化工学报 ›› 2025, Vol. 76 ›› Issue (9): 4944-4959.DOI: 10.11949/0438-1157.20250226
• 能源和环境工程 • 上一篇
张茹1( ), 朱传强2(
), 朱传强2( ), 张栋1, 黄政1, 肖雨果1, 李明1, 李长明1(
), 张栋1, 黄政1, 肖雨果1, 李明1, 李长明1( )
)
收稿日期:2025-03-06
修回日期:2025-05-06
出版日期:2025-09-25
发布日期:2025-10-23
通讯作者:
朱传强,李长明
作者简介:张茹(2000—),女,硕士研究生,19861902925@163.com
基金资助:
Ru ZHANG1( ), Chuanqiang ZHU2(
), Chuanqiang ZHU2( ), Dong ZHANG1, Zheng HUANG1, Yuguo XIAO1, Ming LI1, Changming LI1(
), Dong ZHANG1, Zheng HUANG1, Yuguo XIAO1, Ming LI1, Changming LI1( )
)
Received:2025-03-06
Revised:2025-05-06
Online:2025-09-25
Published:2025-10-23
Contact:
Chuanqiang ZHU, Changming LI
摘要:
以高分子非催化还原(PNCR)脱硝剂及该工艺应用过程中产生的垃圾焚烧底灰和飞灰为对象,研究PNCR脱硝剂热分解过程氮元素释放与残留特征,表征垃圾焚烧底灰和飞灰结构,揭示PNCR脱硝工艺应用后垃圾焚烧的底灰和飞灰含氮污染物特征,探究底灰和飞灰对氨的吸附行为及热解释放特征。结果表明,PNCR脱硝剂在高温条件下,氮元素几乎完全分解和释放,底灰和飞灰残留含氮污染物含量低于0.5%(质量分数);GCMS和XPS表征结果表明,底灰和飞灰中含氮物质主要以无机氮盐(硝酸盐、铵盐)和有机氮(吡啶类、吡咯类和腈类)形式存在;通过TG-MS和in-situ IR等表征以及直接热脱附实验发现,加热释放的氨含量显著低于其他热脱附气体,热分解产物主要是NO x,并且飞灰与底灰对游离氨吸附残余容量极低。研究结果将为垃圾焚烧行业固废含氮污染物有效处置提供理论支撑,以促进PNCR脱硝工艺推广和应用。
中图分类号:
张茹, 朱传强, 张栋, 黄政, 肖雨果, 李明, 李长明. 采用高分子非催化还原脱硝的垃圾焚烧工艺伴生固废含氮污染物特征研究[J]. 化工学报, 2025, 76(9): 4944-4959.
Ru ZHANG, Chuanqiang ZHU, Dong ZHANG, Zheng HUANG, Yuguo XIAO, Ming LI, Changming LI. Characterisation of nitrogenous pollutants in solid wastes associated with waste incineration process using polymer non-catalytic reduction denitrification[J]. CIESC Journal, 2025, 76(9): 4944-4959.
| 热解温度/℃ | 质量/g | 工业分析/% | 元素分析/% | |||||
|---|---|---|---|---|---|---|---|---|
| M | A | V | C | H | N | S | ||
| PNCR | 20.0 | 2.00 | 23.84 | 74.15 | 25.78 | 8.98 | 20.34 | 0.19 |
| 200 | 18.9 | 1.13 | 26.64 | 72.23 | 24.12 | 8.37 | 18.47 | 0.25 |
| 400 | 10.3 | 1.07 | 52.39 | 46.54 | 15.32 | 5.42 | 10.55 | 0.14 |
| 600 | 7.6 | 0.77 | 75.76 | 23.47 | 9.54 | 3.33 | 8.78 | 0.24 |
| 700 | 6.1 | 0.87 | 81.33 | 17.80 | 6.21 | 1.89 | 4.32 | 0.34 |
| 800 | 5.3 | 0.96 | 94.87 | 4.17 | 3.67 | 1.12 | 0.49 | 0.27 |
| 900 | 4.5 | 0.35 | 96.46 | 3.19 | 2.64 | 1.42 | 0.46 | 0.21 |
表1 PNCR脱硝剂及其不同热解温度热解残渣样品的工业分析与元素分析
Table 1 Industrial and elemental analysis of PNCR denitrifier and its pyrolysis residue samples at different pyrolysis temperatures
| 热解温度/℃ | 质量/g | 工业分析/% | 元素分析/% | |||||
|---|---|---|---|---|---|---|---|---|
| M | A | V | C | H | N | S | ||
| PNCR | 20.0 | 2.00 | 23.84 | 74.15 | 25.78 | 8.98 | 20.34 | 0.19 |
| 200 | 18.9 | 1.13 | 26.64 | 72.23 | 24.12 | 8.37 | 18.47 | 0.25 |
| 400 | 10.3 | 1.07 | 52.39 | 46.54 | 15.32 | 5.42 | 10.55 | 0.14 |
