化工学报 ›› 2025, Vol. 76 ›› Issue (8): 4030-4041.DOI: 10.11949/0438-1157.20250156
巢欣旖( ), 陈文尧(
), 陈文尧( ), 张晶, 钱刚, 周兴贵, 段学志(
), 张晶, 钱刚, 周兴贵, 段学志( )
)
收稿日期:2025-02-20
修回日期:2025-03-30
出版日期:2025-08-25
发布日期:2025-09-17
通讯作者:
陈文尧,段学志
作者简介:巢欣旖(2000—),女,硕士研究生,Y30220188@mail.ecust.edu.cn
基金资助:
Xinyi CHAO( ), Wenyao CHEN(
), Wenyao CHEN( ), Jing ZHANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN(
), Jing ZHANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN( )
)
Received:2025-02-20
Revised:2025-03-30
Online:2025-08-25
Published:2025-09-17
Contact:
Wenyao CHEN, Xuezhi DUAN
摘要:
丙酸甲酯(MP)作为医药、香料合成等领域的关键有机中间体,其现有生产工艺面临分离能耗高、生产效率低等挑战。基于此,本研究提出一种以甲醇与乙酸甲酯为原料,在原料高分压(60 kPa)条件下在固定床反应器内连续合成MP的新路线。通过Ti改性策略构筑了一系列高性能Ti-Cs/SiO2催化剂,并采用XRD、BET、TEM、XPS、CO2/NH3-TPD和UV-Vis测试对其物理化学特性进行了表征。结果表明,Ti的引入显著调节了催化剂的酸碱特性,弱酸占总酸位点与弱碱占总碱位点比例对羟醛缩合反应活性有重要影响。在一步法制MP的关键步骤(甲醛和乙酸甲酯羟醛缩合反应)中,乙酸甲酯的转化率随Ti的负载量呈火山型趋势变化,当Ti的负载量为4%(质量分数)时,转化率最高(29.4%)。相应地,在甲醇和乙酸甲酯一步法制MP的串联反应中,该催化剂表现出最高的催化活性,MP的收率达23.6%。本研究揭示了Ti改性对催化剂酸碱特性及反应性能的影响机制,为甲醇和乙酸甲酯一步法合成MP的催化剂设计及工艺放大提供了理论依据和技术支撑。
中图分类号:
巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041.
Xinyi CHAO, Wenyao CHEN, Jing ZHANG, Gang QIAN, Xinggui ZHOU, Xuezhi DUAN. Controlled preparation and performance regulation of catalysts for one-step synthesis of methyl propionate from methanol and methyl acetate[J]. CIESC Journal, 2025, 76(8): 4030-4041.
| 催化剂 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| SiO2 | 428.9 | 0.76 | 6.71 |
| Cs/SiO2 | 92.0 | 0.59 | 21.85 |
| 1Ti-Cs/SiO2 | 235.9 | 0.84 | 11.48 |
| 2Ti-Cs/SiO2 | 240.9 | 0.75 | 9.65 |
| 3Ti-Cs/SiO2 | 241.5 | 0.65 | 8.62 |
| 4Ti-Cs/SiO2 | 246.1 | 0.64 | 8.42 |
| 5Ti-Cs/SiO2 | 269.3 | 0.70 | 8.19 |
表1 载体和负载型催化剂的孔结构参数
Table 1 Pore structure parameters of the support and supported catalysts
| 催化剂 | 比表面积/(m2/g) | 孔容/(cm3/g) | 平均孔径/nm |
|---|---|---|---|
| SiO2 | 428.9 | 0.76 | 6.71 |
| Cs/SiO2 | 92.0 | 0.59 | 21.85 |
| 1Ti-Cs/SiO2 | 235.9 | 0.84 | 11.48 |
| 2Ti-Cs/SiO2 | 240.9 | 0.75 | 9.65 |
| 3Ti-Cs/SiO2 | 241.5 | 0.65 | 8.62 |
| 4Ti-Cs/SiO2 | 246.1 | 0.64 | 8.42 |
