化工学报 ›› 2025, Vol. 76 ›› Issue (12): 6718-6728.DOI: 10.11949/0438-1157.20250292
徐世彪1( ), 韦正兵1, 鲍梦凡1, 程怡1, 贾洋刚1, 林娜1, 冒爱琴1,2(
), 韦正兵1, 鲍梦凡1, 程怡1, 贾洋刚1, 林娜1, 冒爱琴1,2( )
)
收稿日期:2025-03-24
修回日期:2025-05-11
出版日期:2025-12-31
发布日期:2026-01-23
通讯作者:
冒爱琴
作者简介:徐世彪(2000—),男,硕士研究生,xushibiao002@163.com
基金资助:
Shibiao XU1( ), Zhengbing WEI1, Mengfan BAO1, Yi CHENG1, Yanggang JIA1, Na LIN1, Aiqin MAO1,2(
), Zhengbing WEI1, Mengfan BAO1, Yi CHENG1, Yanggang JIA1, Na LIN1, Aiqin MAO1,2( )
)
Received:2025-03-24
Revised:2025-05-11
Online:2025-12-31
Published:2026-01-23
Contact:
Aiqin MAO
摘要:
高熵氧化物(HEOs)因其优异的循环稳定性和较高的理论比容量在储能领域备受关注,但其本征电导率较低限制了其应用。本研究通过调控La(Cr0.2Fe0.2Mn0.2Ni0.2Cu x □0.2-x )O3-δ (x=0、0.1和0.2)中Cu含量,优化阳离子/氧空位,在稳定钙钛矿结构的同时协同改善微观/电子结构,提升电子/离子传输速率,增强电化学性能。结果表明,La(Cr0.2Fe0.2Mn0.2Ni0.2Cu0.1□0.1)O3-δ 具有优异的高倍率储锂性能:200 mA·g-1时循环250圈后比容量达1174.3 mAh·g-1,较x=0.2的样品提升1.3倍;在3000 mA·g-1高倍率下仍保持200.1 mAh·g-1的比容量(容量保持率相较100 mA·g-1时为49.6%),倍率性能提升4倍。本研究通过调控阳离子/氧空位缺陷,有效提升了钙钛矿型HEO的电化学性能,为开发高性能HEOs负极材料提供了新的设计策略。
中图分类号:
徐世彪, 韦正兵, 鲍梦凡, 程怡, 贾洋刚, 林娜, 冒爱琴. Cu阳离子空位提升钙钛矿型高熵氧化物储锂性能[J]. 化工学报, 2025, 76(12): 6718-6728.
Shibiao XU, Zhengbing WEI, Mengfan BAO, Yi CHENG, Yanggang JIA, Na LIN, Aiqin MAO. Cu cation vacancies enhance the lithium storage performance of perovskite-type high-entropy oxides[J]. CIESC Journal, 2025, 76(12): 6718-6728.
| 样品 | 晶胞参数 | 可靠性因子 | ||||||
|---|---|---|---|---|---|---|---|---|
| a/Å | b/Å | c/Å | α=β=γ/(°) | Vm/Å3 | Rp/% | Rwp/% | χ2 | |
| Cu0 | 3.897 | 3.897 | 3.897 | 90 | 59.12 | 9.18 | 7.01 | 3.74 |
| Cu0.1 | 3.892 | 3.892 | 3.892 | 90 | 58.99 | 7.63 | 5.74 | 2.79 |
| Cu0.2 | 3.894 | 3.894 | 3.894 | 90 | 59.05 | 7.75 | 5.88 | 3.13 |
表1 样品的晶胞参数和精修的可靠性因子
Table 1 Lattice parameter of samples and the reliability factors of the Rietveld refinement
| 样品 | 晶胞参数 | 可靠性因子 | ||||||
|---|---|---|---|---|---|---|---|---|