| 600 | 7.6 | 0.77 | 75.76 | 23.47 | 9.54 | 3.33 | 8.78 | 0.24 |
| 700 | 6.1 | 0.87 | 81.33 | 17.80 | 6.21 | 1.89 | 4.32 | 0.34 |
| 800 | 5.3 | 0.96 | 94.87 | 4.17 | 3.67 | 1.12 | 0.49 | 0.27 |
| 900 | 4.5 | 0.35 | 96.46 | 3.19 | 2.64 | 1.42 | 0.46 | 0.21 |

图4 PNCR脱硝剂在不同热解温度下NH3释放量(a)、CO2释放量(b)、CO2/NH3释放量比值(c)和CO2/NH3峰值浓度比值(d)
Fig.4 NH3 release (a), CO2 release (b), CO2/NH3 release ratio (c) and CO2/NH3 peak concentration ratio (d) from PNCR denitrator at different pyrolysis temperatures
| 样品 | 工业分析/% | 元素分析/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mar | Aar | Var | Car | Har | Nar | Sar | C烧 | H烧 | N烧 | S烧 | |
| 底灰 | 1.81 | 88.60 | 8.52 | 1.57 | 0.84 | 0.16 | 1.15 | 0.17 | 0.68 | 0 | 1.78 |
| 飞灰 | 2.93 | 86.37 | 10.84 | 2.54 | 1.33 | 0.37 | 2.64 | 0.31 | 1.32 | 0.01 | 3.36 |
表2 底灰与飞灰样品的工业分析与元素分析
Table 2 Industrial and elemental analyses of bottom ash and fly ash samples
| 样品 | 工业分析/% | 元素分析/% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mar | Aar | Var | Car | Har | Nar | Sar | C烧 | H烧 | N烧 | S烧 | |
| 底灰 | 1.81 | 88.60 | 8.52 | 1.57 | 0.84 | 0.16 | 1.15 | 0.17 | 0.68 | 0 | 1.78 |
| 飞灰 | 2.93 | 86.37 | 10.84 | 2.54 | 1.33 | 0.37 | 2.64 | 0.31 | 1.32 | 0.01 | 3.36 |
| 样品 | 质量分数/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | SO3 | MgO | TiO2 | K2O | Na2O | |
| 底灰 | 48.46 | 21.43 | 12.62 | 7.67 | 2.02 | 2.21 | 1.14 | 0.83 | 0.82 |
| 飞灰 | 42.46 | 20.13 | 13.35 | 8.37 | 1.94 | 2.73 | 1.06 | 4.41 | 2.73 |
表3 底灰与飞灰样品的成分分析
Table 3 Compositional analysis of bottom ash and fly ash samples
| 样品 | 质量分数/% | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | CaO | Al2O3 | Fe2O3 | SO3 | MgO | TiO2 | K2O | Na2O | |
| 底灰 | 48.46 | 21.43 | 12.62 | 7.67 | 2.02 | 2.21 | 1.14 | 0.83 | 0.82 |
| 飞灰 | 42.46 | 20.13 | 13.35 | 8.37 | 1.94 | 2.73 | 1.06 | 4.41 | 2.73 |
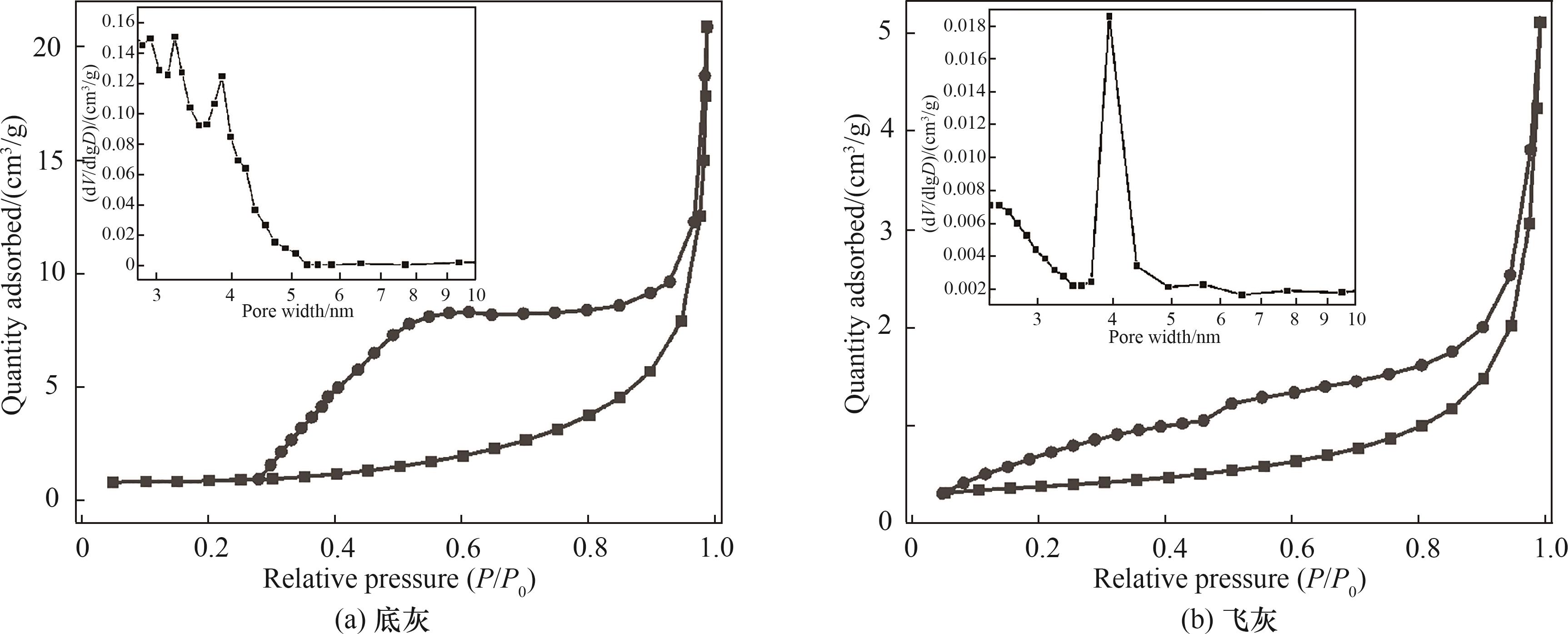
图7 底灰和飞灰样品的N2吸脱附曲线(插图为孔径分布曲线)
Fig.7 N2 adsorption and desorption curves for bottom ash and fly ash samples (Insets show pore size distribution curves)
| 元素峰 | 形态分布 | 相对含量/% | |
|---|---|---|---|
| 底灰 | 飞灰 | ||
| C 1s | 芳香碳和烷烃(C—H、C—C) | 74.79 | 75.99 |
| 碳氧单键碳(C—O) | 11.39 | 14.48 | |
| 羰基碳(C=O) | 4.14 | 1.48 | |
| 羧基碳(COO—) | 9.68 | 1.32 | |
| N 1s | 吡啶氮(N-6) | 17.78 | 10.78 |
| 吡咯氮(N-5) | 29.78 | 36.04 | |
| 铵盐(NH4+) | 17.72 | 25.58 | |
| 硝酸盐(NO3-) | 34.72 | 27.6 | |
| S 2p | 亚砜型硫(R—S=O—R') | 10.92 | 13.72 |
| 硫酸盐(SO4—S) | 89.08 | 86.28 | |
表4 样品表面基团的组成