| 5Ti-Cs/SiO2 | 269.3 | 0.70 | 8.19 |
| 催化剂 | Ti3+/Ti | Ti4+/Ti | Olatt/O |
|---|---|---|---|
| Cs/SiO2 | — | — | 14.2% |
| 1Ti-Cs/SiO2 | 32.18% | 67.82% | 14.4% |
| 2Ti-Cs/SiO2 | 36.60% | 63.40% | 16.4% |
| 3Ti-Cs/SiO2 | 40.78% | 59.22% | 17.0% |
| 4Ti-Cs/SiO2 | 47.06% | 52.94% | 17.3% |
| 5Ti-Cs/SiO2 | 50.52% | 49.48% | 17.6% |
表2 催化剂中的Ti3+、Ti4+和Olatt的比例
Table 2 Ratios of Ti3+, Ti4+ and Olatt in the catalysts
| 催化剂 | Ti3+/Ti | Ti4+/Ti | Olatt/O |
|---|---|---|---|
| Cs/SiO2 | — | — | 14.2% |
| 1Ti-Cs/SiO2 | 32.18% | 67.82% | 14.4% |
| 2Ti-Cs/SiO2 | 36.60% | 63.40% | 16.4% |
| 3Ti-Cs/SiO2 | 40.78% | 59.22% | 17.0% |
| 4Ti-Cs/SiO2 | 47.06% | 52.94% | 17.3% |
| 5Ti-Cs/SiO2 | 50.52% | 49.48% | 17.6% |
| 催化剂 | 总酸量/ (μmol/g) | 不同强度酸量/(μmol/g) | 总碱量/ (μmol/g) | 不同强度碱量/(μmol/g) | ||||
|---|---|---|---|---|---|---|---|---|
| 弱 | 中强 | 强 | 弱 | 中强 | 强 | |||
| 1Ti-Cs/SiO2 | 87 | 36 | 37 | 15 | 188 | 90 | 60 | 36 |
| 2Ti-Cs/SiO2 | 101 | 43 | 40 | 17 | 164 | 84 | 59 | 22 |
| 3Ti-Cs/SiO2 | 115 | 52 | 48 | 15 | 142 | 77 | 47 | 19 |
| 4Ti-Cs/SiO2 | 130 | 64 | 51 | 16 | 123 | 73 | 37 | 13 |
| 5Ti-Cs/SiO2 | 134 | 72 | 51 | 11 | 110 | 72 | 35 | 3 |
表3 催化剂的酸碱性质
Table 3 Acid-base properties of catalysts
| 催化剂 | 总酸量/ (μmol/g) | 不同强度酸量/(μmol/g) | 总碱量/ (μmol/g) | 不同强度碱量/(μmol/g) | ||||
|---|---|---|---|---|---|---|---|---|
| 弱 | 中强 | 强 | 弱 | 中强 | 强 | |||
| 1Ti-Cs/SiO2 | 87 | 36 | 37 | 15 | 188 | 90 | 60 | 36 |
| 2Ti-Cs/SiO2 | 101 | 43 | 40 | 17 | 164 | 84 | 59 | 22 |
| 3Ti-Cs/SiO2 | 115 | 52 | 48 | 15 | 142 | 77 | 47 | 19 |
| 4Ti-Cs/SiO2 | 130 | 64 | 51 | 16 | 123 | 73 | 37 | 13 |
| 5Ti-Cs/SiO2 | 134 | 72 | 51 | 11 | 110 | 72 | 35 | 3 |
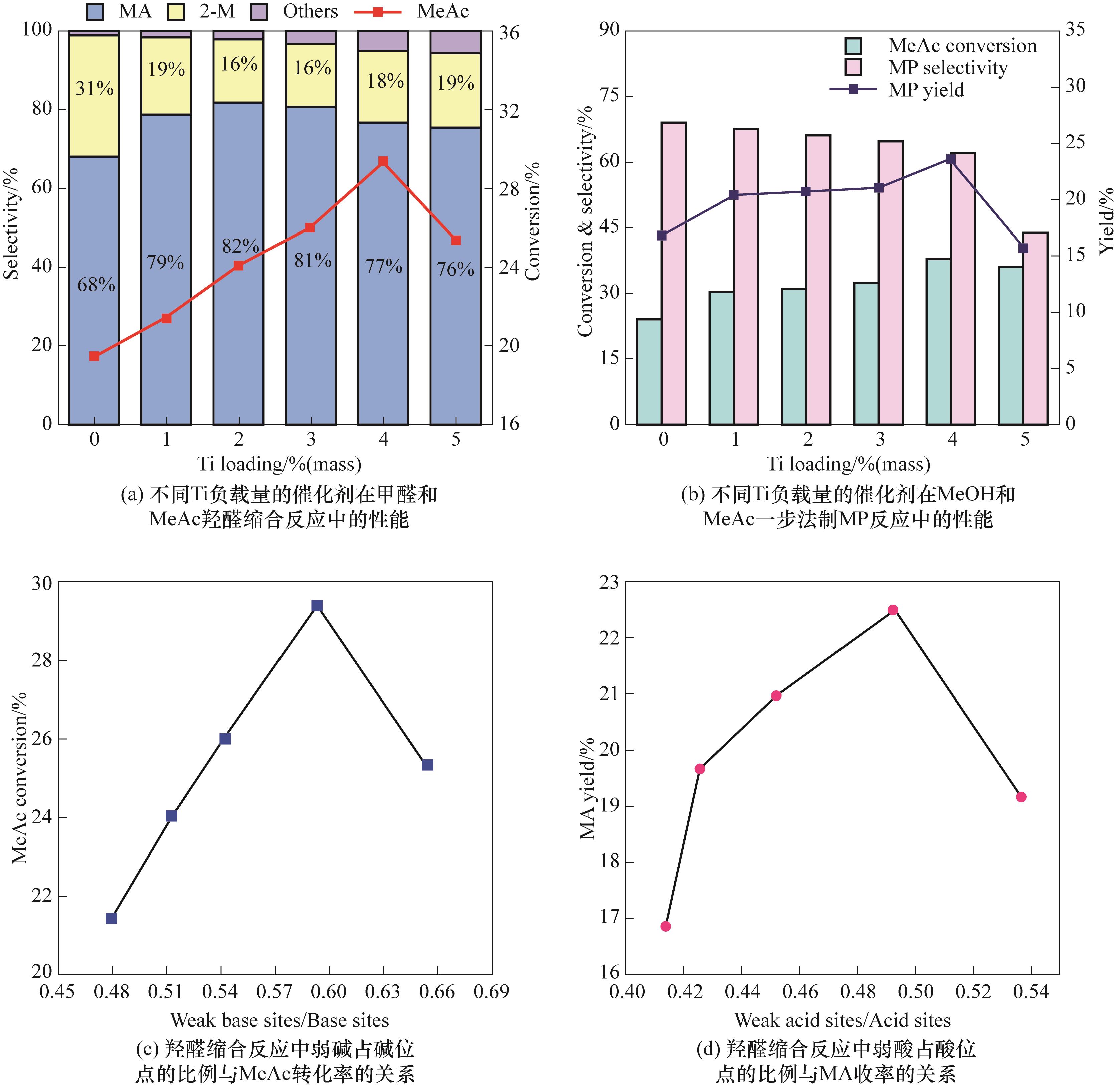
图7 xTi-Cs/SiO2催化剂的固定床评价结果以及酸碱位点比例与羟醛缩合性能的关系
Fig.7 Catalytic performance of xTi-Cs/SiO2 catalysts in fixed-bed reactor and correlation between weak acid/base site proportion and aldol condensation performance
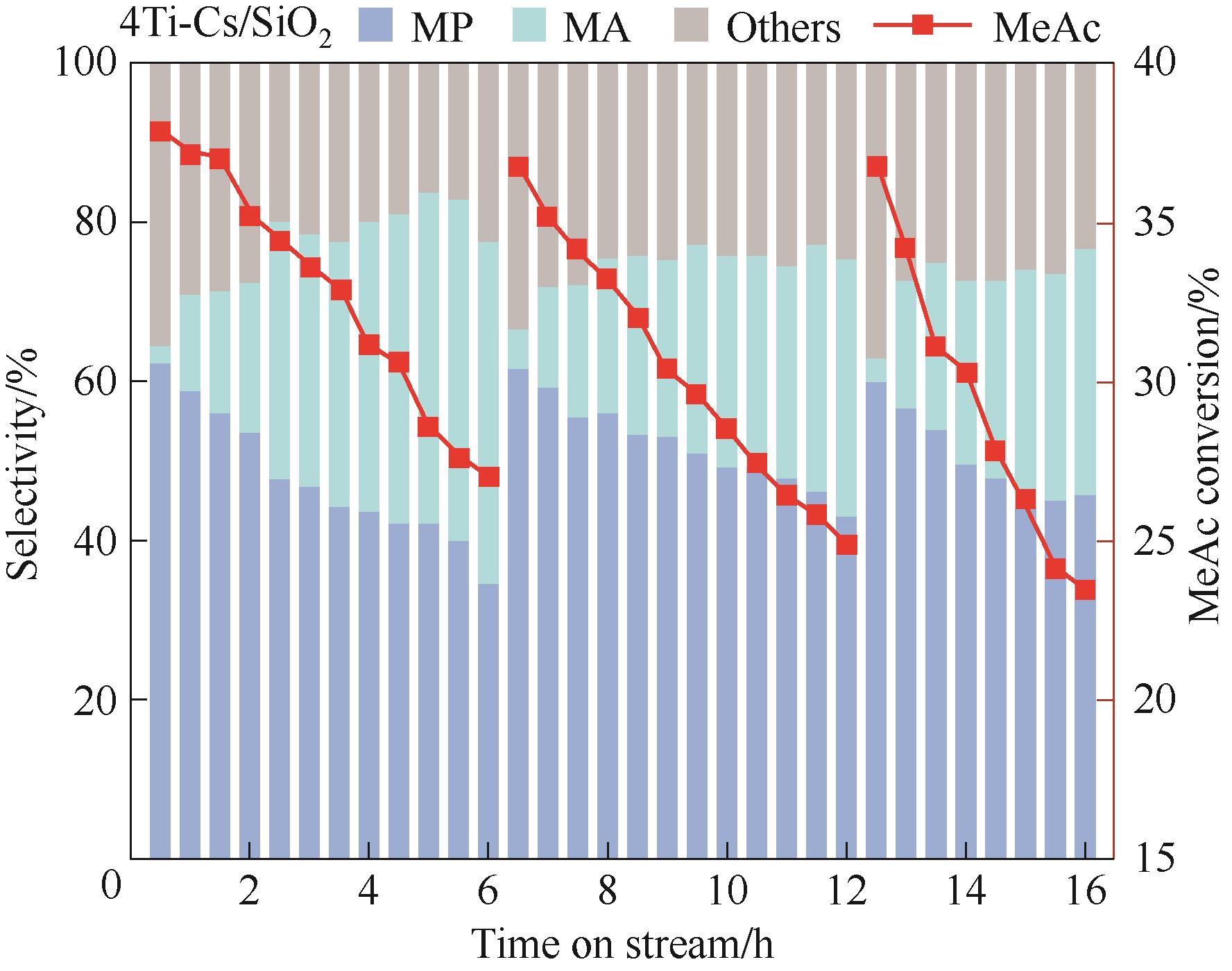
图8 4Ti-Cs/SiO2催化剂在一步法制MP反应中的稳定性与再生性能
Fig.8 Cyclic stability and regenerability of 4Ti-Cs/SiO2 catalyst in one-step synthesis of MP from methanol/methyl acetate
