化工学报 ›› 2025, Vol. 76 ›› Issue (9): 4449-4461.DOI: 10.11949/0438-1157.20250383
胡国祥1( ), 朱忆魁2, 龙华1, 刘晓雯1(
), 朱忆魁2, 龙华1, 刘晓雯1( ), 熊勤钢1(
), 熊勤钢1( )
)
收稿日期:2025-04-14
修回日期:2025-06-30
出版日期:2025-09-25
发布日期:2025-10-23
通讯作者:
刘晓雯,熊勤钢
作者简介:胡国祥(2000—),男,硕士研究生,872234256@qq.com
基金资助:
Guoxiang HU1( ), Yikui ZHU2, Hua LONG1, Xiaowen LIU1(
), Yikui ZHU2, Hua LONG1, Xiaowen LIU1( ), Qingang XIONG1(
), Qingang XIONG1( )
)
Received:2025-04-14
Revised:2025-06-30
Online:2025-09-25
Published:2025-10-23
Contact:
Xiaowen LIU, Qingang XIONG
摘要:
本文针对氯化胆碱-乳酸低共熔溶剂(ChCl-LA)的碱木质素溶解度随乳酸摩尔比增大而提升的实验现象,从原子、分子尺度探究ChCl-LA组分配比影响碱木质素溶解度的本质机理。基于密度泛函理论,考察了不同组分配比ChCl-LA的分子表面静电势、组分间的相互作用、氢键键能,分析了这些因素与ChCl-LA溶解木质素能力的内在关系。利用分子动力学模拟,研究了不同组分配比ChCl-LA、纯乳酸与木质素的相互作用,统计分析了各组分与木质素之间的氢键数、相互作用能、径向分布函数、空间分布函数对木质素溶解的影响规律。结果表明,ChCl-LA的组分配比决定了ChCl-LA内的活性质子和氢键性质,进而影响了ChCl-LA与木质素形成氢键、催化木质素解聚的能力,从而改变了ChCl-LA的木质素溶解度。以上结果为调控ChCl-LA溶解木质素的能力提供了理论支撑,对于ChCl-LA的制备和应用以及绿色、高效溶解木质素具有重要意义。
中图分类号:
胡国祥, 朱忆魁, 龙华, 刘晓雯, 熊勤钢. 组分配比影响氯化胆碱-乳酸低共熔溶剂碱木质素溶解度的底层机理研究[J]. 化工学报, 2025, 76(9): 4449-4461.
Guoxiang HU, Yikui ZHU, Hua LONG, Xiaowen LIU, Qingang XIONG. Study on the underlying mechanism of choline chloride-lactic acid molar ratio influencing alkali lignin solubility in choline chloride-lactic acid deep eutectic solvents[J]. CIESC Journal, 2025, 76(9): 4449-4461.
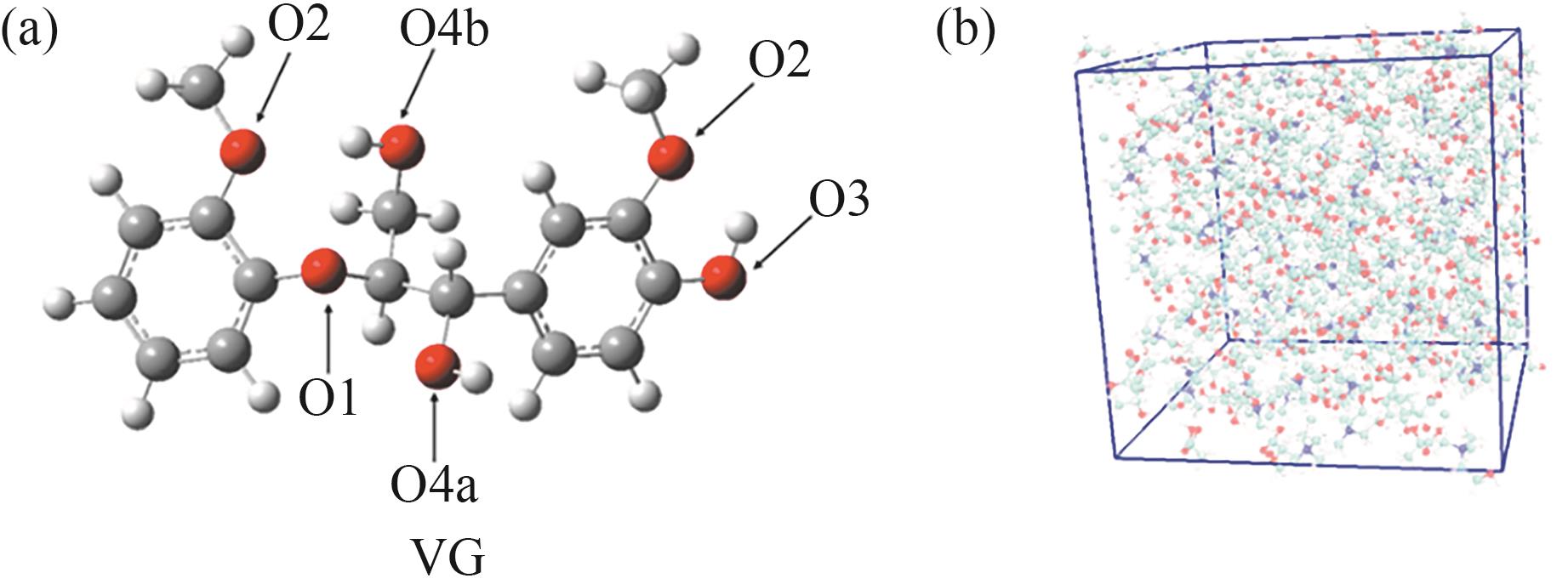
图2 (a) DFT优化后的VG结构;(b) 包含VG和ChCl-LA的MD模拟初始模型(D1-VG)
Fig.2 (a) Optimized geometry of VG using DFT; (b) Initial model for MD simulation including VG and ChCl-LA(D1-VG)
| ChCl-LA | 氢键 | 类型 | BCP | ρ/(a.u.) | (∇2ρ)/(a.u.) | 键能/(kcal/mol) |
|---|---|---|---|---|---|---|
| D1 | O20—H21⋯Cl | Ch+-Cl- | 87 | 0.023 | 0.070 | -4.39 |
| O23—H24⋯Cl | Cl--LA | 85 | 0.046 | 0.098 | -9.52 | |
| C6—H8⋯O26 | Ch+-LA | 42 | 0.017 | 0.063 | -3.05 | |
| C2—H5⋯ O26 | Ch+-LA | 60 | 0.014 | 0.048 | -2.38 | |
| C13—H14⋯ O26 | Ch+-LA | 62 | 0.011 | 0.038 | -1.71 | |
| D2 | O20—H21⋯Cl | Ch+-Cl- | 57 | 0.020 | 0.065 | -3.72 |
| O23—H24⋯Cl | Cl--LA | 54 | 0.036 | 0.091 | -7.29 | |
| O35—H46⋯Cl | Cl--LA | 51 | 0.033 | 0.087 | -6.62 | |
| C17—H19⋯ O43 | Ch+-LA | 82 | 0.014 | 0.045 | -2.38 | |
| C2—H3⋯ O43 | Ch+-LA | 91 | 0.017 | 0.057 | -3.05 | |
| C6—H8⋯ O26 | Ch+-LA | 111 | 0.013 | 0.050 | -2.16 | |
| C13—H14⋯ O26 | Ch+-LA | 93 | 0.012 | 0.042 | -1.93 | |
