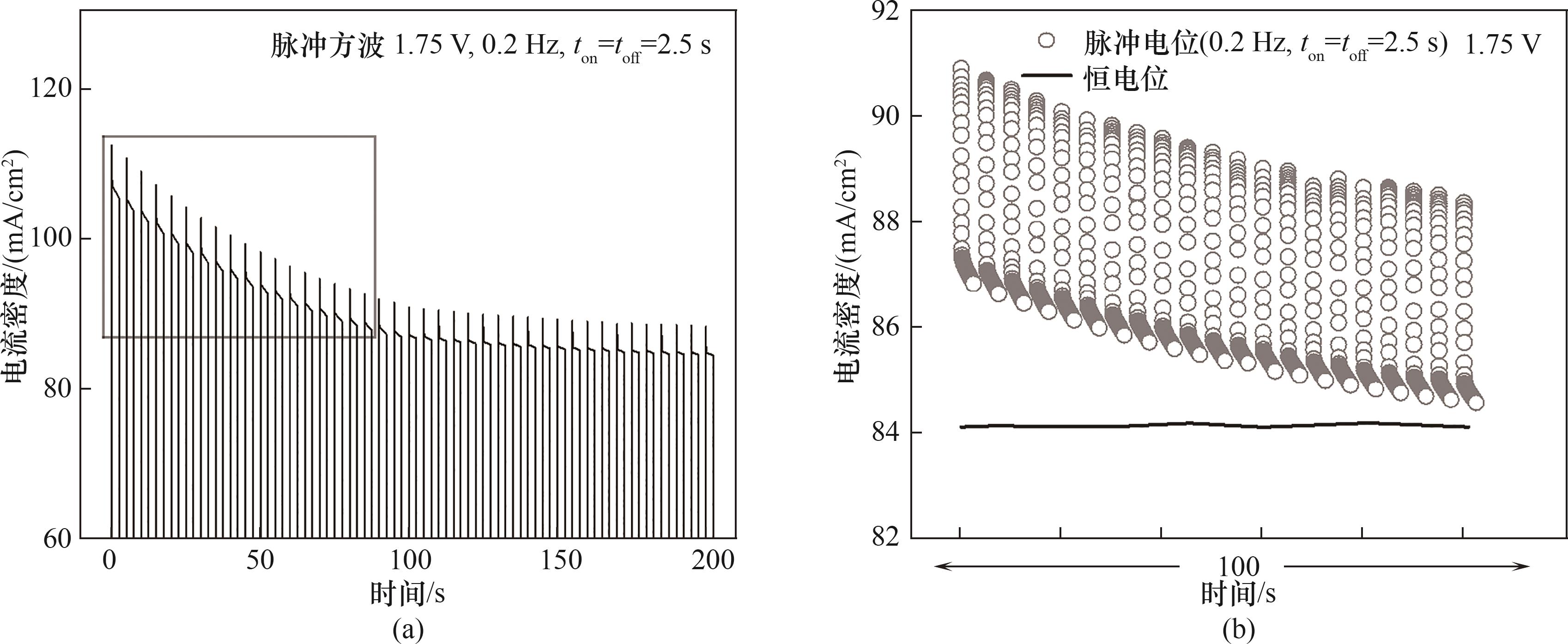
图3 (a)1.75 V、0.2 Hz、50%占空比条件下的电流密度;(b)0.2 Hz脉冲方波与恒电位的电流密度
Fig.3 (a) Current density at 1.75 V, 0.2 Hz, and 50% duty cycle; (b) Current density under a 0.2 Hz pulse square wave and constant potential
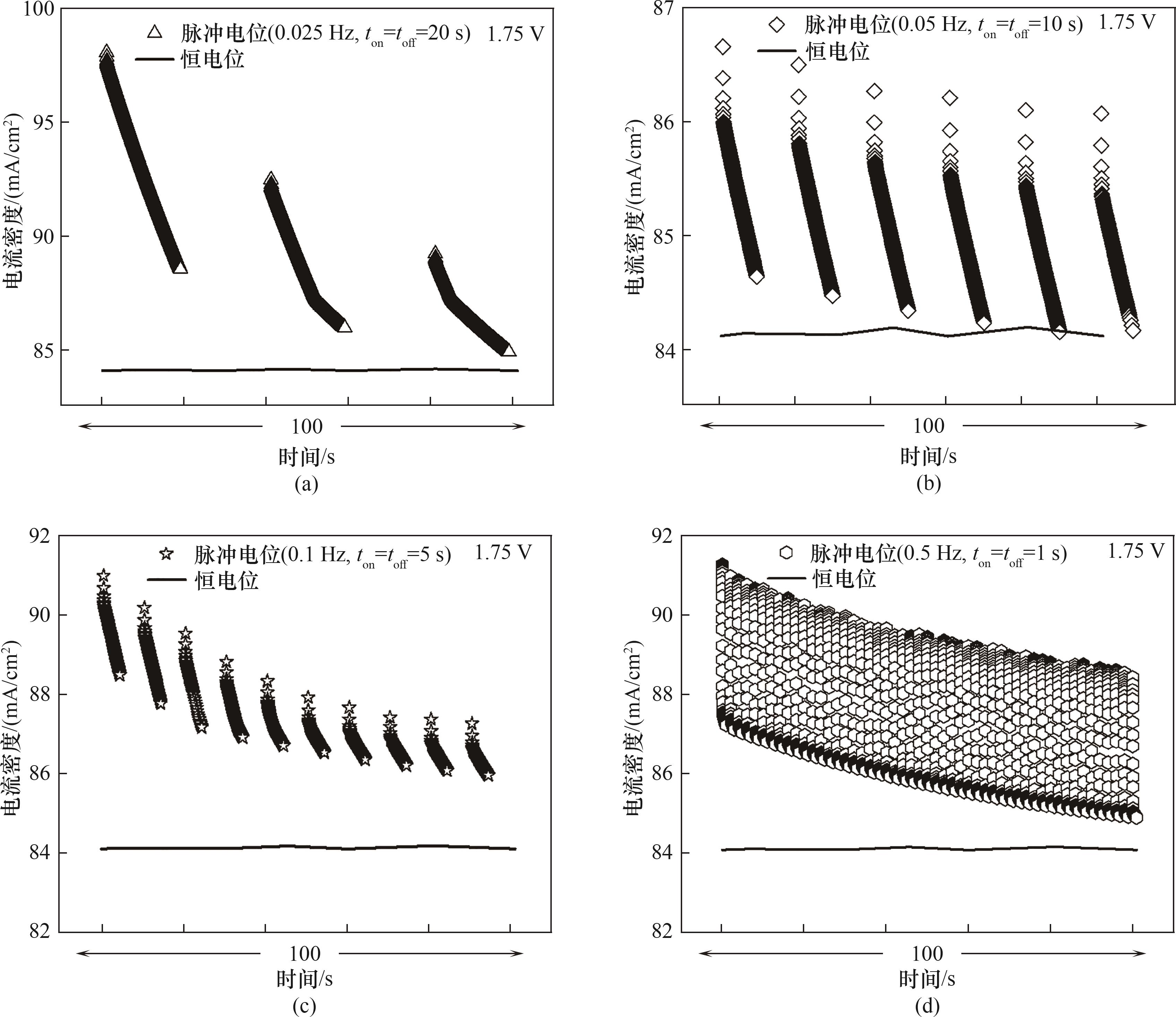
图4 不同频率(0.025、0.05、0.1、0.5 Hz)下方波脉冲与恒电位的电流密度
Fig.4 Current density under square wave pulses at different frequencies (0.025, 0.05, 0.1 and 0.5 Hz) compared to constant potential
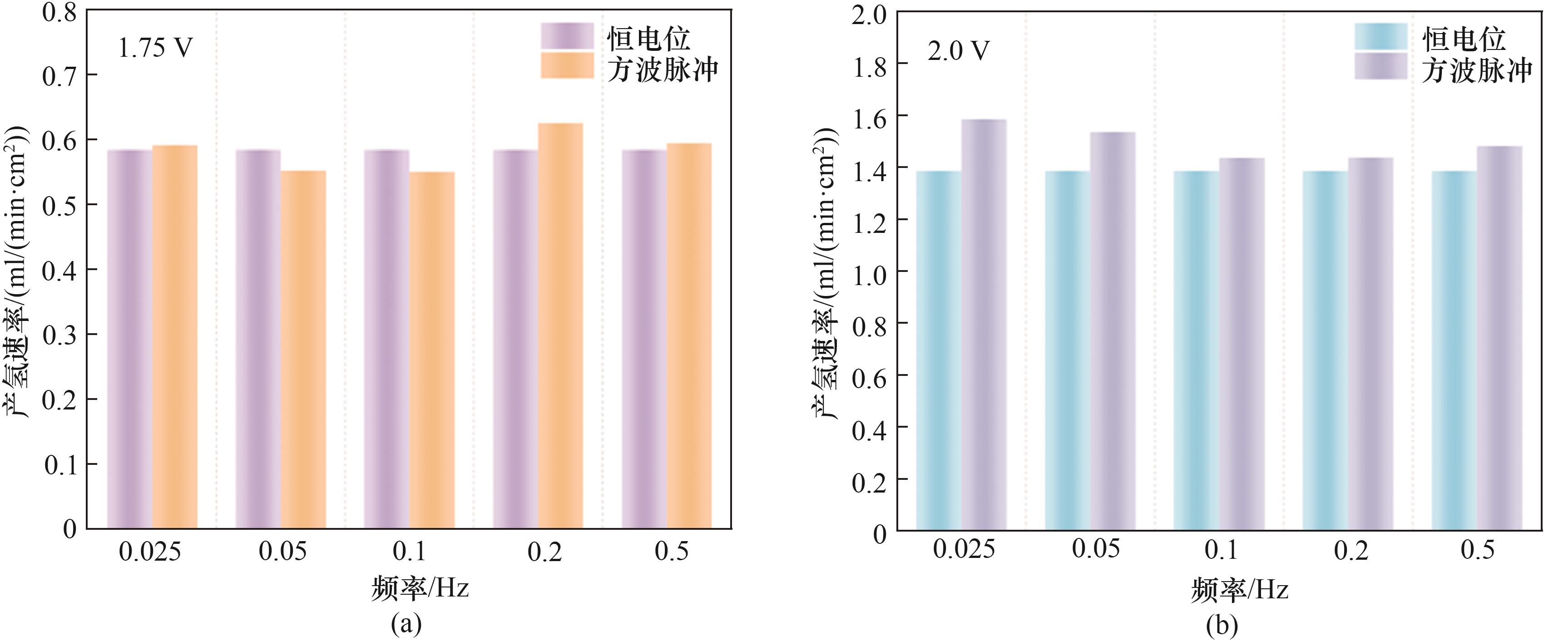
图6 (a)1.75 V、50%占空比时不同频率方波脉冲和恒电位的产氢速率;(b)2.0 V、50%占空比时不同频率方波脉冲和恒电位的产氢速率
Fig.6 (a) Hydrogen evolution rate at 1.75 V with 50% duty cycle for square wave pulses at different frequencies and constant potential; (b) Hydrogen evolution rate at 2.0 V with 50% duty cycle for square wave pulses at different frequencies and constant potential
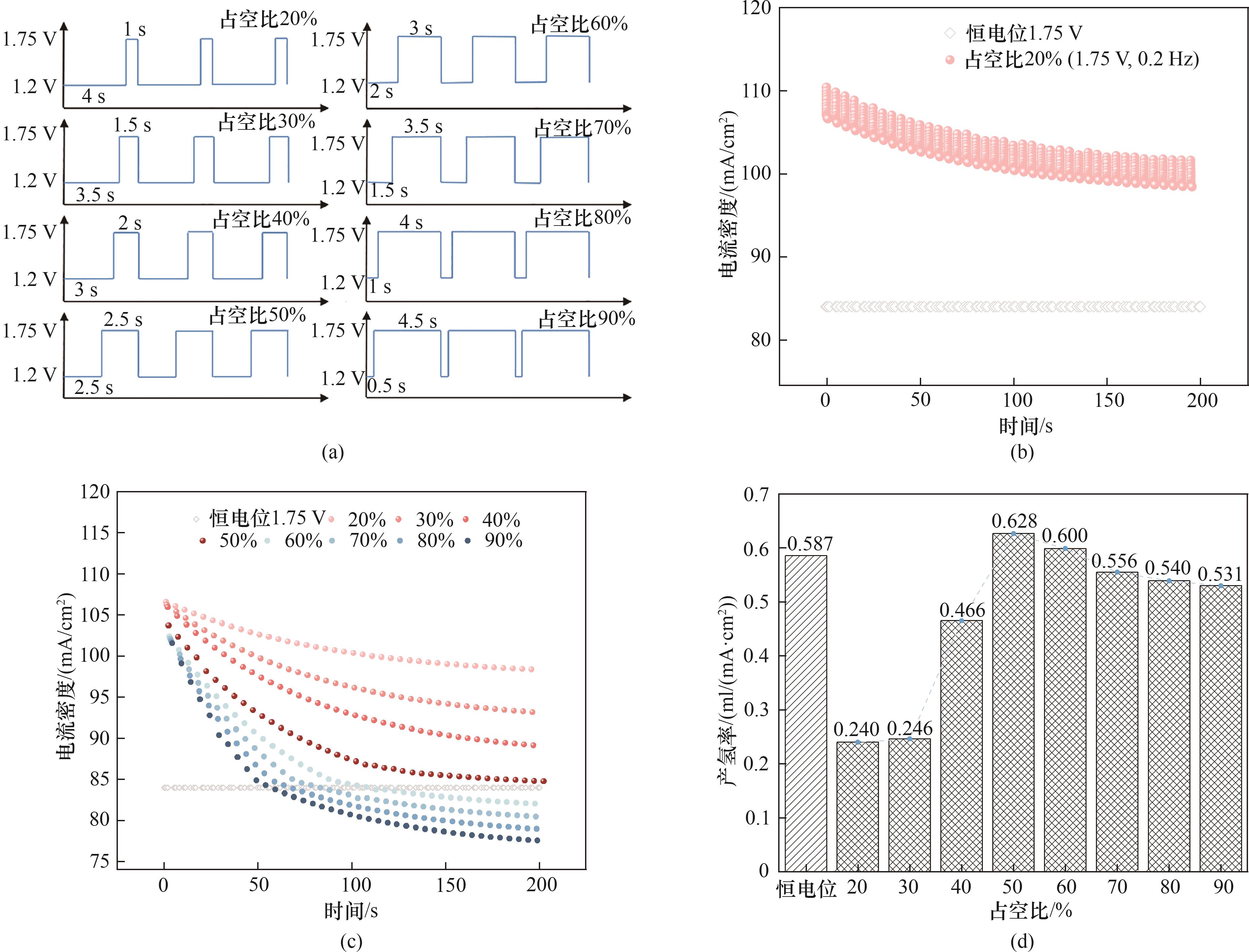
图7 (a) 1.75 V、0.2 Hz时不同占空比的脉冲波形;(b) 1.75 V、0.2 Hz、20%占空比与恒电位的电流密度;(c) 1.75 V、0.2 Hz时不同占空比法拉第电流密度最小值与恒电位的电流密度;(d) 1.75 V、0.2 Hz时不同占空比与恒电位的产氢速率
Fig.7 (a) Pulse waveforms with different duty cycles at 1.75 V and 0.2 Hz; (b) Current density at 1.75 V and 0.2 Hz with 20% duty cycle pulse and constant potential; (c) Minimum current density under different duty cycles at 1.75 V and 0.2 Hz compared to the constant potential current density; (d) Hydrogen evolution rate under different duty cycles at 1.75 V and 0.2 Hz compared to constant potential