| [1] | Zhou X P, Dong J C, Zhao Y, et al. Synergy of photo- and photothermal-catalytic synthesis of methyl propionate from ethylene and carbon dioxide over B–TiO2/Ru[J]. ACS Sustainable Chemistry & Engineering, 2023, 11(24): 9255-9263. |
| [2] | Wang L M, Zhang G L, Zhao H, et al. Encapsulation of phosphine-palladium complex in USY zeolite for hydroesterification of ethylene to methyl propionate[J]. Chemical Engineering Science, 2025, 304: 120976. |
| [3] | 王鲁明, 李增喜. 丙酸甲酯催化合成过程研究进展[J]. 工程研究——跨学科视野中的工程, 2024, 16(5): 481-499. |
| Wang L M, Li Z X. Research progress on the catalytic synthesis process of methyl propionate[J]. Journal of Engineering Studies, 2024, 16(5): 481-499. | |
| [4] | Sun T, Wang G, Guo X P, et al. A highly active NiMoAl catalyst prepared by a solvothermal method for the hydrogenation of methyl acrylate[J]. Catalysts, 2022, 12(10): 1118. |
| [5] | Liu G L, Li G, Song H Y. Direct synthesis of methyl propionate from n-propyl alcohol and methanol using gold catalysts[J]. Catalysis Letters, 2009, 128(3): 493-501. |
| [6] | Zhang B, Yuan H Y, Liu Y, et al. Ambient-pressure alkoxycarbonylation for sustainable synthesis of ester[J]. Nature Communications, 2024, 15(1): 7837. |
| [7] | Sivakumar G, Kumar R, Yadav V, et al. Multi-functionality of methanol in sustainable catalysis: beyond methanol economy[J]. ACS Catalysis, 2023, 13(22): 15013-15053. |
| [8] | Chen W Y, Zuo J, Sang K, et al. Leveraging the proximity and distribution of Cu-Cs sites for direct conversion of methanol to esters/aldehydes[J]. Angewandte Chemie (International Ed), 2024, 63(1): e202314288. |
| [9] | Guan Y N, Ma H Q, Chen W Y, et al. Methyl methacrylate synthesis: thermodynamic analysis for oxidative esterification of methacrolein and aldol condensation of methyl acetate[J]. Industrial & Engineering Chemistry Research, 2020, 59(39): 17408-17416. |
| [10] | 左骥, 罗莉, 谢永锴, 等. 甲醇无氧脱氢制甲醛Cu催化剂的粒径效应[J]. 化工进展, 2025, 44(3): 1347-1354. |