| a/Å | b/Å | c/Å | α=β=γ/(°) | Vm/Å3 | Rp/% | Rwp/% | χ2 | |
| Cu0 | 3.897 | 3.897 | 3.897 | 90 | 59.12 | 9.18 | 7.01 | 3.74 |
| Cu0.1 | 3.892 | 3.892 | 3.892 | 90 | 58.99 | 7.63 | 5.74 | 2.79 |
| Cu0.2 | 3.894 | 3.894 | 3.894 | 90 | 59.05 | 7.75 | 5.88 | 3.13 |
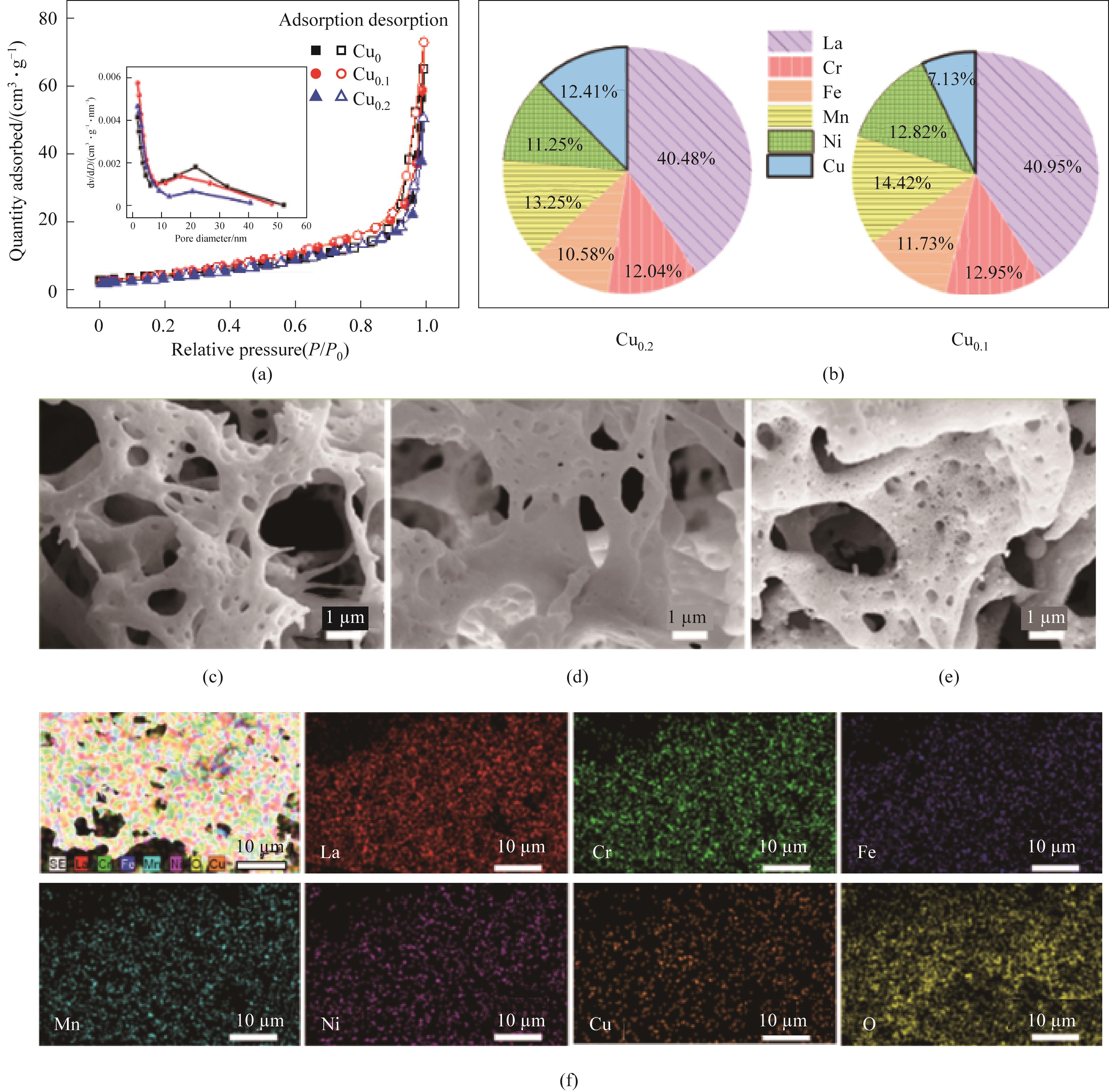
图2 N2吸/脱附等温曲线及BJH孔径分布(a);ICP测量的Cu0.2和Cu0.1样品中金属元素的摩尔比(b);Cu0(c)、Cu0.1(d)和Cu0.2(e)的SEM图;Cu0.1样品的EDS mapping图(f)
Fig.2 N2 absorption-desorption isothermal curves and BJH pore size distribution of the samples (a); The metal element contents of the Cu0.1 and Cu0.2 samples measured by ICP (b);SEM patterns Cu0 (c), Cu0.1 (d) and Cu0.2 (e); EDS mapping patterns of Cu0.1 sample (f)
| 样品 | 比表面积/(m2·g-1) | 孔体积/(cm3·g-1) | 平均孔径/nm | 最可几 孔径/nm |
|---|---|---|---|---|
| Cu0 | 17.23 | 0.104 | 20.83 | 2.771 |
| Cu0.1 | 18.84 | 0.119 | 20.78 | 3.047 |
| Cu0.2 | 14.90 | 0.083 | 19.28 | 2.324 |
表2 样品的BET比表面积、孔体积、平均孔径和最可几孔径
Table 2 BET surface areas, pore volume, average pore size and the most probable pore size of the samples
| 样品 | 比表面积/(m2·g-1) | 孔体积/(cm3·g-1) | 平均孔径/nm | 最可几 孔径/nm |
|---|---|---|---|---|
| Cu0 | 17.23 | 0.104 | 20.83 | 2.771 |
| Cu0.1 | 18.84 | 0.119 | 20.78 | 3.047 |
| Cu0.2 | 14.90 | 0.083 | 19.28 | 2.324 |
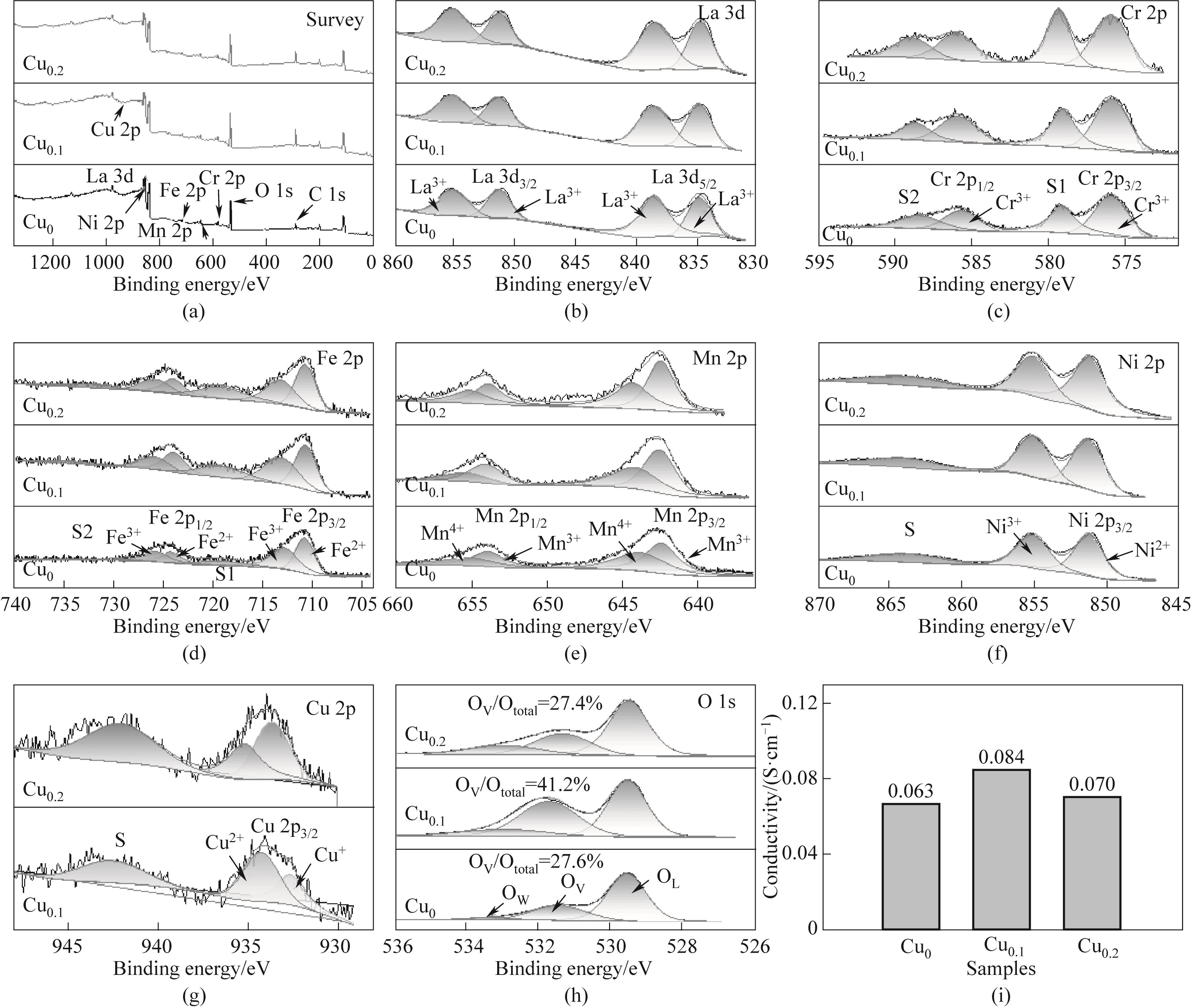
图3 XPS测量光谱(a);样品中所有元素的高分辨率XPS光谱(b)~(h);四探针电导率(i)
Fig.3 XPS survry spectras(a); High resolution XPS spectrums of all elements of the samples (b)—(h); Four probes conductivity(i)
| 样品 | 阳离子价态浓度 | OV /% | |||||
|---|---|---|---|---|---|---|---|
| La3+ | Cr3+ | Fe2+/Fe3+ | Mn3+/Mn4+ | Ni2+/Ni3+ | Cu+/Cu2+ | ||
| Cu0 | 1 | 1 | 46.1/53.9 | 53.5/46.5 | 54.2/45.8 | 0 | 27.6 |
| Cu0.1 | 1 | 1 | 53.6/46.4 | 55.8/44.2 | 54.9/45.1 | 42.4/57.6 | 41.2 |
| Cu0.2 | 1 | 1 | 52.3/47.7 | 54.8/45.2 | 61.5/38.5 | 54.9/45.1 | 27.4 |
表3 XPS中阳离子价态浓度比及氧空位浓度