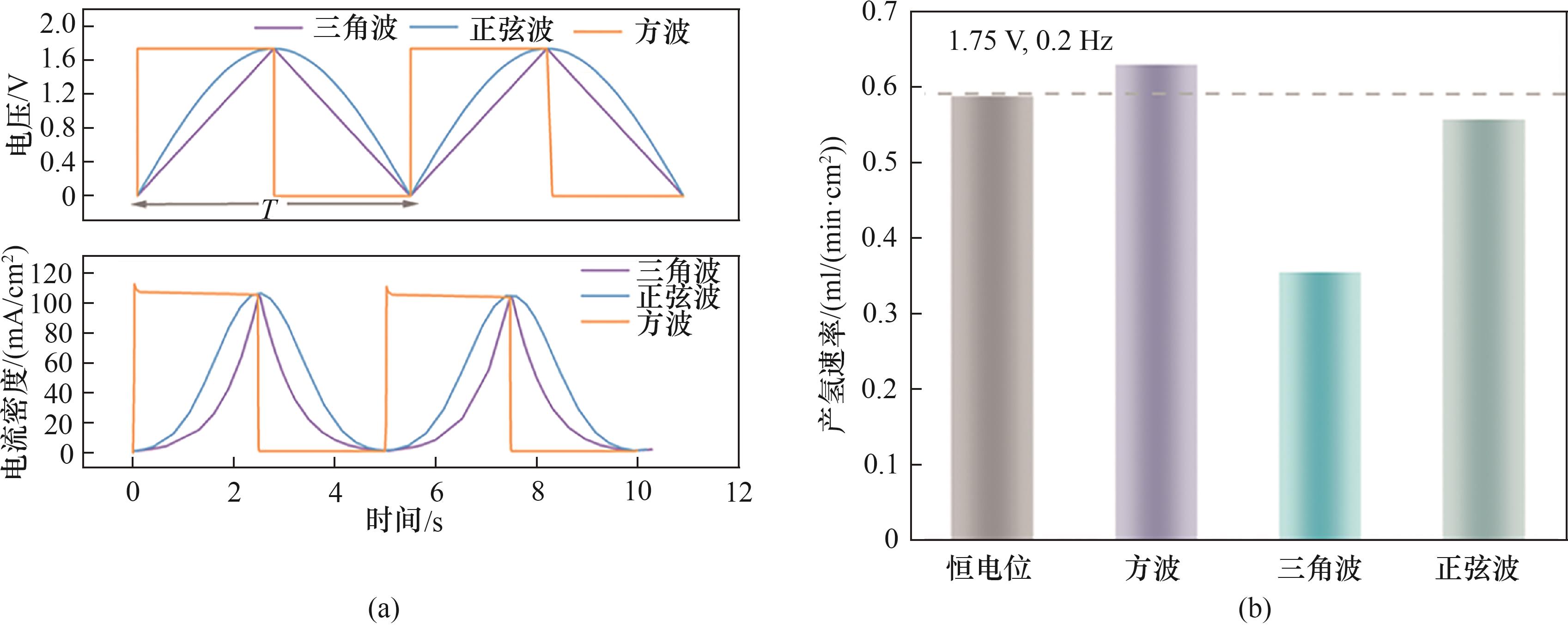
图8 (a) PEMWE中施加的电压波形和响应的电流密度曲线;(b) 1.75 V、0.2 Hz、50%占空比下不同波形的产氢速率
Fig.8 (a) Voltage waveforms applied in PEMWE and the corresponding current density responses; (b) Hydrogen evolution rate under different waveforms at 1.75 V, 0.2 Hz and 50% duty cycle
| [1] | Chong W K, Ng B J, Lee Y J, et al. Self-activated superhydrophilic green ZnIn2S4 realizing solar-driven overall water splitting: close-to-unity stability for a full daytime[J]. Nature Communications, 2023, 14(1): 7676. |
| [2] | Mohideen M M, Subramanian B, Sun J Y, et al. Techno-economic analysis of different shades of renewable and non-renewable energy-based hydrogen for fuel cell electric vehicles[J]. Renewable and Sustainable Energy Reviews, 2023, 174: 113153. |
| [3] | Glenk G, Reichelstein S. Economics of converting renewable power to hydrogen[J]. Nature Energy, 2019, 4(3): 216-222. |
| [4] | Nikolaidis P, Poullikkas A. A comparative overview of hydrogen production processes[J]. Renewable and Sustainable Energy Reviews, 2017, 67: 597-611. |
| [5] | Gu J J, Guo B, Hu S, et al. Experimental studies on dynamic performance of 250-kW alkaline electrolytic system[J]. Journal of Power Sources, 2024, 592: 233920. |