| Zuo J, Luo L, Xie Y K, et al. Effect of Cu catalyst particle size on methanol nonoxidative dehydrogenation to formaldehyde[J]. Chemical Industry and Engineering Progress, 2025, 44(3): 1347-1354. | |
| [11] | Wang G, Li Z X, Li C S. Recent progress in one-step synthesis of acrylic acid and methyl acrylate via aldol reaction: catalyst, mechanism, kinetics and separation[J]. Chemical Engineering Science, 2022, 247: 117052. |
| [12] | Feng X Z, Sun B, Yao Y, et al. Renewable production of acrylic acid and its derivative: new insights into the aldol condensation route over the vanadium phosphorus oxides[J]. Journal of Catalysis, 2014, 314: 132-141. |
| [13] | Wang Y M, Wang Z L, Hao X, et al. Nb-doped vanadium phosphorus oxide catalyst for the aldol condensation of acetic acid with formaldehyde to acrylic acid[J]. Industrial & Engineering Chemistry Research, 2018, 57(36): 12055-12060. |
| [14] | He T, Qu Y X, Wang J D. Aldol condensation reaction of methyl acetate and formaldehyde over cesium oxide supported on silica gel: an experimental and theoretical study[J]. Catalysis Letters, 2019, 149(2): 373-389. |
| [15] | Ai M. Formation of methyl methacrylate by condensation of methyl propionate with formaldehyde over silica-supported cesium hydroxide catalysts[J]. Applied Catalysis A: General, 2005, 288(1/2): 211-215. |
| [16] | Tai J R, Davis R J. Synthesis of methacrylic acid by aldol condensation of propionic acid with formaldehyde over acid-base bifunctional catalysts[J]. Catalysis Today, 2007, 123(1/2/3/4): 42-49. |
| [17] | Wang Y N, Yan R Y, Lv Z P, et al. Lanthanum and cesium-loaded SBA-15 catalysts for MMA synthesis by aldol condensation of methyl propionate and formaldehyde[J]. Catalysis Letters, 2016, 146(9): 1808-1818. |
| [18] | Xu L, Wang X P, Song J H, et al. Acid promoter-modified Cs/Al2O3 catalyst for methyl methacrylate production by aldol condensation of methyl propionate with formaldehyde[J]. Industrial & Engineering Chemistry Research, 2023, 62(49): 21130-21139. |
