化工学报 ›› 2025, Vol. 76 ›› Issue (S1): 418-425.DOI: 10.11949/0438-1157.20241279
• 能源和环境工程 • 上一篇
收稿日期:2024-11-11
修回日期:2024-11-19
出版日期:2025-06-25
发布日期:2025-06-26
通讯作者:
何婷
作者简介:何婷(1995—),女,博士,副研究员,heting199503@163.com
基金资助:
Ting HE1( ), Kai ZHANG1, Wensheng LIN2, Liqiong CHEN1, Jiafu CHEN1
), Kai ZHANG1, Wensheng LIN2, Liqiong CHEN1, Jiafu CHEN1
Received:2024-11-11
Revised:2024-11-19
Online:2025-06-25
Published:2025-06-26
Contact:
Ting HE
摘要:
大力发展沼气是缓解我国天然气供应紧张的重要途径,但是沼气中CO2含量高达20%以上,严重影响热值和储运。沼气脱碳后制LNG可显著提高热值和储运便捷性。本研究提出一种沼气超临界压力低温脱碳-液化耦合流程,以解决现有沼气低温脱碳技术中精馏易冻堵、低压凝华能耗高的难题。采用HYSYS软件和遗传算法对所提出的流程进行了建模和优化,结果表明,沼气在8.5 MPa下冷却到-131℃时可将CO2脱除至0.5%。当CO2含量在10%~30%时,系统比功耗为0.5295~0.6149 kWh/kg LNG,系统㶲效率为57.5%~62.1%。与基于双塔精馏、化学吸收、低压凝华等的工艺相比,在LNG密度仅降低11.9%时,可实现比功耗降低60%以上。
中图分类号:
何婷, 张开, 林文胜, 陈利琼, 陈家富. 沼气超临界压力低温脱碳-液化耦合流程研究[J]. 化工学报, 2025, 76(S1): 418-425.
Ting HE, Kai ZHANG, Wensheng LIN, Liqiong CHEN, Jiafu CHEN. Research on integrated process of cryogenic CO2 removal under supercritical pressure and liquefaction for biogas[J]. CIESC Journal, 2025, 76(S1): 418-425.
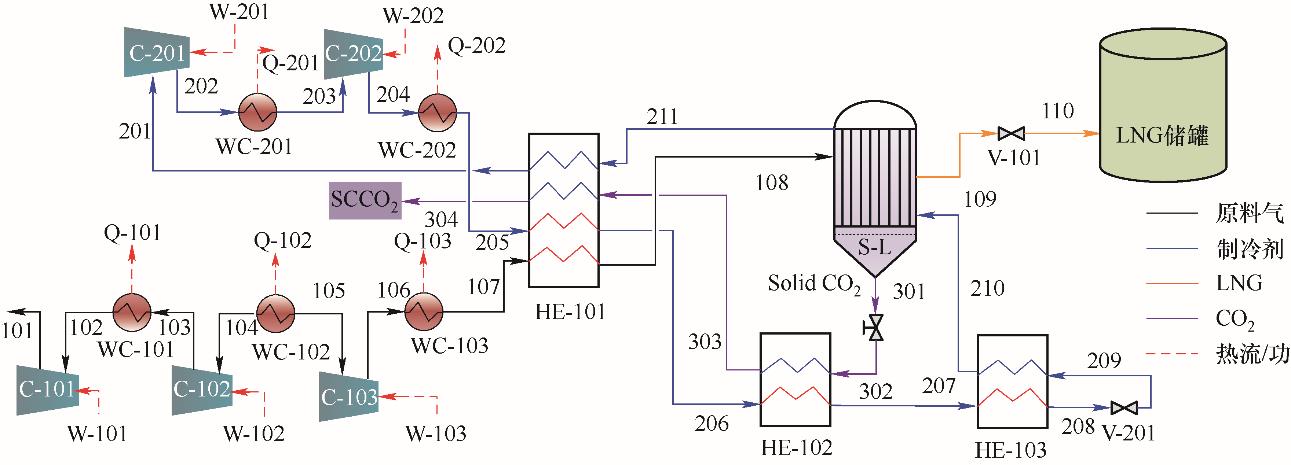
图3 沼气超临界压力低温脱碳-液化耦合流程图C—压缩机;HE—换热器;Q—热量;S-L—液固分离器;V—阀;W—功;WC—水冷器
Fig.3 Integrated cryogenic CO2 removal under supercritical pressure and liquefaction process for biogasC—compressor;HE—heat exchanger;Q—heat flow;S-L—solid-liquid separator;V—valve;W—work;WC—water-cooler
| 参数 | 数值 | |
|---|---|---|
| 假设条件 | 水冷器出口温度 | 35℃ |
| 设备压降 | 0 | |
| 最小换热温差 | 3℃ | |
| 压缩机绝热效率 | 85% | |
| 遗传算法参数 | ||
| 交叉概率 | ||
表1 优化参数设置
Table 1 Parameter settings for process optimization
| 参数 | 数值 | |
|---|---|---|
| 假设条件 | 水冷器出口温度 | 35℃ |
| 设备压降 | 0 | |
| 最小换热温差 | 3℃ | |
| 压缩机绝热效率 | 85% | |
| 遗传算法参数 | ||
| 交叉概率 | ||
| 优化变量 | 下限 | 上限 |
|---|---|---|
| N201-C1 /(kmol/h) | 0 | 600 |
| N201-C2 /(kmol/h) | 0 | 600 |
| N201-C3 /(kmol/h) | 0 | 600 |
| N201-C4 /(kmol/h) | 0 | 600 |
| P204/kPa | 1800 | 3500 |
| P208/kPa | 110 | 250 |
| T206/℃ | -50 | -10 |
| T208/℃ | -135 | -100 |
表2 优化参数的上下限
Table 2 Upper and lower bounds of parameters to be optimized
| 优化变量 | 下限 | 上限 |
|---|---|---|
| N201-C1 /(kmol/h) | 0 | 600 |
| N201-C2 /(kmol/h) | 0 | 600 |
| N201-C3 /(kmol/h) | 0 | 600 |
| N201-C4 /(kmol/h) | 0 | 600 |
| P204/kPa | 1800 | 3500 |
| P208/kPa | 110 | 250 |
| T206/℃ | -50 | -10 |
| T208/℃ | -135 | -100 |
| 物流 | 温度/℃ | 压力/kPa | 摩尔流量/(kmol/h) | CO2 含量/% | CH4 含量/% | |
|---|---|---|---|---|---|---|
| 原料气侧 | 101 | 35 | 101 | 1000 | 30 | 70 |