| C2—H5⋯ O26 | Ch+-LA | 97 | 0.014 | 0.048 | -2.38 | |
| D3 | O20—H21⋯Cl | Ch+-Cl- | 78 | 0.019 | 0.063 | -3.50 |
| O47—H58⋯Cl | Cl--LA | 139 | 0.024 | 0.071 | -4.61 | |
| O35—H46⋯Cl | Cl--LA | 108 | 0.029 | 0.081 | -5.73 | |
| O23—H24⋯Cl | Cl--LA | 120 | 0.029 | 0.083 | -5.73 | |
| C17—H19⋯ O43 | Ch+-LA | 79 | 0.013 | 0.042 | -2.16 | |
| C2—H3⋯ O43 | Ch+-LA | 97 | 0.017 | 0.058 | -3.05 | |
| C6—H8⋯ O26 | Ch+-LA | 90 | 0.011 | 0.042 | -1.71 | |
| C2—H5⋯ O26 | Ch+-LA | 106 | 0.018 | 0.062 | -3.27 | |
| C13—H14⋯ O26 | Ch+-LA | 87 | 0.010 | 0.036 | -1.49 | |
| C2—H5⋯ O26 | Ch+-LA | 133 | 0.014 | 0.052 | -2.38 | |
| D4 | O20—H21⋯Cl | Ch+-Cl- | 121 | 0.018 | 0.060 | -3.27 |
| O35—H36⋯Cl | Cl--LA | 131 | 0.023 | 0.068 | -4.39 | |
| O47—H48⋯Cl | Cl--LA | 160 | 0.026 | 0.076 | -5.06 | |
| O59—H61⋯Cl | Cl--LA | 161 | 0.028 | 0.079 | -5.50 | |
| O23—H26⋯Cl | Cl--LA | 150 | 0.014 | 0.051 | -2.38 | |
| C17—H19⋯ O44 | Ch+-LA | 85 | 0.014 | 0.047 | -2.38 | |
| C2—H3⋯ O44 | Ch+-LA | 101 | 0.016 | 0.055 | -2.83 | |
| C2—H3⋯ O50 | Ch+-LA | 133 | 0.011 | 0.047 | -1.71 | |
| C6—H8⋯ O62 | Ch+-LA | 149 | 0.015 | 0.054 | -2.60 | |
| C2—H5⋯ O62 | Ch+-LA | 156 | 0.015 | 0.049 | -2.60 | |
| C13—H14⋯ O25 | Ch+-LA | 132 | 0.013 | 0.048 | -2.16 | |
| C27—H28⋯ O20 | Ch+-LA | 89 | 0.011 | 0.042 | -1.71 | |
| D6 | O20—H21⋯Cl | Ch+-Cl- | 191 | 0.017 | 0.056 | -3.05 |
| O35—H36⋯Cl | Cl--LA | 197 | 0.025 | 0.074 | -4.83 | |
| O47—H48⋯Cl | Cl--LA | 153 | 0.021 | 0.063 | -3.94 | |
| O59—H61⋯Cl | Cl--LA | 154 | 0.022 | 0.065 | -4.17 | |
| O23—H26⋯Cl | Cl--LA | 140 | 0.013 | 0.045 | -2.15 | |
| C17—H19⋯ O44 | Ch+-LA | 104 | 0.016 | 0.055 | -2.83 | |
| C2—H3⋯ O44 | Ch+-LA | 178 | 0.013 | 0.043 | -2.16 | |
| C2—H3⋯ O50 | Ch+-LA | 179 | 0.014 | 0.045 | -2.38 | |
| C6—H8⋯ O62 | Ch+-LA | 163 | 0.017 | 0.058 | -3.05 | |
| C2—H5⋯ O62 | Ch+-LA | 206 | 0.015 | 0.047 | -2.60 | |
| C13—H14⋯ O25 | Ch+-LA | 141 | 0.009 | 0.032 | -1.26 | |
| C27—H28⋯ O20 | Ch+-LA | 145 | 0.016 | 0.055 | -2.83 | |
| C6—H8⋯ O65 | Ch+-LA | 198 | 0.012 | 0.042 | -1.93 | |
| O45—H46⋯ O91 | LA-LA | 221 | 0.017 | 0.058 | -3.05 | |
| C85—H86⋯ O80 | LA-LA | 215 | 0.009 | 0.032 | -1.26 | |
| C73—H74⋯ O45 | LA-LA | 165 | 0.012 | 0.042 | -1.93 | |