| [19] | Sararuk C, Yang D, Zhang G L, et al. One-step aldol condensation of ethyl acetate with formaldehyde over Ce and P modified cesium supported alumina catalyst[J]. Journal of Industrial and Engineering Chemistry, 2017, 46: 342-349. |
| [20] | Wu Z Y, Wang L M, Li Z X, et al. Unveiling the promotion of Brønsted acid sites in Cs dispersion and consequential Si-O-Cs species formation for methyl acrylate synthesis from methyl acetate and formaldehyde over Cs/Beta zeolite catalyst[J]. Chemical Engineering Journal, 2023, 474: 145655. |
| [21] | Guo Z J, Zhang G L, Wang L, et al. Fe-modified Cs–P/γ-Al2O3 catalyst for synthesis of methyl methacrylate from methyl propionate and formaldehyde[J]. Industrial & Engineering Chemistry Research, 2020, 59(8): 3334-3341. |
| [22] | Deng S L, Yan T T, Ran R, et al. Influence of Al oxides on Cs-SiO2 catalysts for vapor phase aldol condensation of methyl acetate and formaldehyde[J]. Industrial & Engineering Chemistry Research, 2022, 61(17): 5766-5777. |
| [23] | Bao Q, Qi H, Zhang C L, et al. Highly catalytic activity of Ba/γ-Ti-Al2O3 catalyst for aldol condensation of methyl acetate with formaldehyde[J]. Catalysis Letters, 2018, 148(11): 3402-3412. |
| [24] | Rekoske J E, Barteau M A. Kinetics, selectivity, and deactivation in the aldol condensation of acetaldehyde on anatase titanium dioxide[J]. Industrial & Engineering Chemistry Research, 2011, 50(1): 41-51. |
| [25] | Zhang Z Y, Berdugo-Díaz C E, Bregante D T, et al. Aldol condensation and esterification over Ti-substituted *BEA zeolite: mechanisms and effects of pore hydrophobicity[J]. ACS Catalysis, 2022, 12(2): 1481-1496. |
| [26] | Idriss H, Kim K S, Barteau M A. Carbon-Carbon bond formation via aldolization of acetaldehyde on single crystal and polycrystalline TiO2 surfaces[J]. Journal of Catalysis, 1993, 139(1): 119-133. |
| [27] | Gao B Z, Zhu Q R, Yi Y H, et al. Catalytic performance of amorphous Ti/SiO2 in the gas-phase epoxidation of propylene with H2O2 [J]. European Journal of Inorganic Chemistry, 2023, 26(22): e202200661. |