Table 4 Composition of surface groups of the samples
| 元素峰 | 形态分布 | 相对含量/% | |
|---|---|---|---|
| 底灰 | 飞灰 | ||
| C 1s | 芳香碳和烷烃(C—H、C—C) | 74.79 | 75.99 |
| 碳氧单键碳(C—O) | 11.39 | 14.48 | |
| 羰基碳(C=O) | 4.14 | 1.48 | |
| 羧基碳(COO—) | 9.68 | 1.32 | |
| N 1s | 吡啶氮(N-6) | 17.78 | 10.78 |
| 吡咯氮(N-5) | 29.78 | 36.04 | |
| 铵盐(NH4+) | 17.72 | 25.58 | |
| 硝酸盐(NO3-) | 34.72 | 27.6 | |
| S 2p | 亚砜型硫(R—S=O—R') | 10.92 | 13.72 |
| 硫酸盐(SO4—S) | 89.08 | 86.28 | |
| 序号 | 化合物名称 | 分子式 | 分子量 | 底灰 | 飞灰 |
|---|---|---|---|---|---|
| 1 | 2-吡咯烷酮 | C4H7NO | 85 | √ | √ |
| 2 | 1-乙烯基-2-吡咯烷酮 | C6H9NO | 111 | √ | √ |
| 3 | 十八烷腈 | C18H35N | 265 | √ | √ |
| 4 | 十六腈 | C16H31N | 237 | × | √ |
| 5 | 芥酸酰胺 | C22H43NO | 338 | × | √ |
| 6 | 2,4-二叔丁基酚 | C14H22O | 206 | √ | × |
| 7 | (Z)-十八-9-烯醇 | C18H36O | 268 | √ | × |
| 8 | 6-叔丁基对甲酚 | C23H32O2 | 340 | √ | × |
| 9 | 十五醇 | C15H32O | 228 | × | √ |
| 10 | 1,16-十六烷二醇 | C16H34O2 | 258 | × | √ |
| 11 | 十六醛 | C16H32O | 240 | × | √ |
| 12 | 十八烷醛 | C18H36O | 268 | × | √ |
| 13 | 二碘甲烷 | CH2I2 | 268 | √ | √ |
| 14 | 十二烷 | C12H26 | 170 | √ | √ |
| 15 | 1,3-二叔丁基苯 | C14H22 | 190 | √ | √ |
| 16 | 正十五烷 | C15H32 | 212 | √ | √ |
| 17 | 十四烷 | C14H30 | 198 | √ | √ |
| 18 | 氯代十六烷 | C16H33Cl | 261 | √ | × |
| 19 | 正十六烷 | C16H34 | 226 | √ | √ |
| 20 | 正十七烷 | C17H36 | 240 | √ | √ |
| 21 | 正二十一烷 | C21H44 | 297 | √ | × |
| 22 | 十八烯 | C18H36 | 252 | √ | × |
| 23 | 正二十四烷 | C24H50 | 339 | √ | √ |
| 24 | 三十烷 | C30H62 | 423 | √ | × |
| 25 | 2,4-二甲基庚烷 | C9H20 | 128 | × | √ |
| 26 | 乙基苯 | C8H10 | 106 | × | √ |
| 27 | 间二甲苯 | C8H10 | 106 | × | √ |
| 28 | 二甲硫基甲烷 | C3H8S2 | 108 | × | √ |
| 29 | 2,2-二甲基戊烷 | C7H16 | 100 | × | √ |
| 30 | 3-乙基辛烷 | C10H22 | 142 | × | √ |
| 31 | (+)-柠檬烯 | C10H16 | 136 | × | √ |
| 32 | 萘 | C10H8 | 128 | × | √ |
| 33 | 十二烷 | C12H26 | 170 | × | √ |
| 34 | 5-甲基十一烷 | C12H26 | 170 | × | √ |
| 35 | 2,6-二甲基十一烷 | C13H28 | 184 | × | √ |
| 36 | 3,3-二甲基已烷 | C8H18 | 114 | × | √ |
| 37 | 碘十一烷 | C11H23I | 282 | × | √ |
| 38 | 正二十烷 | C20H42 | 283 | × | √ |
| 39 | 正十八烷 | C18H38 | 254 | × | √ |
| 40 | 正二十烷 | C20H42 | 283 | × | √ |
表5 飞灰和底灰中有机物相应组分的种类与名称
Table 5 Types and names of corresponding components of organic matter in fly ash and bottom ash
| 序号 | 化合物名称 | 分子式 | 分子量 | 底灰 | 飞灰 |
|---|---|---|---|---|---|
| 1 | 2-吡咯烷酮 | C4H7NO | 85 | √ | √ |
| 2 | 1-乙烯基-2-吡咯烷酮 | C6H9NO | 111 | √ | √ |
| 3 | 十八烷腈 | C18H35N | 265 | √ | √ |
| 4 | 十六腈 | C16H31N | 237 | × | √ |
| 5 | 芥酸酰胺 | C22H43NO | 338 | × | √ |
| 6 | 2,4-二叔丁基酚 | C14H22O | 206 | √ | × |