Table 3 Concentration ratio of cationic valence states in XPS and oxygen vacancy concentration
| 样品 | 阳离子价态浓度 | OV /% | |||||
|---|---|---|---|---|---|---|---|
| La3+ | Cr3+ | Fe2+/Fe3+ | Mn3+/Mn4+ | Ni2+/Ni3+ | Cu+/Cu2+ | ||
| Cu0 | 1 | 1 | 46.1/53.9 | 53.5/46.5 | 54.2/45.8 | 0 | 27.6 |
| Cu0.1 | 1 | 1 | 53.6/46.4 | 55.8/44.2 | 54.9/45.1 | 42.4/57.6 | 41.2 |
| Cu0.2 | 1 | 1 | 52.3/47.7 | 54.8/45.2 | 61.5/38.5 | 54.9/45.1 | 27.4 |
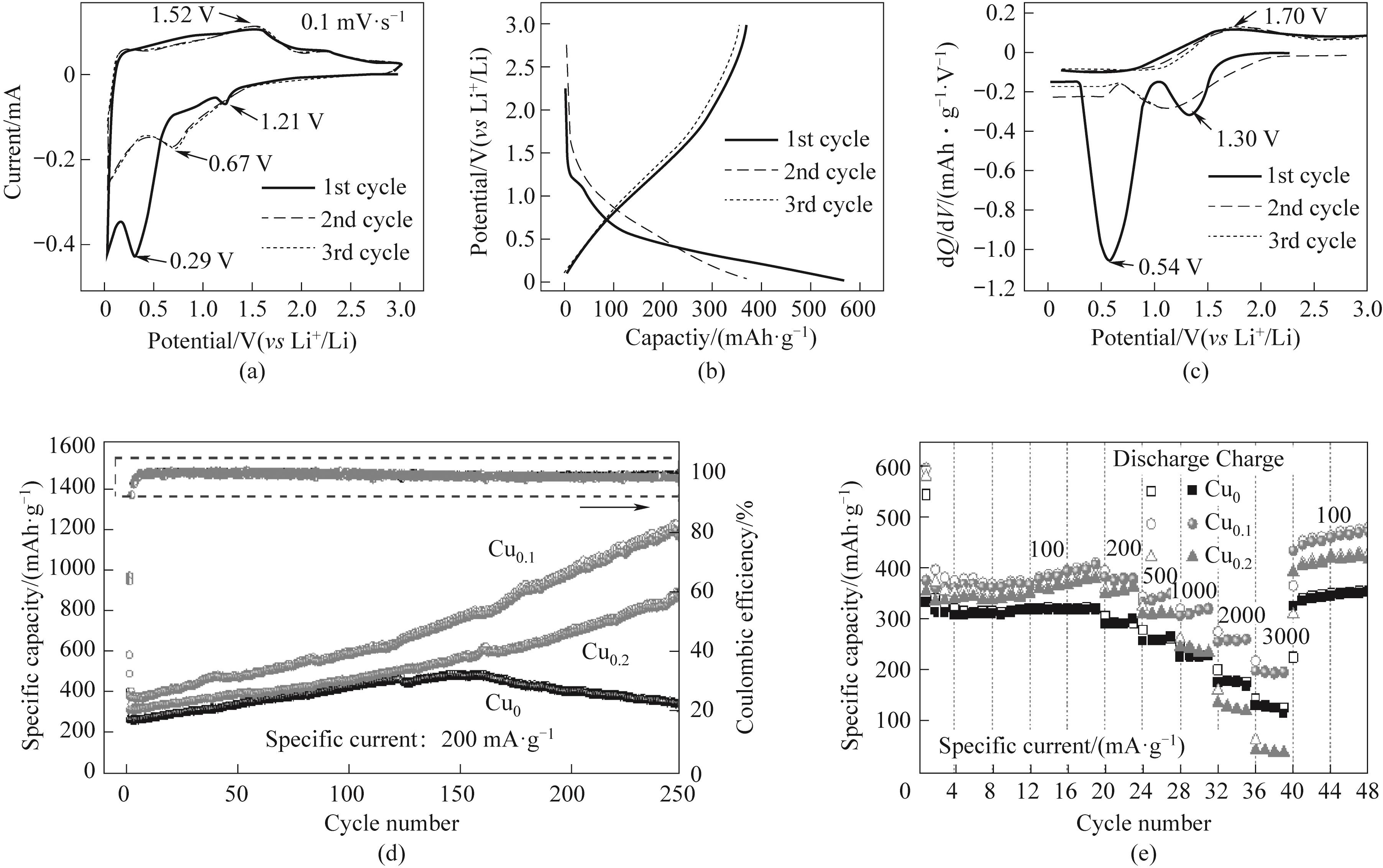
图4 Cu0.1电极在0.1 mV·s-1描扫速率下的CV图(a)、在200 mA·g-1下的充放电曲线图(b)以及微分容量曲线(c);各电极在200 mA·g-1下的循环性能/库仑效率(d)和倍率性能(e)
Fig.4 CV curves at 0.1 mV·s-1 sweep rate (a) and charge/discharge profiles(b) and dQ/dV plots(c) at 200 mA·g-¹ of the Cu0.1 electrode; cycling performance/Coulombic efficiency (d) and rate performance of each electrode (e)
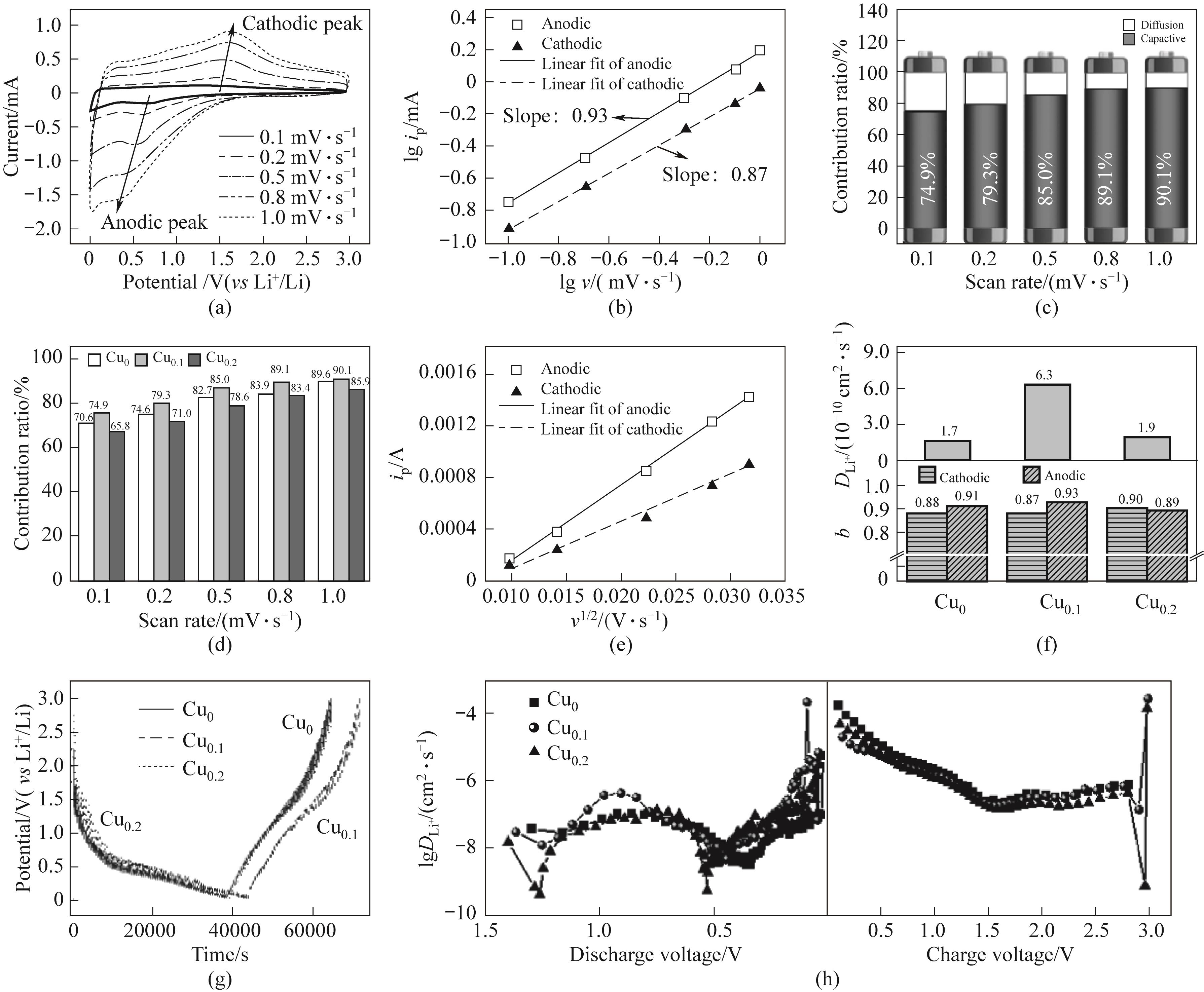
图5 Cu0.1电极在不同扫速下的CV曲线(a);lgip与lgv的关系曲线(b);Cu0.1电极不同扫描速率下的赝电容贡献率(c);各电极不同扫描速率下的赝电容贡献率(d);ip与v1/2的关系图(e);赝电容b值和循环伏安法DLi+(f);GITT测试过程中的充电/放电曲线(g)和充电/放电过程中的DLi+(h)
Fig.5 CV curves of Cu0.1 electrode at different scan rates (a); The relationship between lgip and lgv (b); Contribution ratios of pseudocapacitive capacities at different scan rates of the Cu0.1 electrode (c); Contribution ratios of the electrodes (d); The relationship between ip and v1/2(e); The b-value of pseudocapacitive and lithium-ion diffusion coefficient calculated from CV(f); Charge/discharge curves during the GITT test (g) and DLi+ during the charge/discharge process (h)
| [1] | Anandkumar M, Trofimov E. Synthesis, properties, and applications of high-entropy oxide ceramics: current progress and future perspectives[J]. Journal of Alloys and Compounds, 2023, 960: 170690. |
| [2] | 黄俊达, 朱宇辉, 冯煜, 等. 二次电池研究进展[J]. 物理化学学报, 2022, 38(12): 2208008. |