| [28] | Guo Y J, Hwang S J, Katz A. Hydrothermally robust Ti/SiO2 epoxidation catalysts via surface modification with oligomeric PMHS[J]. Molecular Catalysis, 2019, 477: 110509. |
| [29] | Liu J Y, Li Z X, Bian Y H, et al. Promotional effect of Ti on catalytic performance of Cs/Ti-SiO2 for conversion of methyl propionate and formaldehyde to methyl methacrylate[J]. Chemical Engineering Science, 2024, 283: 119441. |
| [30] | Pham T N, Shi D C, Sooknoi T, et al. Aqueous-phase ketonization of acetic acid over Ru/TiO2/carbon catalysts[J]. Journal of Catalysis, 2012, 295: 169-178. |
| [31] | Jia B Y, Wu M J, Zhang H, et al. Ti functionalized hierarchical-pore UiO-66(Zr/Ti) catalyst for the transesterification of phenyl acetate and dimethyl carbonate[J]. New Journal of Chemistry, 2019, 43(43): 16981-16989. |
| [32] | Arillo M A, López M L, Pico C, et al. Surface characterisation of spinels with Ti(Ⅳ) distributed in tetrahedral and octahedral sites[J]. Journal of Alloys and Compounds, 2001, 317: 160-163. |
| [33] | Biesinger M C, Lau L W M, Gerson A R, et al. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn[J]. Applied Surface Science, 2010, 257(3): 887-898. |
| [34] | Lin Y L, Wang T J, Jin Y. Surface characteristics of hydrous silica-coated TiO2 particles[J]. Powder Technology, 2002, 123(2/3): 194-198. |
| [35] | Hong Z, Zhao G Q, Huang F T, et al. Enhancing the side-chain alkylation of toluene with methanol to styrene over the Cs-modified X zeolite by the assistance of basic picoline as a co-catalyst[J]. Green Energy & Environment, 2022, 7(6): 1241-1252. |
| [36] | Li Q, Deng S L, Liu J Y, et al. PEG-assisted synthesis of highly dispersed Cs/Zr-SiO2 catalyst for aldol condensation of methyl acetate with formaldehyde[J]. Chemical Engineering Science, 2025, 301: 120716. |
| [37] | Shintaku H, Nakajima K, Kitano M, et al. Lewis acid catalysis of TiO4 tetrahedra on mesoporous silica in water[J]. ACS Catalysis, 2014, 4(4): 1198-1204. |