| 7 | (Z)-十八-9-烯醇 | C18H36O | 268 | √ | × |
| 8 | 6-叔丁基对甲酚 | C23H32O2 | 340 | √ | × |
| 9 | 十五醇 | C15H32O | 228 | × | √ |
| 10 | 1,16-十六烷二醇 | C16H34O2 | 258 | × | √ |
| 11 | 十六醛 | C16H32O | 240 | × | √ |
| 12 | 十八烷醛 | C18H36O | 268 | × | √ |
| 13 | 二碘甲烷 | CH2I2 | 268 | √ | √ |
| 14 | 十二烷 | C12H26 | 170 | √ | √ |
| 15 | 1,3-二叔丁基苯 | C14H22 | 190 | √ | √ |
| 16 | 正十五烷 | C15H32 | 212 | √ | √ |
| 17 | 十四烷 | C14H30 | 198 | √ | √ |
| 18 | 氯代十六烷 | C16H33Cl | 261 | √ | × |
| 19 | 正十六烷 | C16H34 | 226 | √ | √ |
| 20 | 正十七烷 | C17H36 | 240 | √ | √ |
| 21 | 正二十一烷 | C21H44 | 297 | √ | × |
| 22 | 十八烯 | C18H36 | 252 | √ | × |
| 23 | 正二十四烷 | C24H50 | 339 | √ | √ |
| 24 | 三十烷 | C30H62 | 423 | √ | × |
| 25 | 2,4-二甲基庚烷 | C9H20 | 128 | × | √ |
| 26 | 乙基苯 | C8H10 | 106 | × | √ |
| 27 | 间二甲苯 | C8H10 | 106 | × | √ |
| 28 | 二甲硫基甲烷 | C3H8S2 | 108 | × | √ |
| 29 | 2,2-二甲基戊烷 | C7H16 | 100 | × | √ |
| 30 | 3-乙基辛烷 | C10H22 | 142 | × | √ |
| 31 | (+)-柠檬烯 | C10H16 | 136 | × | √ |
| 32 | 萘 | C10H8 | 128 | × | √ |
| 33 | 十二烷 | C12H26 | 170 | × | √ |
| 34 | 5-甲基十一烷 | C12H26 | 170 | × | √ |
| 35 | 2,6-二甲基十一烷 | C13H28 | 184 | × | √ |
| 36 | 3,3-二甲基已烷 | C8H18 | 114 | × | √ |
| 37 | 碘十一烷 | C11H23I | 282 | × | √ |
| 38 | 正二十烷 | C20H42 | 283 | × | √ |
| 39 | 正十八烷 | C18H38 | 254 | × | √ |
| 40 | 正二十烷 | C20H42 | 283 | × | √ |
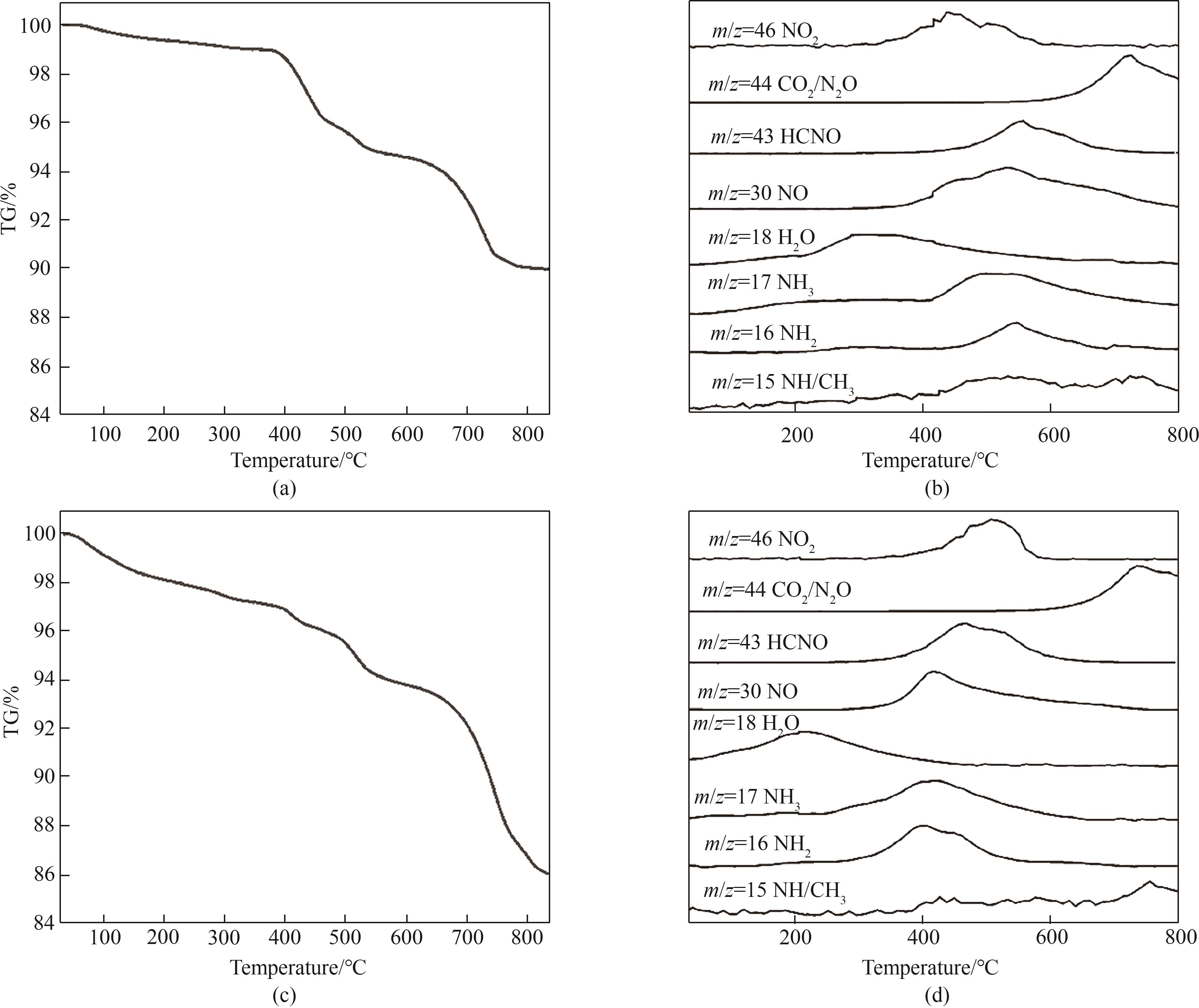
图10 底灰[(a)、(b)]与飞灰[(c)、(d)]样品TG曲线及TG-MS分解产物质谱
Fig.10 TG curves and TG-MS mass spectra of decomposition products of bottom ash [(a), (b)] and fly ash [(c), (d)] samples
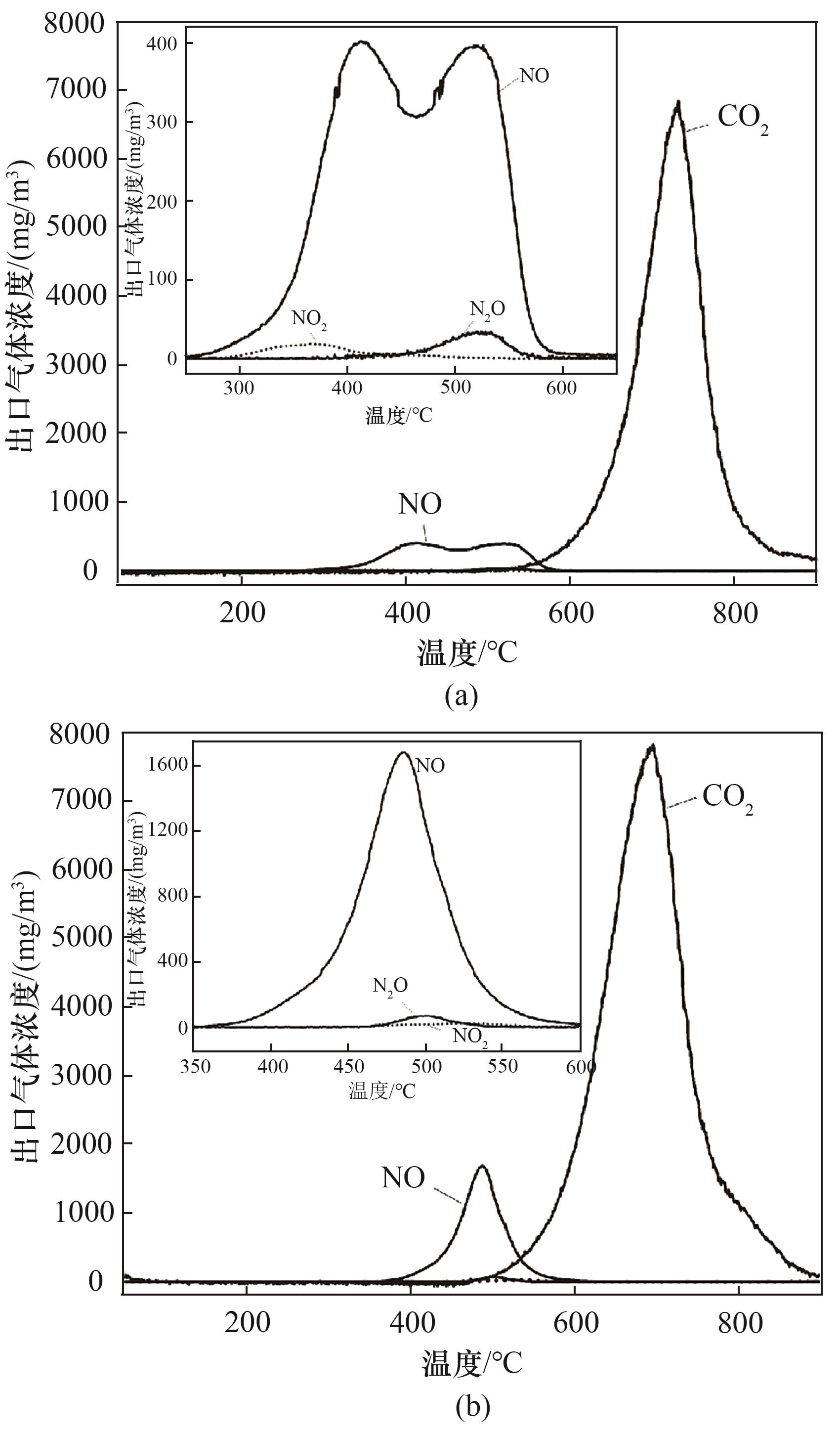
图12 底灰(a)与飞灰(b)样品程序升温过程中NO、NO2、N2O、CO2释放曲线
Fig.12 NO, NO2, N2O and CO2 release curves during the programmed warming of bottom ash (a) and fly ash (b) samples
| [1] | 张焕亨. PNCR脱硝技术及其试验研究[J]. 锅炉技术, 2021, 52(4): 65-68. |
| Zhang H H. PNCR denitration technology and its experimental research[J]. Boiler Technology, 2021, 52(4): 65-68. | |
| [2] | 王沛, 杨婷婷, 王常春, 等. 垃圾焚烧电厂高分子非催化还原(PNCR)脱硝技术的应用[J]. 江西化工, 2022, 38(6): 109-112. |
| Wang P, Yang T T, Wang C C, et al. Application of polymer non catalytic reduction (PNCR) denitrification technology in waste incineration power plant[J]. Jiangxi Chemical Industry, 2022, 38(6): 109-112. | |
| [3] | 邓靖, 罗慧, 刘玉坤. 生活垃圾焚烧烟气脱硝技术对比[J]. 节能与环保, 2021(7): 66-68. |
| Deng J, Luo H, Liu Y K. Comparison of DeNO x technology in domestic waste incineration[J]. Energy Conservation & Environmental Protection, 2021(7): 66-68. | |