| Huang J D, Zhu Y H, Feng Y, et al. Research progress on key materials and technologies for secondary batteries[J]. Acta Physico-Chimica Sinica, 2022, 38(12): 2208008. | |
| [3] | Lim S, Kim J H, Yamada Y, et al. Improvement of rate capability by graphite foam anode for Li secondary batteries[J]. Journal of Power Sources, 2017, 355: 164-170. |
| [4] | Sarkar A, Velasco L, Wang D, et al. High entropy oxides for reversible energy storage[J]. Nature Communications, 2018, 9: 3400. |
| [5] | Chen G J, Li C W, Jia H M, et al. A novel approach for the composition design of high-entropy fluorite oxides with low thermal conductivity[J]. Journal of Advanced Ceramics, 2024, 13(9): 1369-1381. |
| [6] | Bai Y H, Li J R, Lu H, et al. Ultrafast high-temperature sintering of high-entropy oxides with refined microstructure and superior lithium-ion storage performance[J]. Journal of Advanced Ceramics, 2023, 12(10): 1857-1871. |
| [7] | Nguyen T X, Tsai C C, Patra J, et al. Co-free high entropy spinel oxide anode with controlled morphology and crystallinity for outstanding charge/discharge performance in lithium-ion batteries[J]. Chemical Engineering Journal, 2022, 430: 132658. |
| [8] | Patra J, Nguyen T X, Tsai C C, et al. Effects of elemental modulation on phase purity and electrochemical properties of Co-free high-entropy spinel oxide anodes for lithium-ion batteries[J]. Advanced Functional Materials, 2022, 32(17): 2110992. |
| [9] | Xiao B, Wu G, Wang T D, et al. High-entropy oxides as advanced anode materials for long-life lithium-ion batteries[J]. Nano Energy, 2022, 95: 106962. |
| [10] | Wang K, Hua W B, Huang X H, et al. Synergy of cations in high entropy oxide lithium ion battery anode[J]. Nature Communications, 2023, 14(1): 1487. |
| [11] | Luo X F, Patra J, Chuang W T, et al. Charge-discharge mechanism of high‐entropy Co-free spinel oxide toward Li+ storage examined using operando quick-scanning X-ray absorption spectroscopy[J]. Advanced Science, 2022, 9(21): 2201219. |