| [6] | Buttler A, Spliethoff H. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: a review[J]. Renewable and Sustainable Energy Reviews, 2018, 82: 2440-2454. |
| [7] | Dang J, Yang F Y, Li Y Y, et al. Experiments and microsimulation of high-pressure single-cell PEM electrolyzer[J]. Applied Energy, 2022, 321: 119351. |
| [8] | Carmo M, Fritz D L, Mergel J, et al. A comprehensive review on PEM water electrolysis[J]. International Journal of Hydrogen Energy, 2013, 38(12): 4901-4934. |
| [9] | Masaud Z, Liu G H, Roseng L E, et al. Progress on pulsed electrocatalysis for sustainable energy and environmental applications[J]. Chemical Engineering Journal, 2023, 475: 145882. |
| [10] | Dharmaraj C H, Adishkumar S. Economical hydrogen production by electrolysis using nano[J]. International Journal of Energy and Environment, 2012, 3(1): 129-136. |
| [11] | Vincent I, Choi B, Nakoji M, et al. Pulsed current water splitting electrochemical cycle for hydrogen production[J]. International Journal of Hydrogen Energy, 2018, 43(22): 10240-10248. |
| [12] | Rocha F, Proost J. Discriminating between the effect of pulse width and duty cycle on the hydrogen generation performance of 3-D electrodes during pulsed water electrolysis[J]. International Journal of Hydrogen Energy, 2021, 46(57): 28925-28935. |