| 107 | 35 | 8500 | 1000 | 30 | 70 | |
| 108 | -40 | 8500 | 1000 | 30 | 70 | |
| 109 | -131 | 8500 | 703.5 | 0.05 | 99.95 | |
| 110 | -130 | 1000 | 703.5 | 0.05 | 99.95 | |
| 301 | -131 | 8500 | 296.5 | 100 | 0 | |
| 304 | 30 | 8500 | 296.5 | 100 | 0 | |
| 混合制冷剂 | 201 | 32 | 130 | 1064 | ||
| 205 | 35 | 2560 | 1064 | |||
| 206 | -30 | 2560 | 1064 | |||
| 208 | -130 | 2560 | 1064 | |||
| 209 | -135.9 | 130 | 1064 | |||
| 211 | -43.2 | 130 | 1064 | |||
表3 流程关键节点参数
Table 3 Key process parameters
| 物流 | 温度/℃ | 压力/kPa | 摩尔流量/(kmol/h) | CO2 含量/% | CH4 含量/% | |
|---|---|---|---|---|---|---|
| 原料气侧 | 101 | 35 | 101 | 1000 | 30 | 70 |
| 107 | 35 | 8500 | 1000 | 30 | 70 | |
| 108 | -40 | 8500 | 1000 | 30 | 70 | |
| 109 | -131 | 8500 | 703.5 | 0.05 | 99.95 | |
| 110 | -130 | 1000 | 703.5 | 0.05 | 99.95 | |
| 301 | -131 | 8500 | 296.5 | 100 | 0 | |
| 304 | 30 | 8500 | 296.5 | 100 | 0 | |
| 混合制冷剂 | 201 | 32 | 130 | 1064 | ||
| 205 | 35 | 2560 | 1064 | |||
| 206 | -30 | 2560 | 1064 | |||
| 208 | -130 | 2560 | 1064 | |||
| 209 | -135.9 | 130 | 1064 | |||
| 211 | -43.2 | 130 | 1064 | |||
| 流程 | 比功耗/(kWh/kg LNG) | 比功耗降低幅度/% |
|---|---|---|
| 本研究 | 0.6149 | — |
| 双塔精馏[ | 2.07 | 70.3 |
| 化学吸收[ | 1.54 | 60.1 |
| 低压凝华[ | 1.574 | 60.9 |
表4 本研究和其他研究的对比
Table 4 Comparison between this study and other studies
| 流程 | 比功耗/(kWh/kg LNG) | 比功耗降低幅度/% |
|---|---|---|
| 本研究 | 0.6149 | — |
| 双塔精馏[ | 2.07 | 70.3 |
| 化学吸收[ | 1.54 | 60.1 |
| 低压凝华[ | 1.574 | 60.9 |
| CO2含量/% | 混合制冷剂流量/(kmol/h) | 制冷剂组分含量 | |||
|---|---|---|---|---|---|
| 甲烷 | 乙烷 | 丙烷 | 正丁烷 | ||
| 10 | 1340 | 0.220149 | 0.35597 | 0.10597 | 0.31791 |
| 15 | 1279 | 0.248632 | 0.308835 | 0.111024 | 0.331509 |
| 20 | 1210 | 0.247934 | 0.293388 | 0.123967 | 0.334711 |
| 25 | 1140 | 0.245614 | 0.280702 | 0.131579 | 0.342105 |
| 30 | 1064 | 0.23496241 | 0.28195489 | 0.13345865 | 0.34962406 |
表5 不同CO2含量下制冷循环的参数
Table 5 Parameters of the refrigeration cycle under different CO2 contents
| CO2含量/% | 混合制冷剂流量/(kmol/h) | 制冷剂组分含量 | |||
|---|---|---|---|---|---|
| 甲烷 | 乙烷 | 丙烷 | 正丁烷 | ||
| 10 | 1340 | 0.220149 | 0.35597 | 0.10597 | 0.31791 |
| 15 | 1279 | 0.248632 | 0.308835 | 0.111024 | 0.331509 |
| 20 | 1210 | 0.247934 | 0.293388 | 0.123967 | 0.334711 |
| 25 | 1140 | 0.245614 | 0.280702 | 0.131579 | 0.342105 |
| 30 | 1064 | 0.23496241 | 0.28195489 | 0.13345865 | 0.34962406 |