| [4] | 罗晨, 马素霞, 冯于川, 等. 选择性非催化还原烟气脱硝技术的研究现状与发展[J]. 电力科技与环保, 2024, 40(6): 603-614. |
| Luo C, Ma S X, Feng Y X, et al. Research status and development of flue gas denitrification technology in selective non-catalytic reduction[J]. Electric Power Technology and Environmental Protection, 2024, 40(6): 603-614. | |
| [5] | Baleta J, Mikulčić H, Vujanović M, et al. Numerical simulation of urea based selective non-catalytic reduction deNO x process for industrial applications[J]. Energy Conversion and Management, 2016, 125: 59-69. |
| [6] | Liu C X, Wang H J, Zhang Z Y, et al. The latest research progress of NH3-SCR in the SO2 resistance of the catalyst in low temperatures for selective catalytic reduction of NO x [J]. Catalysts, 2020, 10(9): 1034. |
| [7] | 朱传强, 茹晋波, 孙亭亭, 等. 固体高分子脱硝剂选择性非催化还原NO x 特性[J]. 化工进展, 2023, 42(9): 4939-4946. |
| Zhu C Q, Ru J B, Sun T T, et al. Characteristics of selective non-catalytic reduction of NO x with solid polymer denitration agent[J]. Chemical Industry and Engineering Progress, 2023, 42(9): 4939-4946. | |
| [8] | 刘超, 施艇, 瞿国栋. PNCR脱硝剂的研发与性能测试及联合脱硝工艺研究[J]. 造纸装备及材料, 2023, 52(8): 115-117. |
| Liu C, Shi T, Qu G D. Development and performance test of PNCR denitrifying agent and study on combined denitrifying process[J]. Papermaking Equipment & Materials, 2023, 52(8): 115-117. | |
| [9] | 杨进, 向植刚, 李欣华. SNCR+PNCR工艺在燃煤热风炉烟气脱硝中的应用[J]. 广东化工, 2020, 47(6): 182-184. |
| Yang J, Xiang Z G, Li X H. Application of SNCR + PNCR process in flue gas denitrification of coal-fired hot blast furnace[J]. Guangdong Chemical Industry, 2020, 47(6): 182-184. | |
| [10] | 刘焕联, 庞博. 垃圾焚烧烟气脱硝工艺选择及案例分析[J]. 环境卫生工程, 2018, 26(6): 19-22. |
| Liu H L, Pang B. Process selection and case analysis on denitration of waste incineration flue gas[J]. Environmental Sanitation Engineering, 2018, 26(6): 19-22. | |
| [11] | 袁伯若, 程虎. 垃圾焚烧烟气超低排放全流程工艺选择[J]. 有色冶金节能, 2021, 37(5): 1-4, 12. |
| Yuan B R, Cheng H. Whole process selection of ultra-low off-gas emission of waste incineration[J]. Energy Saving of Nonferrous Metallurgy, 2021, 37(5): 1-4, 12. | |
| [12] | 朱传强, 茹晋波, 扈明东, 等. 垃圾焚烧电厂高分子非催化还原(PNCR)脱硝技术应用分析[J]. 工程热物理学报, 2021, 42(6): 1600-1607. |
| Zhu C Q, Ru J B, Hu M D, et al. Application analysis of polymer non-catalytic reduction of NO x in waste incineration[J]. Journal of Engineering Thermophysics, 2021, 42(6): 1600-1607. | |
| [13] | Liu W, Wu B B, Bai X X, et al. Migration and emission characteristics of ammonia/ammonium through flue gas cleaning devices in coal-fired power plants of China[J]. Environmental Science & Technology, 2020, 54(1): 390-399. |