| D8 | O20—H21⋯Cl | Ch+-Cl- | 265 | 0.016 | 0.05 | -2.82 |
| O35—H36⋯Cl | Cl--LA | 228 | 0.018 | 0.060 | -3.27 | |
| O47—H48⋯Cl | Cl--LA | 253 | 0.020 | 0.06 | -3.72 | |
| O59—H61⋯Cl | Cl--LA | 202 | 0.023 | 0.067 | -4.39 | |
| O23—H26⋯Cl | Cl--LA | 242 | 0.021 | 0.063 | -3.94 | |
| C17—H19⋯ O44 | Ch+-LA | 247 | 0.013 | 0.043 | -2.16 | |
| C2—H3⋯ O44 | Ch+-LA | 232 | 0.015 | 0.047 | -2.60 | |
| C2—H3⋯ O50 | Ch+-LA | 179 | 0.014 | 0.045 | -2.38 | |
| C6—H8⋯ O62 | Ch+-LA | 251 | 0.010 | 0.036 | -1.49 | |
| C2—H5⋯ O62 | Ch+-LA | 159 | 0.016 | 0.055 | -2.83 | |
| C13—H14⋯ O25 | Ch+-LA | 226 | 0.015 | 0.047 | -2.60 | |
| C27—H28⋯ O20 | Ch+-LA | 167 | 0.013 | 0.043 | -2.16 | |
| C10—H11⋯ O44 | Ch+-LA | 189 | 0.014 | 0.045 | -2.38 | |
| C10—H15⋯ O44 | Ch+-LA | 162 | 0.009 | 0.032 | -1.26 | |
| C87—H89⋯ O79 | LA-LA | 138 | 0.019 | 0.063 | -3.50 | |
| C73—H74⋯ O45 | LA-LA | 129 | 0.012 | 0.042 | -1.93 | |
| O83—H94⋯ O45 | LA-LA | 187 | 0.018 | 0.062 | -3.27 | |
| O45—H46⋯ O91 | LA-LA | 315 | 0.009 | 0.032 | -1.26 | |
| C85—H86⋯ O80 | LA-LA | 180 | 0.010 | 0.036 | -1.49 | |
| D10 | O20—H21⋯Cl | Ch+-Cl- | 193 | 0.015 | 0.054 | -2.60 |
| O35—H36⋯Cl | Cl--LA | 172 | 0.019 | 0.063 | -3.50 | |
| O47—H48⋯Cl | Cl--LA | 204 | 0.017 | 0.056 | -3.05 | |
| O59—H61⋯Cl | Cl--LA | 183 | 0.021 | 0.063 | -3.94 | |
| O23—H26⋯Cl | Cl--LA | 197 | 0.022 | 0.065 | -4.17 | |
| C17—H19⋯ O44 | Ch+-LA | 280 | 0.010 | 0.036 | -1.49 | |
| C2—H3⋯ O44 | Ch+-LA | 219 | 0.014 | 0.045 | -2.38 | |
| C2—H3⋯ O50 | Ch+-LA | 264 | 0.016 | 0.055 | -2.83 | |
| C6—H8⋯ O62 | Ch+-LA | 260 | 0.013 | 0.045 | -2.15 | |
| C2—H5⋯ O62 | Ch+-LA | 228 | 0.013 | 0.048 | -2.16 | |
| C13—H14⋯ O25 | Ch+-LA | 222 | 0.014 | 0.045 | -2.38 | |
| C27—H28⋯ O20 | Ch+-LA | 282 | 0.014 | 0.045 | -2.38 | |
| C10—H11⋯ O44 | Ch+-LA | 291 | 0.015 | 0.047 | -2.60 | |
| C10—H15⋯ O44 | Ch+-LA | 248 | 0.013 | 0.048 | -2.16 | |
| C87—H89⋯ O79 | LA-LA | 233 | 0.010 | 0.036 | -1.49 | |
| C73—H74⋯ O45 | LA-LA | 158 | 0.014 | 0.045 | -2.38 | |
| O83—H94⋯ O45 | LA-LA | 197 | 0.016 | 0.055 | -2.83 | |
| O45—H46⋯ O91 | LA-LA | 353 | 0.013 | 0.048 | -2.16 | |
| C85—H86⋯ O80 | LA-LA | 376 | 0.009 | 0.032 | -1.26 | |
| O71—H82⋯ O104 | LA-LA | 365 | 0.014 | 0.045 | -2.38 | |
| O116—H117⋯ O35 | LA-LA | 279 | 0.009 | 0.032 | -1.26 |