| 13 | Baccioli A, Antonelli M, Frigo S, et al. Small scale bio-LNG plant: comparison of different biogas upgrading techniques[J]. Applied Energy, 2018, 217: 328-335. |
| 14 | Ryan J M, Schaffert F W. CO2 recovery by the Ryan/Holmes process[J]. Chemical Engineering Progress, 1984, 80(10): 53-56. |
| 15 | Berstad D, Anantharaman R, Nekså P. Low-temperature CO2 capture technologies — applications and potential[J]. International Journal of Refrigeration, 2013, 36(5): 1403-1416. |
| 16 | Roussanaly S, Anantharaman R, Lindqvist K. Multi-criteria analyses of two solvent and one low-temperature concepts for acid gas removal from natural gas[J]. Journal of Natural Gas Science and Engineering, 2014, 20: 38-49. |
| 17 | Pellegrini L A. Process for the removal of CO2 from acid gas: EP13774252.4[P]. 2015-09-16. |
| 18 | Valencia J A, Denton R D. Method and apparatus for separating carbon dioxide and other acid gases from methane by the use of distillation and a controlled freezing zone: US04533372A[P]. 1985-08-06. |
| 19 | Thomas E R, Denton R D. Conceptual studies for CO2/natural gas separation using the controlled freeze zone (CFZ) process[J]. Gas Separation & Purification, 1988, 2(2): 84-89. |
| 20 | Northrop P S, Valencia J A. The CFZTM process: a cryogenic method for handling high-CO2 and H2S gas reserves and facilitating geosequestration of CO2 and acid gases[J]. Energy Procedia, 2009, 1(1): 171-177. |
| 21 | Hart A, Gnanendran N. Cryogenic CO2 capture in natural gas[J]. Energy Procedia, 2009, 1(1): 697-706. |
| 22 | Babar M, Bustam M A, Maulud A S, et al. Enhanced cryogenic packed bed with optimal CO2 removal from natural gas; a joint computational and experimental approach[J]. Cryogenics, 2020, 105: 103010. |
| 23 | Baccanelli M, Langé S, Rocco M V, et al. Low temperature techniques for natural gas purification and LNG production: an energy and exergy analysis[J]. Applied Energy, 2016, 180: 546-559. |
| 24 | Naquash A, Qyyum M A, Haider J, et al. Renewable LNG production: biogas upgrading through CO2 solidification integrated with single-loop mixed refrigerant biomethane liquefaction process[J]. Energy Conversion Management, 2021, 243: 114363. |
| 1 | 曾金繁. 醇胺和膜分离结合的沼气脱碳工艺流程模拟研究[D]. 上海: 上海交通大学, 2021. |
| Zeng J F. Process simulation and parameter optimization of CO2 removal by alkanolamine and membrane for biogas purification[D]. Shanghai: Shanghai Jiao Tong University, 2021. | |
| 2 | 中国沼气学会. 中国沼气行业“双碳”发展报告[R]. 北京: 中国沼气协会, 2021. |