| [13] | Zhang X W, Zhou W, Huang Y M, et al. Enhanced hydrogen production enabled by pulsed potential coupled sulfite electrooxidation water electrolysis system[J]. Renewable Energy, 2024, 227: 120464. |
| [14] | Zhang X W, Zhou W, Huang Y M, et al. Pulsed dynamic electrolysis enhanced PEMWE hydrogen production: revealing the effects of pulsed electric fields on protons mass transport and hydrogen bubble escape[J]. Journal of Energy Chemistry, 2025, 100: 201-214. |
| [15] | Järvinen L, Puranen P, Kosonen A, et al. Automized parametrization of PEM and alkaline water electrolyzer polarisation curves[J]. International Journal of Hydrogen Energy, 2022, 47(75): 31985-32003. |
| [16] | Jiang Y Y, Li Y Y, Ding Y J, et al. Simulation and experiment study on two-phase flow characteristics of proton exchange membrane electrolysis cell[J]. Journal of Power Sources, 2023, 553: 232303. |
| [17] | Toghyani S, Afshari E, Baniasadi E, et al. Thermal and electrochemical performance assessment of a high temperature PEM electrolyzer[J]. Energy, 2018, 152: 237-246. |
| [18] | Eikerling M, Kulikovsky A. Polymer Electrolyte Fuel Cells: Physical Principles of Materials and Operation[M]. London: CRC Press, 2014. |
| [19] | Bard A J, Faulkner L R, White H S. Electrochemical Methods: Fundamentals and Applications[M]. 3rd ed. Hoboken, NJ: John Wiley & Sons, Ltd., 2022. |