表1 不同ChCl-LA的临界点拓扑参数
Table 1 Structure and topological parameters of key critical points in different ChCl-LA
| ChCl-LA | 氢键 | 类型 | BCP | ρ/(a.u.) | (∇2ρ)/(a.u.) | 键能/(kcal/mol) |
|---|---|---|---|---|---|---|
| D1 | O20—H21⋯Cl | Ch+-Cl- | 87 | 0.023 | 0.070 | -4.39 |
| O23—H24⋯Cl | Cl--LA | 85 | 0.046 | 0.098 | -9.52 | |
| C6—H8⋯O26 | Ch+-LA | 42 | 0.017 | 0.063 | -3.05 | |
| C2—H5⋯ O26 | Ch+-LA | 60 | 0.014 | 0.048 | -2.38 | |
| C13—H14⋯ O26 | Ch+-LA | 62 | 0.011 | 0.038 | -1.71 | |
| D2 | O20—H21⋯Cl | Ch+-Cl- | 57 | 0.020 | 0.065 | -3.72 |
| O23—H24⋯Cl | Cl--LA | 54 | 0.036 | 0.091 | -7.29 | |
| O35—H46⋯Cl | Cl--LA | 51 | 0.033 | 0.087 | -6.62 | |
| C17—H19⋯ O43 | Ch+-LA | 82 | 0.014 | 0.045 | -2.38 | |
| C2—H3⋯ O43 | Ch+-LA | 91 | 0.017 | 0.057 | -3.05 | |
| C6—H8⋯ O26 | Ch+-LA | 111 | 0.013 | 0.050 | -2.16 | |
| C13—H14⋯ O26 | Ch+-LA | 93 | 0.012 | 0.042 | -1.93 | |
| C2—H5⋯ O26 | Ch+-LA | 97 | 0.014 | 0.048 | -2.38 | |
| D3 | O20—H21⋯Cl | Ch+-Cl- | 78 | 0.019 | 0.063 | -3.50 |
| O47—H58⋯Cl | Cl--LA | 139 | 0.024 | 0.071 | -4.61 | |
| O35—H46⋯Cl | Cl--LA | 108 | 0.029 | 0.081 | -5.73 | |
| O23—H24⋯Cl | Cl--LA | 120 | 0.029 | 0.083 | -5.73 | |
| C17—H19⋯ O43 | Ch+-LA | 79 | 0.013 | 0.042 | -2.16 | |
| C2—H3⋯ O43 | Ch+-LA | 97 | 0.017 | 0.058 | -3.05 | |
| C6—H8⋯ O26 | Ch+-LA | 90 | 0.011 | 0.042 | -1.71 | |
| C2—H5⋯ O26 | Ch+-LA | 106 | 0.018 | 0.062 | -3.27 | |
| C13—H14⋯ O26 | Ch+-LA | 87 | 0.010 | 0.036 | -1.49 | |
| C2—H5⋯ O26 | Ch+-LA | 133 | 0.014 | 0.052 | -2.38 | |
| D4 | O20—H21⋯Cl | Ch+-Cl- | 121 | 0.018 | 0.060 | -3.27 |
| O35—H36⋯Cl | Cl--LA | 131 | 0.023 | 0.068 | -4.39 | |
| O47—H48⋯Cl | Cl--LA | 160 | 0.026 | 0.076 | -5.06 | |
| O59—H61⋯Cl | Cl--LA | 161 | 0.028 | 0.079 | -5.50 | |
| O23—H26⋯Cl | Cl--LA | 150 | 0.014 | 0.051 | -2.38 | |
| C17—H19⋯ O44 | Ch+-LA | 85 | 0.014 | 0.047 | -2.38 | |
| C2—H3⋯ O44 | Ch+-LA | 101 | 0.016 | 0.055 | -2.83 | |
| C2—H3⋯ O50 | Ch+-LA | 133 | 0.011 | 0.047 | -1.71 | |
| C6—H8⋯ O62 | Ch+-LA | 149 | 0.015 | 0.054 | -2.60 | |
| C2—H5⋯ O62 | Ch+-LA | 156 | 0.015 | 0.049 | -2.60 | |
| C13—H14⋯ O25 | Ch+-LA | 132 | 0.013 | 0.048 | -2.16 | |
| C27—H28⋯ O20 | Ch+-LA | 89 | 0.011 | 0.042 | -1.71 | |
| D6 | O20—H21⋯Cl | Ch+-Cl- | 191 | 0.017 | 0.056 | -3.05 |