| [12] | Li Y N, Wang B B, Wang Y L, et al. Modulating crystal structure and lithium-ion storage performance of high-entropy oxide (CrMnFeCoNiZn)3O4 by single element extraction[J]. Composites Part B: Engineering, 2025, 294: 112175. |
| [13] | Gao P, Chen Z, Gong Y X, et al. The role of cation vacancies in electrode materials for enhanced electrochemical energy storage: synthesis, advanced characterization, and fundamentals[J]. Advanced Energy Materials, 2020, 10(14): 1903780. |
| [14] | Yan J H, Wang D, Zhang X Y, et al. A high-entropy perovskite titanate lithium-ion battery anode[J]. Journal of Materials Science, 2020, 55(16): 6942-6951. |
| [15] | Hou S S, Su L, Wang S, et al. Unlocking the origins of highly reversible lithium storage and stable cycling in a spinel high-entropy oxide anode for lithium-ion batteries[J]. Advanced Functional Materials, 2024, 34(4): 2307923. |
| [16] | 张欣, 韩登宝, 陈小梅, 等. 钙钛矿材料制备中的溶剂配位效应[J]. 物理化学学报, 2021, 37(4): 2008055. |
| Zhang X, Han D B, Chen X M, et al. Effects of solvent coordination on perovskite crystallization[J]. Acta Physico- Chimica Sinica, 2021, 37(4): 2008055. | |
| [17] | Liu X F, Xing Y Y, Xu K, et al. Kinetically accelerated lithium storage in high‐entropy (LiMgCoNiCuZn)O enabled by oxygen vacancies[J]. Small, 2022, 18(18): 2200524. |
| [18] | Zhang R, Liu Z L, Gao T N, et al. A solvent‐polarity‐induced interface self‐assembly strategy towards mesoporous triazine‐based carbon materials[J]. Angewandte Chemie International Edition, 2021, 60(45): 24299-24305. |
| [19] | Feng D Y, Dong Y B, Zhang L L, et al. Holey lamellar high-entropy oxide as an ultra-high-activity heterogeneous catalyst for solvent-free aerobic oxidation of benzyl alcohol[J]. Angewandte Chemie International Edition, 2020, 59(44): 19503-19509. |