| [20] | Bockris J, Reddy A, Gamboa-Aldeco M. Modern Electrochemistry 2A: Fundamentals of Electrodics[M]. New York: Springer US, 2000. |
| [21] | COMSOL Multiphysics Reference Manual. Version COMSOL 6.2 [CP/OL]. 2023. |
| [22] | Pasaogullari U, Wang C Y. Two-phase modeling and flooding prediction of polymer electrolyte fuel cells[J]. Journal of the Electrochemical Society, 2005, 152(2): A380. |
| [23] | Qian X, Kim K, Jung S. Multiphase, multidimensional modeling of proton exchange membrane water electrolyzer[J]. Energy Conversion and Management, 2022, 268: 116070. |
| [24] | Ziegler C, Yu H M, Schumacher J O. Two-phase dynamic modeling of PEMFCs and simulation of cyclo-voltammograms[J]. Journal of the Electrochemical Society, 2005, 152(8): A1555. |
| [25] | Lin N, Zausch J. 1D multiphysics modelling of PEM water electrolysis anodes with porous transport layers and the membrane[J]. Chemical Engineering Science, 2022, 253: 117600. |
| [26] | Bear J, Bachmat Y. Introduction to Modeling of Transport Phenomena in Porous Media[M]. Dordrecht: Springer Netherlands, 1990. |
| [27] | Rho K H, Na Y, Ha T, et al. Performance analysis of polymer electrolyte membrane water electrolyzer using OpenFOAM®: two-phase flow regime, electrochemical model[J]. Membranes, 2020, 10(12): 441. |