| China Biogas Society. Report on the “double carbon” development of China's biogas industry[R]. Beijing: China Biogas Society, 2021. | |
| 3 | 尹龙天. 基于MEA-乙醇吸收的旋转床用于沼气中CO2脱除性能与模拟研究[D]. 北京: 北京化工大学, 2021. |
| Yin L T. Study on the performance and simulation of CO2 removal from biogas by a rotating bed based on MEA-ethanol absorption[D]. Beijing: Beijing University of Chemical Technology, 2021. | |
| 4 | 曾金繁, 巨永林. 采用醇胺法的沼气脱碳工艺流程模拟及优化[J]. 现代化工, 2021, 41(8): 224-229. |
| Zeng J F, Ju Y L. Process simulation and parameter optimization of alkanolamine route for removing CO2 from biogas[J]. Modern Chemical Industry, 2021, 41(8): 224-229. | |
| 5 | 周淑霞. 沼气液化制取生物质LNG关键技术研究[D]. 济南: 山东大学, 2012. |
| Zhou S X. Research on key technologies on liquefied production of biomass LNG from biogas[D]. Jinan: Shandong University, 2013. | |
| 6 | He T, Si B, Gundersen T, et al. Integrated ethane recovery and cryogenic carbon capture in a dual mixed refrigerant natural gas liquefaction process [J]. Energy, 2024, 290: 130125. |
| 7 | 洪宗平, 叶楚梅, 吴洪, 等. 天然气脱碳技术研究进展[J]. 化工学报, 2021, 72(12): 6030-6048. |
| Hong Z P, Ye C M, Wu H, et al. Research progress in CO2 removal technology of natural gas[J]. CIESC Journal, 2021, 72(12): 6030-6048. | |
| 8 | He T, Liu Z, Son H, et al. Comparative analysis of cryogenic distillation and chemical absorption for carbon capture in integrated natural gas liquefaction processes[J]. Journal of Cleaner Production, 2024, 383: 135264. |
| 9 | 何婷, 林文胜. 基于余热利用的活化MDEA法脱除CO2的天然气液化系统[J]. 化工学报, 2021, 72(S1): 453-460. |
| He T, Lin W S. Natural gas liquefaction system with activated MDEA method for CO2 removal based on waste heat utilization[J]. CIESC Journal, 2021, 72(S1): 453-460. | |
| 10 | Baena-Moreno F M, Saché E, Pastor-Pérez L, et al. Membrane-based technologies for biogas upgrading: a review[J]. Environmental Chemistry Letters, 2020, 18(5): 1649-1658. |
| 11 | Yusuf N, Almomani F. Recent advances in biogas purifying technologies: process design and economic considerations[J]. Energy, 2023, 265: 126163. |
| 12 | Bi Y J, Ju Y L. Review on cryogenic technologies for CO2 removal from natural gas[J]. Frontiers in Energy, 2022, 16(5): 793-811. |
| 25 | Hashemi S E, Sarker S, Lien K M, et al. Cryogenic vs. absorption biogas upgrading in liquefied biomethane production — an energy efficiency analysis[J]. Fuel, 2019, 245: 294-304. |