| [20] | Li X L, Lin Z F, Jin N, et al. Perovskite‐type SrVO3 as high‐performance anode materials for lithium-ion batteries[J]. Advanced Materials, 2022, 34(46): 2107262. |
| [21] | Yang Y, Zhu J W, Wang P Y, et al. NH2-MIL-125 (Ti) derived flower-like fine TiO2 nanoparticles implanted in N-doped porous carbon as an anode with high activity and long cycle life for lithium-ion batteries[J]. Acta Physico-Chimica Sinica, 2022, 38(6): 2106002. |
| [22] | Petrovičovà B, Xu W L, Musolino M G, et al. High-entropy spinel oxides produced via sol-gel and electrospinning and their evaluation as anodes in Li-ion batteries[J]. Applied Sciences, 2022, 12(12): 5965. |
| [23] | Howng W Y, Thorn R J. Investigation of the electronic structure of L a 1 - x ( M 2 + ) x C r O 3 , Cr2O3 and La2O3 by X-ray photoelectron spectroscopy[J]. Journal of Physics and Chemistry of Solids, 1980, 41(1): 75-81. |
| [24] | Jia Y G, Chen S J, Shao X, et al. Synergetic effect of lattice distortion and oxygen vacancies on high-rate lithium-ion storage in high-entropy perovskite oxides[J]. Journal of Advanced Ceramics, 2023, 12(6): 1214-1227. |
| [25] | Xiao B, Wu G, Wang T D, et al. Enhanced Li-ion diffusion and cycling stability of Ni-free high-entropy spinel oxide anodes with high-concentration oxygen vacancies[J]. ACS Applied Materials & Interfaces, 2023, 15(2): 2792-2803. |
| [26] | Tian K H, Duan C Q, Ma Q, et al. High-entropy chemistry stabilizing spinel oxide (CoNiZnXMnLi)3O4 (X = Fe, Cr) for high-performance anode of Li-ion batteries[J]. Rare Metals, 2022, 41(4): 1265-1275. |
| [27] | Wei S, Wan C C, Zhang L Y, et al. N-doped and oxygen vacancy-rich NiCo2O4 nanograss for supercapacitor electrode [J]. Chemical Engineering Journal, 2022, 429: 132242. |