| [28] | Wang Z M, Xu C, Wang X Y, et al. Numerical investigation of water and temperature distributions in a proton exchange membrane electrolysis cell[J]. Science China Technological Sciences, 2021, 64(7): 1555-1566. |
| [29] | Zhang G B, Qu Z G, Wang Y. Proton exchange membrane fuel cell of integrated porous bipolar plate-gas diffusion layer structure: entire morphology simulation[J]. eTransportation, 2023, 17: 100250. |
| [30] | Kang Z Y, Alia S M, Young J L, et al. Effects of various parameters of different porous transport layers in proton exchange membrane water electrolysis[J]. Electrochimica Acta, 2020, 354: 136641. |
| [31] | García-Salaberri P A. 1D two-phase, non-isothermal modeling of a proton exchange membrane water electrolyzer: an optimization perspective[J]. Journal of Power Sources, 2022, 521: 230915. |
| [32] | Lv H, Wang S, Sun Y W, et al. Anode catalyst layer with hierarchical pore size distribution for highly efficient proton exchange membrane water electrolysis[J]. Journal of Power Sources, 2023, 564: 232878. |
| [33] | Lopata J S, Weidner J W, Cho H S, et al. Adjusting porous media properties to enhance the gas-phase OER for PEM water electrolysis in 3D simulations[J]. Electrochimica Acta, 2022, 424: 140625. |
| [1] | 赵子祥, 段钟弟, 孙浩然, 薛鸿祥. 大温差两相流动诱导水锤冲击的数值模型[J]. 化工学报, 2025, 76(S1): 170-180. |