| 26 | Pellegrini L A, De Guido G, Langé S. Biogas to liquefied biomethane via cryogenic upgrading technologies[J]. Renewable Energy, 2018, 124: 75-83. |
| 27 | Spitoni M, Pierantozzi M, Comodi G, et al. Theoretical evaluation and optimization of a cryogenic technology for carbon dioxide separation and methane liquefaction from biogas[J]. Journal of Natural Gas Science and Engineering, 2019, 62: 132-143. |
| 28 | Xiong X J, Lin W S, Gu A Z. Integration of CO2 cryogenic removal with a natural gas pressurized liquefaction process using gas expansion refrigeration[J]. Energy, 2015, 93: 1-9. |
| 29 | Babar M, Bustam M A, Ali A, et al. Thermodynamic data for cryogenic carbon dioxide capture from natural gas: a review[J]. Cryogenics, 2019, 102: 85-104. |
| 30 | Peng D Y, Robinson D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals, 1976, 15: 59-64. |
| 31 | Smith J M, Ness H, Abbott M M. Introduction to Chemical Engineering Thermodynamics[M]. New York: McGraw-Hill, 1975. |
| [1] | 孙云龙, 徐肖肖, 黄永方, 郭纪超, 陈卫卫. 水平光滑管内CO2流动沸腾的非绝热可视化研究[J]. 化工学报, 2025, 76(S1): 230-236. |
| [2] | 郭纪超, 徐肖肖, 孙云龙. 基于植物工厂中的CO2浓度气流模拟及优化研究[J]. 化工学报, 2025, 76(S1): 237-245. |
| [3] | 石一帆, 柯钢, 陈浩, 黄孝胜, 叶芳, 李成娇, 郭航. 大型高低温环境实验室温度控制仿真[J]. 化工学报, 2025, 76(S1): 268-280. |
| [4] | 孔繁臣, 张硕, 唐明生, 邹慧明, 胡舟航, 田长青. 二氧化碳直线压缩机气体轴承模拟[J]. 化工学报, 2025, 76(S1): 281-288. |
| [5] | 何婷, 黄舒阳, 黄坤, 陈利琼. 基于余热利用的天然气化学吸收脱碳-高温热泵耦合流程研究[J]. 化工学报, 2025, 76(S1): 297-308. |
| [6] | 沙鑫权, 胡然, 丁磊, 蒋珍华, 吴亦农. 空间用单机两级有阀线性压缩机研制及测试[J]. 化工学报, 2025, 76(S1): 114-122. |
| [7] | 燕子腾, 詹飞龙, 丁国良. 空调用套管式分流器结构设计及分流效果验证[J]. 化工学报, 2025, 76(S1): 152-159. |
| [8] | 汪思远, 刘国强, 熊通, 晏刚. 窗式空调器轴流风机的风速非均匀分布特性及其对冷凝器流路优化设计的影响规律[J]. 化工学报, 2025, 76(S1): 205-216. |
| [9] | 马瑞洁, 黄子轩, 关雪倩, 陈光进, 刘蓓. ZIF-8/DMPU浆液分离C2H6/ CH4混合气研究[J]. 化工学报, 2025, 76(5): 2262-2269. |
| [10] | 陈建兵, 常昊, 高明, 邢兵, 张磊, 刘奇磊. 基于反应模板与分子动力学的胺基相变吸收剂分相预测方法[J]. 化工学报, 2025, 76(5): 2387-2396. |
| [11] | 郭明钢, 杨晓航, 代岩, 米盼盼, 马世鑫, 贺高红, 肖武, 崔福军. 贫氦管输天然气提氦多元化产品耦合工艺优化设计[J]. 化工学报, 2025, 76(5): 2251-2261. |
| [12] | 产文, 余万, 王岗, 苏华山, 黄芬霞, 胡涛. 改进回热布局的Allam循环热力、经济性能分析和双目标优化[J]. 化工学报, 2025, 76(4): 1680-1692. |
| [13] | 霍军良, 唐治国, 邱宗君, 冯玉华, 蒋旭, 王乐怡, 杨宇, 乔帆帆, 赫一凡, 喻健良. 节流作用下CO2管道放空过程的冻堵风险实验研究[J]. 化工学报, 2025, 76(4): 1898-1908. |
| [14] | 吴罗长, 杨泽宇, 颜建国, 朱旭涛, 陈阳, 王子辰. 微小方形通道内近超临界压力二氧化碳流动换热特性实验研究[J]. 化工学报, 2025, 76(4): 1583-1594. |
| [15] | 刘璐, 万开, 王文玥, 王太, 汤建成, 王少恒. 基于氦膨胀制冷的正仲氢转化耦合流动换热研究[J]. 化工学报, 2025, 76(4): 1513-1522. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号