| [28] | Nguyen T X, Patra J, Tsai C C, et al. Secondary-phase-induced charge-discharge performance enhancement of Co-free high entropy spinel oxide electrodes for Li-ion batteries[J]. Advanced Functional Materials, 2023, 33(30): 2300509. |
| [29] | 冯炜程, 于景成, 杨溢澜, 等. 调控双钙钛矿中高熵组分促进高温析氧反应[J]. 物理化学学报, 2024, 40(6): 2306013. |
| Feng W C, Yu J C, Yang Y L, et al. Regulating the high entropy component of double perovskite for high-temperature oxygen evolution reaction[J]. Acta Physico-Chimica Sinica, 2024, 40(6): 2306013. | |
| [30] | Yang X D, Li F, Liu W, et al. Oxygen vacancy-induced spin polarization of tungsten oxide nanowires for efficient photocatalytic reduction and immobilization of uranium(Ⅵ) under simulated solar light[J]. Applied Catalysis B-Environmental, 2023, 324: 122202. |
| [31] | Duan C Q, Tian K H, Li X L, et al. New spinel high-entropy oxides (FeCoNiCrMnXLi)3O4 (X= Cu, Mg, Zn) as the anode material for lithium-ion batteries[J]. Ceramics International, 2021, 47(22): 32025-32032. |
| [32] | 杨毅, 闫崇, 黄佳琦. 锂电池中固体电解质界面研究进展[J]. 物理化学学报, 2021, 37(11): 62-74. |
| Yang Y, Yan C, Huang J Q, et al. Research progress of solid electrolyte interphase in lithium batteries[J]. Acta Physico-Chimica Sinica, 2021, 37(11): 62-74. | |
| [33] | Zhai F Y, Zhu X Y, Zhang W F, et al. Insight of the evolution of structure and energy storage mechanism of (FeCoNiCrMn)3O4 spinel high entropy oxide in life-cycle span as lithium-ion battery anode[J]. Journal of Power Sources, 2024, 603: 234418. |
| [34] | Kim H, Choi W, Yoon J, et al. Exploring anomalous charge storage in anode materials for next-generation Li rechargeable batteries[J]. Chemical Reviews, 2020, 120(14): 6934-6976. |
| [35] | Li X L, Lin Z F, Jin N, et al. Boosting the lithium-ion storage performance of perovskite Sr x VO3- δ via Sr cation and O anion deficient engineering[J]. Chinese Science Bulletin, 2022, 67(22): 2305-2315. |