| O35—H36⋯Cl | Cl--LA | 197 | 0.025 | 0.074 | -4.83 | |
| O47—H48⋯Cl | Cl--LA | 153 | 0.021 | 0.063 | -3.94 | |
| O59—H61⋯Cl | Cl--LA | 154 | 0.022 | 0.065 | -4.17 | |
| O23—H26⋯Cl | Cl--LA | 140 | 0.013 | 0.045 | -2.15 | |
| C17—H19⋯ O44 | Ch+-LA | 104 | 0.016 | 0.055 | -2.83 | |
| C2—H3⋯ O44 | Ch+-LA | 178 | 0.013 | 0.043 | -2.16 | |
| C2—H3⋯ O50 | Ch+-LA | 179 | 0.014 | 0.045 | -2.38 | |
| C6—H8⋯ O62 | Ch+-LA | 163 | 0.017 | 0.058 | -3.05 | |
| C2—H5⋯ O62 | Ch+-LA | 206 | 0.015 | 0.047 | -2.60 | |
| C13—H14⋯ O25 | Ch+-LA | 141 | 0.009 | 0.032 | -1.26 | |
| C27—H28⋯ O20 | Ch+-LA | 145 | 0.016 | 0.055 | -2.83 | |
| C6—H8⋯ O65 | Ch+-LA | 198 | 0.012 | 0.042 | -1.93 | |
| O45—H46⋯ O91 | LA-LA | 221 | 0.017 | 0.058 | -3.05 | |
| C85—H86⋯ O80 | LA-LA | 215 | 0.009 | 0.032 | -1.26 | |
| C73—H74⋯ O45 | LA-LA | 165 | 0.012 | 0.042 | -1.93 | |
| D8 | O20—H21⋯Cl | Ch+-Cl- | 265 | 0.016 | 0.05 | -2.82 |
| O35—H36⋯Cl | Cl--LA | 228 | 0.018 | 0.060 | -3.27 | |
| O47—H48⋯Cl | Cl--LA | 253 | 0.020 | 0.06 | -3.72 | |
| O59—H61⋯Cl | Cl--LA | 202 | 0.023 | 0.067 | -4.39 | |
| O23—H26⋯Cl | Cl--LA | 242 | 0.021 | 0.063 | -3.94 | |
| C17—H19⋯ O44 | Ch+-LA | 247 | 0.013 | 0.043 | -2.16 | |
| C2—H3⋯ O44 | Ch+-LA | 232 | 0.015 | 0.047 | -2.60 | |
| C2—H3⋯ O50 | Ch+-LA | 179 | 0.014 | 0.045 | -2.38 | |
| C6—H8⋯ O62 | Ch+-LA | 251 | 0.010 | 0.036 | -1.49 | |
| C2—H5⋯ O62 | Ch+-LA | 159 | 0.016 | 0.055 | -2.83 | |
| C13—H14⋯ O25 | Ch+-LA | 226 | 0.015 | 0.047 | -2.60 | |
| C27—H28⋯ O20 | Ch+-LA | 167 | 0.013 | 0.043 | -2.16 | |
| C10—H11⋯ O44 | Ch+-LA | 189 | 0.014 | 0.045 | -2.38 | |
| C10—H15⋯ O44 | Ch+-LA | 162 | 0.009 | 0.032 | -1.26 | |
| C87—H89⋯ O79 | LA-LA | 138 | 0.019 | 0.063 | -3.50 | |
| C73—H74⋯ O45 | LA-LA | 129 | 0.012 | 0.042 | -1.93 | |
| O83—H94⋯ O45 | LA-LA | 187 | 0.018 | 0.062 | -3.27 | |
| O45—H46⋯ O91 | LA-LA | 315 | 0.009 | 0.032 | -1.26 | |
| C85—H86⋯ O80 | LA-LA | 180 | 0.010 | 0.036 | -1.49 | |
| D10 | O20—H21⋯Cl | Ch+-Cl- | 193 | 0.015 | 0.054 | -2.60 |
| O35—H36⋯Cl | Cl--LA | 172 | 0.019 | 0.063 | -3.50 | |
| O47—H48⋯Cl | Cl--LA | 204 | 0.017 | 0.056 | -3.05 | |
| O59—H61⋯Cl | Cl--LA | 183 | 0.021 | 0.063 | -3.94 | |
| O23—H26⋯Cl | Cl--LA | 197 | 0.022 | 0.065 | -4.17 | |
| C17—H19⋯ O44 | Ch+-LA | 280 | 0.010 | 0.036 | -1.49 | |