| [38] | Liang X H, Peng X X, Xia C J, et al. Improving Ti incorporation into the BEA framework by employing ethoxylated chlorotitanate as Ti precursor: postsynthesis, characterization, and incorporation mechanism[J]. Industrial & Engineering Chemistry Research, 2021, 60(3): 1219-1230. |
| [39] | Guidotti M, Ravasio N, Psaro R, et al. Epoxidation on titanium-containing silicates: do structural features really affect the catalytic performance?[J]. Journal of Catalysis, 2003, 214(2): 242-250. |
| [40] | Li W Q, Qiu M H, Li W T, et al. Au supported defect free TS-1 for enhanced performance of gas phase propylene epoxidation with H2 and O2[J]. Sustainable Energy & Fuels, 2022, 6(10): 2462-2470. |
| [41] | Hu J, Lu Z P, Yin H B, et al. Aldol condensation of acetic acid with formaldehyde to acrylic acid over SiO2-, SBA-15-, and HZSM-5-supported V-P-O catalysts[J]. Journal of Industrial and Engineering Chemistry, 2016, 40: 145-151. |
| [42] | Yan T T, Deng S L, Ran R, et al. Cesium loaded on an Al-modified silica support catalyst for methyl acrylate synthesis by aldol condensation of methyl acetate and formaldehyde[J]. Industrial & Engineering Chemistry Research, 2022, 61(7): 2748-2758. |
| [43] | Wang B, Deng S L, Bian Y H, et al. Aldol condensation of methyl propionate and formaldehyde: thermodynamics, reaction process, and network[J]. Industrial & Engineering Chemistry Research, 2022: acs.iecr.2c02570. |
| [44] | Zuo C C, Li C S, Ge T T, et al. Spherical P-modified catalysts for heterogeneous cross-aldol condensation of formaldehyde with methyl acetate for methyl acrylate production[J]. The Canadian Journal of Chemical Engineering, 2017, 95(11): 2104-2111. |
| [45] | Ma H Q, Guan Y N, Chen W Y, et al. Support effects of Cs/Al2O3 catalyzed aldol condensation of methyl acetate with formaldehyde[J]. Catalysis Today, 2021, 365: 310-317. |
| [46] | 李晓云, 孙彦民, 于海斌. 甲醇催化脱氢制无水甲醛研究进展[C]//全国工业催化信息站, 工业催化杂志社.第七届全国工业催化技术及应用年会论文集. 天津: 中国海油天津化工研究设计院催化技术重点实验室, 2010: 134-136. |