| [2] | 黄灏, 王文, 贺隆坤. LNG船薄膜型液货舱预冷过程模拟与分析[J]. 化工学报, 2025, 76(S1): 187-194. |
| [3] | 汪思远, 刘国强, 熊通, 晏刚. 窗式空调器轴流风机的风速非均匀分布特性及其对冷凝器流路优化设计的影响规律[J]. 化工学报, 2025, 76(S1): 205-216. |
| [4] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [5] | 孙九春, 桑运龙, 王海涛, 贾浩, 朱艳. 泥水盾构仓体内射流对泥浆输送特性影响研究[J]. 化工学报, 2025, 76(S1): 246-257. |
| [6] | 何婷, 黄舒阳, 黄坤, 陈利琼. 基于余热利用的天然气化学吸收脱碳-高温热泵耦合流程研究[J]. 化工学报, 2025, 76(S1): 297-308. |
| [7] | 马爱华, 赵帅, 王林, 常明慧. 太阳能吸收制冷循环动态特性仿真方法研究[J]. 化工学报, 2025, 76(S1): 318-325. |
| [8] | 段浩磊, 陈浩远, 梁坤峰, 王林, 陈彬, 曹勇, 张晨光, 李硕鹏, 朱登宇, 何亚茹, 杨大鹏. 纯电动车热管理系统低GWP工质替代方案性能分析与综合评价[J]. 化工学报, 2025, 76(S1): 54-61. |
| [9] | 王俊鹏, 冯佳琪, 张恩搏, 白博峰. 曲折式与阵列式迷宫阀芯结构内流动与空化特性研究[J]. 化工学报, 2025, 76(S1): 93-105. |
| [10] | 吴天灏, 叶霆威, 林延, 黄振. 生物质化学链气化原位补氢制H2/CO可控合成气[J]. 化工学报, 2025, 76(7): 3498-3508. |
| [11] | 陈培强, 郑群, 姜玉廷, 熊春华, 陈今茂, 王旭东, 黄龙, 阮曼, 徐万里. 电液流量及电流密度对海水激活电池输出特性的影响[J]. 化工学报, 2025, 76(7): 3235-3245. |
| [12] | 龚宇, 王胜利, 孙金菊, 海阔, 黄文. 微型多级压缩机充气系统的热力学模型及规律探究[J]. 化工学报, 2025, 76(7): 3626-3638. |
| [13] | 娄嘉诚, 常福城, 刘也铭, 李志斌, 李熙, 李会雄. 1000 MW超超临界直流锅炉水冷壁瞬态响应特性的建模与仿真研究[J]. 化工学报, 2025, 76(6): 2638-2651. |
| [14] | 向晓彤, 段旭东, 王斯民. 多目标优化驱动的PEM电解槽性能研究[J]. 化工学报, 2025, 76(6): 2626-2637. |
| [15] | 廖鹏伟, 刘庆辉, 潘安, 王嘉岳, 符小贵, 杨思宇, 余皓. 考虑不确定性的风电制氢系统:多时间尺度运行策略[J]. 化工学报, 2025, 76(6): 2743-2754. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号