| C2—H3⋯ O44 | Ch+-LA | 219 | 0.014 | 0.045 | -2.38 | |
| C2—H3⋯ O50 | Ch+-LA | 264 | 0.016 | 0.055 | -2.83 | |
| C6—H8⋯ O62 | Ch+-LA | 260 | 0.013 | 0.045 | -2.15 | |
| C2—H5⋯ O62 | Ch+-LA | 228 | 0.013 | 0.048 | -2.16 | |
| C13—H14⋯ O25 | Ch+-LA | 222 | 0.014 | 0.045 | -2.38 | |
| C27—H28⋯ O20 | Ch+-LA | 282 | 0.014 | 0.045 | -2.38 | |
| C10—H11⋯ O44 | Ch+-LA | 291 | 0.015 | 0.047 | -2.60 | |
| C10—H15⋯ O44 | Ch+-LA | 248 | 0.013 | 0.048 | -2.16 | |
| C87—H89⋯ O79 | LA-LA | 233 | 0.010 | 0.036 | -1.49 | |
| C73—H74⋯ O45 | LA-LA | 158 | 0.014 | 0.045 | -2.38 | |
| O83—H94⋯ O45 | LA-LA | 197 | 0.016 | 0.055 | -2.83 | |
| O45—H46⋯ O91 | LA-LA | 353 | 0.013 | 0.048 | -2.16 | |
| C85—H86⋯ O80 | LA-LA | 376 | 0.009 | 0.032 | -1.26 | |
| O71—H82⋯ O104 | LA-LA | 365 | 0.014 | 0.045 | -2.38 | |
| O116—H117⋯ O35 | LA-LA | 279 | 0.009 | 0.032 | -1.26 |
| [1] | Chen Z, Ragauskas A, Wan C X. Lignin extraction and upgrading using deep eutectic solvents[J]. Industrial Crops and Products, 2020, 147: 112241. |
| [2] | Mennani M, Kasbaji M, Ait Benhamou A, et al. Current approaches, emerging developments and functional prospects for lignin-based catalysts-a review[J]. Green Chemistry, 2023, 25(8): 2896-2929. |
| [3] | Kumar A K, Sharma S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: a review[J]. Bioresources and Bioprocessing, 2017, 4(1): 7. |
| [4] | 赵金政, 周国辉, 刘晓敏. 离子液体在生物质溶解分离中的应用与机理研究[J]. 化工学报, 2021, 72(1): 247-258. |
| Zhao J Z, Zhou G H, Liu X M. Study on application and mechanism of ionic liquids in biomass dissolution and separation[J]. CIESC Journal, 2021, 72(1): 247-258. | |
| [5] | Hong S, Shen X J, Xue Z M, et al. Structure-function relationships of deep eutectic solvents for lignin extraction and chemical transformation[J]. Green Chemistry, 2020, 22(21): 7219-7232. |
| [6] | Bao Y S, Wang Y, Yan C Y, et al. Deep eutectic solvents for fractionation and valorization of lignocellulose[J]. Green Chemical Engineering, 2025, 6(1): 21-35. |
| [7] | Lobato-Rodríguez Á, Gullón B, Romaní A, et al. Recent advances in biorefineries based on lignin extraction using deep eutectic solvents: a review[J]. Bioresource Technology, 2023, 388: 129744. |
| [8] | 刘巧玲, 汪洋, 藏启嘉. 低共熔溶剂的分类及其溶解木质素研究进展[J]. 林产化学与工业, 2024, 44(5): 80-90. |