| [14] | Zheng C Q, Li X L, Li J Z, et al. Investigation on the ammonia emission characteristics in coal-fired power plants of China[J]. Fuel, 2022, 314: 123046. |
| [15] | 石磊, 牛国平, 马强,等. 燃煤电厂烟气飞灰吸附氨变化规律[J]. 热力发电, 2019, 48(6): 53-57. |
| Shi L, Niu G P, Ma Q, et al. Ammonia adsorption by fly ash in flue gas of a coal-fired power plant[J]. Thermal Power Generation, 2019, 48(6): 53-57. | |
| [16] | 赵宏, 张发捷, 马云龙, 等. 燃煤电厂SCR脱硝氨逃逸迁移规律试验研究[J]. 中国电力, 2021, 54(1): 196-202. |
| Zhao H, Zhang F J, Ma Y L, et al. Test study on the migration characteristics of slip ammonia from the SCR system in the coal-fired power plant[J]. Electric Power, 2021, 54(1): 196-202. | |
| [17] | Cheng T, Zheng C Q, Yang L J, et al. Effect of selective catalytic reduction denitrification on fine particulate matter emission characteristics[J]. Fuel, 2019, 238: 18-25. |
| [18] | Bao J, Mao L, Zhang Y H, et al. Effect of selective catalytic reduction system on fine particle emission characteristics[J]. Energy & Fuels, 2016, 30(2): 1325-1334. |
| [19] | Zhu C Q, Li C M, Zhao Z C, et al. The reaction characteristics and mechanism of polymer non-catalytic reduction (PNCR) for NO x removal[J]. Fuel Processing Technology, 2023, 252: 108002. |
| [20] | Xiao S, Li C B, Zheng X Y, et al. Application of a low-cost and high-efficiency polymer non-catalytic reduction technology for NOx removal in waste-to-energy plant[J]. Journal of Environmental Sciences, DOI: 10.1016/j.jes.2025.01.026 . |
| [21] | Tan W F, Wang L A, Huang C, et al. Municipal solid waste incineration fly ash sintered lightweight aggregates and kinetics model establishment[J]. International Journal of Environmental Science and Technology, 2013, 10(3): 465-472. |
| [22] | Liu Z, Li J B, Zhu M M, et al. Investigation into scavenging of sodium and ash deposition characteristics during co-combustion of Zhundong lignite with an oil shale semi-coke of high aluminosilicate in a circulating fluidized bed[J]. Fuel, 2019, 257: 116099. |
| [23] | Xing X Y, Han K X, Liu R J, et al. Study on the rheological properties of fly ash modified asphalt mastics[J]. Coatings, 2023, 13(8): 1307. |
| [24] | 黎永伦, 陈维芳, 王叶贵, 等. 城市垃圾焚烧飞灰物理化学性质及重金属风险分析[J]. 能源研究与信息, 2023, 39(1): 1-8. |
| Li Y L, Chen W F, Wang Y G, et al. Physicochemical properties and risk assessment of heavy metals in the incinerated municipal solid waste fly ash[J]. Energy Research and Information, 2023, 39(1): 1-8. | |