| [36] | Du W Q, Zheng Y Q, Liu X Y, et al. Oxygen-enriched vacancy spinel MFe2O4/carbon (M= Ni, Mn, Co) derived from metal-organic frameworks toward boosting lithium storage[J]. Chemical Engineering Journal, 2023, 451(2): 138626. |
| [37] | Lv H L, Wang X J, Yang Y, et al. RGO-coated MOF-derived In2Se3 as a high-performance anode for sodium-ion batteries[J]. Acta Physico-Chimica Sinica, 2023, 39(3): 2210014. |
| [1] | 吴馨, 龚建英, 李祥宇, 王宇涛, 杨小龙, 蒋震. 超声波激励疏水表面液滴运动的实验研究[J]. 化工学报, 2025, 76(S1): 133-139. |
| [2] | 曹庆泰, 郭松源, 李建强, 蒋赞, 汪彬, 耑锐, 吴静怡, 杨光. 负过载下多孔隔板对液氧贮箱蓄液性能的影响研究[J]. 化工学报, 2025, 76(S1): 217-229. |
| [3] | 娄岚浩, 杨立鹏, 杨晓光. 锂离子电池电化学机理模型参数辨识研究综述[J]. 化工学报, 2025, 76(9): 4369-4382. |
| [4] | 曾宁, 郭振江, 陈建华, 张子轩, 曾玉娇, 肖炘, 刘松林, 薛绍秀, 周智武, 卢振明, 王利民. 二水湿法磷酸工艺中非水溶磷的分子动力学模拟[J]. 化工学报, 2025, 76(9): 4539-4550. |
| [5] | 徐佳琪, 张文君, 余燕萍, 苏宝根, 任其龙, 杨启炜. 热等离子体重整炼厂气制合成气过程数值模拟与实验研究[J]. 化工学报, 2025, 76(9): 4462-4473. |
| [6] | 王三一, 黄文来. 电化学合成氨流程建模与优化[J]. 化工学报, 2025, 76(9): 4474-4486. |
| [7] | 王钰, 冯英楠, 王涛, 赵之平. 原位生长构筑纳米复合纳滤膜:膜制备与应用[J]. 化工学报, 2025, 76(9): 4723-4736. |
| [8] | 刘世昌, 李一白, 王靖, 刘永忠. 氢气驱动电化学捕碳系统的模块化设计与优化[J]. 化工学报, 2025, 76(8): 4108-4118. |
| [9] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [10] | 田宇红, 杜壮壮, 徐慧芳, 祝自强, 王宇聪. ZIF-8基多孔液体制备及其SO2吸附性能[J]. 化工学报, 2025, 76(8): 4284-4296. |
| [11] | 李云昊, 徐纯刚, 李小森, 付骏, 王屹, 陈朝阳. 固液复配型促进剂对盐水体系CO2水合物形成影响研究[J]. 化工学报, 2025, 76(8): 4228-4238. |
| [12] | 佘海龙, 胡光忠, 崔晓钰, 柳忠彬, 彭帝, 李航. 不同节流工质下叠层微通道分布式节流制冷器性能研究[J]. 化工学报, 2025, 76(8): 4017-4029. |
| [13] | 刘璐, 杨莹, 杨浩文, 王太, 王腾, 董新宇, 闫润. 星形亲水区组合表面冷凝液滴脱落特性实验研究[J]. 化工学报, 2025, 76(8): 3905-3914. |
| [14] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [15] | 吴阿强, 诸葛祥群, 刘通, 王明星, 罗鲲. 纳米普鲁士蓝悬浮电解液对锂氧电池性能的影响[J]. 化工学报, 2025, 76(8): 4310-4317. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号