| Liu Q L, Wang Y, Zang Q J, et al. Research progress on the classification of deep eutectic solvents and their dissolution of lignin[J]. Chemistry & Industry of Forest Products, 2024, 44(5): 80-90. | |
| [9] | Satlewal A, Agrawal R, Bhagia S, et al. Natural deep eutectic solvents for lignocellulosic biomass pretreatment: Recent developments, challenges and novel opportunities[J]. Biotechnology Advances, 2018, 36(8): 2032-2050. |
| [10] | Shen X J, Wen J L, Mei Q Q, et al. Facile fractionation of lignocelluloses by biomass-derived deep eutectic solvent (DES) pretreatment for cellulose enzymatic hydrolysis and lignin valorization[J]. Green Chemistry, 2019, 21(2): 275-283. |
| [11] | Francisco M, van den Bruinhorst A, Kroon M C. New natural and renewable low transition temperature mixtures (LTTMs): screening as solvents for lignocellulosic biomass processing [J]. Green Chemistry, 2012, 14(8): 2153-2157. |
| [12] | Liu Z, Hou Y, Hu S Q, et al. Possible dissolution mechanism of alkali lignin in lactic acid-choline chloride under mild conditions[J]. RSC Advances, 2020, 10(67): 40649-40657. |
| [13] | Zdanowicz M, Wilpiszewska K, Spychaj T. Deep eutectic solvents for polysaccharides processing. A review[J]. Carbohydrate Polymers, 2018, 200: 361-380. |
| [14] | Hou X D, Li A L, Lin K P, et al. Insight into the structure-function relationships of deep eutectic solvents during rice straw pretreatment[J]. Bioresource Technology, 2018, 249: 261-267. |
| [15] | Chen H Y, Wang A L, Yan C C, et al. Study on the solubility of industrial lignin in choline chloride-based deep eutectic solvents[J]. Sustainability, 2023, 15(9): 7118. |
| [16] | Biernacki K, Souza H K S, Almeida C M R, et al. Physicochemical properties of choline chloride-based deep eutectic solvents with polyols: an experimental and theoretical investigation[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(50): 18712-18728. |
| [17] | Zhang M, Zhao X M, Tang S Y, et al. Structure-properties relationships of deep eutectic solvents formed between choline chloride and carboxylic acids: experimental and computational study[J]. Journal of Molecular Structure, 2023, 1273: 134283. |