| [25] | 张亚朋, 崔龙鹏, 刘艳芳, 等. 3种典型工业固废的CO2矿化封存性能[J]. 环境工程学报, 2021, 15(7): 2344-2355. |
| Zhang Y P, Cui L P, Liu Y F, et al. Comparison of three typical industrial solid wastes on the performance of CO2 mineralization and sequestration[J]. Chinese Journal of Environmental Engineering, 2021, 15(7): 2344-2355. | |
| [26] | 彭磊, 陈兵. 基于同步辐射小角X射线散射和液氮吸附所测分维计算高庙子膨润土膨胀变形[J]. 岩土力学, 2020, 41(8): 2712-2721. |
| Peng L, Chen B. Calculation of swelling deformation of Gaomiaozi bentonite based on fractal dimension measured by synchrotron radiation SAXS and liquid nitrogen adsorption[J]. Rock and Soil Mechanics, 2020, 41(8): 2712-2721. | |
| [27] | 曹潘飞, 吴林. 山西阳煤二矿无烟煤元素赋存特征的XPS研究[J]. 中国矿业, 2022, 31(S1): 198-202. |
| Cao P F, Wu L. XPS study on the occurrence characteristies of anthracite elementsin Yangmei No. 2 Mine, Shanxi Province[J]. China Mining Magazine, 2022, 31(S1): 198-202. | |
| [28] | Dwivedi A, Dwivedi A, Kumar A. Qualitative surface characterization of Indian Permian coal using XPS and FTIR[J]. International Journal of Coal Preparation and Utilization, 2023, 43(7): 1152-1163. |
| [29] | Chen G Y, Li J T, Li K, et al. Nitrogen, sulfur, chlorine containing pollutants releasing characteristics during pyrolysis and combustion of oily sludge[J]. Fuel, 2020, 273: 117772. |
| [30] | Liu W J, Shao Z G, Xu Y. Emission characteristics of nitrogen and sulfur containing pollutants during the pyrolysis of oily sludge with and without catalysis[J]. Journal of Hazardous Materials, 2021, 401: 123820. |
| [31] | 贾进章, 邢迎欢, 李斌. 山西阳煤无烟煤分子结构特征分析[J]. 化学研究与应用, 2022, 34(10): 2311-2320. |
| Jia J Z, Xing Y H, Li B. Analysis of molecular structure characteristics of anthracite in Shanxi Yangquan coal[J]. Chemical Research and Applications, 2022, 34(10): 2311-2320. | |
| [32] | Lin B C, Alhadj Mallah M M, Huang Q X, et al. Effects of temperature and potassium compounds on the transformation behavior of sulfur during pyrolysis of oily sludge[J]. Energy & Fuels, 2017, 31(7): 7004-7014. |
| [33] | 王学军, 庄心生, 齐辉. 薄膜爽滑剂芥酸酰胺研究进展[J]. 塑料工业, 2022, 50(7): 1-5, 11. |
| Wang X J, Zhuang X S, Qi H. Research progress on erucamide as film slip agent[J]. Plastics Industry, 2022, 50(7): 1-5, 11. | |
| [34] | 谷沁洋. 城市生活垃圾焚烧飞灰碳酸化固化特性研究[D]. 南京: 东南大学, 2022. |
| Gu Q Y. Study on the carbonation and solidification characteristics of fly ash from municipal waste incineration [D]. Nanjing: Southeast University, 2022. | |