| Li X Y, Sun Y M, Yu H B. Research progress in catalytic dehydrogenation of methanol to anhydrous formaldehyde[C]// National Industrial Catalysis Information Station, Industrial Catalysis Journal. Proceedings of the 7th National Annual Conference on Industrial Catalytic Technology and Applications. Tianjin: CNOOC Tianjin Chemical Research & Design Institute Key Laboratory of Catalytic Technology, 2010: 134-136. | |
| [47] | Ran R, Zhu W, Zhang G L, et al. Unraveling the coking and deactivation behavior of Al-Cs/SiO2 catalyst in the aldol condensation of methyl propionate with formaldehyde[J]. Industrial & Engineering Chemistry Research, 2024, 63(3): 1286-1297. |
| [48] | Xu L, Wang X P, Song J H, et al. Coking and deactivation behavior study of Ce-modified Cs-Nb/Al2O3 in aldol condensation of methyl propionate with formaldehyde[J]. Molecular Catalysis, 2024, 564: 114323. |
| [1] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [2] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [3] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [4] | 彭梦圆, 李家明, 沙敏, 张丁. 季铵盐氟碳表面活性剂复配体系的性能研究[J]. 化工学报, 2025, 76(8): 4177-4184. |
| [5] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [6] | 周媚, 曾浩桀, 蒋火炎, 蒲婷, 曾星星, 刘宝玉. 二次晶化法改性合成MTW分子筛及其在苯和环己烯烷基化反应中的催化性能[J]. 化工学报, 2025, 76(8): 4071-4080. |
| [7] | 吴天灏, 叶霆威, 林延, 黄振. 生物质化学链气化原位补氢制H2/CO可控合成气[J]. 化工学报, 2025, 76(7): 3498-3508. |
| [8] | 赵世颖, 左志帅, 贺梦颖, 安华良, 赵新强, 王延吉. Co-Pt/HAP的制备及其催化1,2-丙二醇氨化反应[J]. 化工学报, 2025, 76(7): 3305-3315. |
| [9] | 刘沁雯, 叶恒冰, 张逸伟, 朱法华, 钟文琪. 煤与禽类粪便混合燃料的加压富氧燃烧特性研究[J]. 化工学报, 2025, 76(7): 3487-3497. |
| [10] | 郭铮铮, 赵一丹, 王辅强, 裴璐, 靳彦岭, 任芳, 任鹏刚. 异质结构MoS2/RGO/NiFe2O4复合材料的构筑及电磁波吸收性能研究[J]. 化工学报, 2025, 76(7): 3719-3732. |
| [11] | 乔亮, 李尚, 刘新亮, 王明, 张沛, 侯影飞. 三元共聚物稠油降黏剂的合成及分子模拟研究[J]. 化工学报, 2025, 76(7): 3686-3695. |
| [12] | 陆学瑞, 周帼彦, 方琦, 俞孟正, 张秀成, 涂善东. 固体氧化物燃料电池外重整器积炭效应数值模拟研究[J]. 化工学报, 2025, 76(7): 3295-3304. |
| [13] | 梁碧麟, 余倩, 贾思琦, 李芳, 李其明. Ni-MOF-74金属有机框架膜的结构调变及气体分离性能研究[J]. 化工学报, 2025, 76(6): 2714-2721. |
| [14] | 郭乃胜, 朱小波, 王双, 陈平, 褚召阳, 王志臣. 聚氨酯改性沥青高低温性能及影响因素的研究进展[J]. 化工学报, 2025, 76(6): 2505-2523. |
| [15] | 李愽龙, 蒋雨希, 任傲天, 秦雯琪, 傅杰, 吕秀阳. TS-1/In-TS-1催化果糖一步法醇解制备乳酸甲酯连续化试验[J]. 化工学报, 2025, 76(6): 2678-2686. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号