| [35] | 黄一萌, 马晓春, 张海洲, 等. Ni0.09Ti0.91O2纳米管负载铜的催化脱硝性能和机理[J]. 稀有金属材料与工程, 2024, 53(5): 1417-1428. |
| Huang Y M, Ma X C, Zhang H Z, et al. Catalytic denitration performance and mechanism of copper-loaded Ni0.09Ti0.91O2 nanotube[J]. Rare Metal Materials and Engineering, 2024, 53(5): 1417-1428. | |
| [36] | Li C M, Zeng H, Liu P L, et al. The recycle of red mud as excellent SCR catalyst for removal of NO x [J]. RSC Advances, 2017, 7(84): 53622-53630. |
| [37] | Zheng J F, Wang J, Yang F L, et al. Adsorption and catalytic oxidation of residual NH3 on coal ash after selective non-catalytic reduction in coal-fired boilers[J]. Chemosphere, 2023, 317: 137765. |
| [38] | Zheng J F, Wang J, Yang F L, et al. Influence and mechanism of the adsorption and reactions of residual NH3, NO, and O2 on coal ash after the selective noncatalytic reduction process[J]. Fuel, 2023, 343: 127826. |
| [1] | 史松伟, 赵诚, 刘帅, 应雨轩, 严密. 富铁飞灰耦合Fe-Zn/Al2O3脱除沼气H2S研究[J]. 化工学报, 2025, 76(8): 4239-4247. |
| [2] | 王小令, 王绍清, 赵云刚, 常方哲, 穆瑞峰. 基于ReaxFF MD模拟的煤加氢热解有机Ca转化机制研究[J]. 化工学报, 2025, 76(8): 4297-4309. |
| [3] | 王树宇, 薛志亮, 朱静, 付鑫, 周永刚, 胡一鸣, 黄群星. 废弃全钢胎颗粒热解过程中质量和形态变化研究[J]. 化工学报, 2025, 76(7): 3459-3467. |
| [4] | 龚丽芳, 任美慧, 蒋吉春, 郭光召, 胡红云, 黄永达, 姚洪. 垃圾焚烧烟气中芳香烃化合物在线监测和选择性催化还原脱除研究[J]. 化工学报, 2025, 76(6): 3018-3028. |
| [5] | 刘亮, 吴佳俊, 卿梦霞, 周光亚, 贺梓航. 落地油泥热解特性及工艺系统能量平衡分析[J]. 化工学报, 2025, 76(4): 1779-1787. |
| [6] | 徐东菱, 马跃, 龚露, 马桂丽, 王金可, 郭丰志, 王浩伦, 李思佳, 李术元, 岳长涛. 油页岩与烟煤混合流化热解实验研究[J]. 化工学报, 2025, 76(4): 1742-1753. |
| [7] | 徐芳, 张锐, 崔达, 王擎. ReaxFF-MD揭示木质素热解反应机制的分子动力学研究[J]. 化工学报, 2025, 76(3): 1253-1263. |
| [8] | 姚国家, 王志, 苏昂, 冯东阁, 唐宏, 孙灵芳. 空气系数对煤粉预热解燃烧特性的影响分析[J]. 化工学报, 2025, 76(3): 1243-1252. |
| [9] | 李中青, 王志远, 栾小建, 梁四凯, 王凯. 电沉积-低氧分压法制备MnO涂层及其抑制石脑油热裂解结焦性能研究[J]. 化工学报, 2025, 76(3): 1050-1063. |
| [10] | 丁湧, 李文建, 陈昭宇, 曹立辉, 刘轩铭, 任强强, 胡松, 向军. 废旧晶体硅光伏组件EVA有氧热解动力学与产物特性[J]. 化工学报, 2024, 75(9): 3310-3319. |
| [11] | 吴学红, 韦新, 侯加文, 吕财, 刘勇, 刘鹤, 常志娟. 热解法制备碳纳米管及其在散热涂层中的应用研究[J]. 化工学报, 2024, 75(9): 3360-3368. |
| [12] | 黄正梁, 冯铭瑞, 宋琦, 任聪静, 杨遥, 孙婧元, 王靖岱, 阳永荣. 预混进料对废树脂流化裂解反应中颗粒团聚的抑制作用[J]. 化工学报, 2024, 75(9): 3094-3102. |
| [13] | 王舒英, 左涛, 石志伟, 范小明, 张卫新. 阳离子交换树脂基介孔石墨化碳合成与储钠性能[J]. 化工学报, 2024, 75(9): 3338-3347. |
| [14] | 姚宏哲, 黄飞宇, 杨松, 钟梅, 代正华. 重质油高温快速热解自动反应网络的动力学建模[J]. 化工学报, 2024, 75(7): 2644-2655. |
| [15] | 晁惠雨, 白振敏, 侯汉青, 田立志, 李洪, 房晓权, 石晓华. 液相法合成三聚氰酸体系热力学分析[J]. 化工学报, 2024, 75(6): 2157-2165. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号