| [18] | Li Q W, Dong Y, Hammond K D, et al. Revealing the role of hydrogen bonding interactions and supramolecular complexes in lignin dissolution by deep eutectic solvents[J]. Journal of Molecular Liquids, 2021, 344: 117779. |
| [19] | Wang W X, Zhu B P, Xu Y, et al. Mechanism study of ternary deep eutectic solvents with protonic acid for lignin fractionation[J]. Bioresource Technology, 2022, 363: 127887. |
| [20] | Lynam J G, Kumar N, Wong M J. Deep eutectic solvents' ability to solubilize lignin, cellulose, and hemicellulose; thermal stability; and density[J]. Bioresource Technology, 2017, 238: 684-689. |
| [21] | Tan Y T, Ngoh G C, Chua A S M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin[J]. Bioresource Technology, 2019, 281: 359-366. |
| [22] | Frisch M J., Trucks G W., Schlegel H B, et al. Gaussian 16 Revision B.01 [CP].Wallingford CT: Gaussian Inc., 2016. |
| [23] | Hemelsoet K, De Vleeschouwer F, Van Speybroeck V, et al. Validation of DFT-based methods for predicting qualitative thermochemistry of large polyaromatics[J]. Chemphyschem, 2011, 12(6): 1100-1108. |
| [24] | Parr R G. Density functional theory of atoms and molecules[M]// Horizons of Quantum Chemistry. Dordrecht: Springer Netherlands, 1980: 5-15 |
| [25] | Lu T. Molculs program, Version 1.12[EB/OL].[2025-04-13]. . |
| [26] | Lu T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn[J]. The Journal of Chemical Physics, 2024, 161(8): 082503. |
| [27] | Lu T, Chen Q X. Interaction region indicator: a simple real space function clearly revealing both chemical bonds and weak interactions[J]. Chemistry–Methods, 2021, 1(5): 231-239. |
| [28] | Almas M, Khan A S, Ullah S, et al. Fast and efficient extraction of phenol from aqueous phase using deep eutectic solvents: experimental and density functional theory investigation for interactions studies[J]. Journal of Molecular Liquids, 2024, 404: 124942. |
| [29] | Zhu Y K, Li L, Hou Y S, et al. Investigation into the characteristics of bagasse processed with 3C-DES and the production of chemimechanical pulp for pulp molded[J]. Journal of Cleaner Production, 2024, 447: 141652. |
| [30] | Mishra D K, Gopakumar G, Pugazhenthi G, et al. Molecular and spectroscopic insights into a metal salt-based deep eutectic solvent: a combined quantum theory of atoms in molecules, noncovalent interaction, and density functional theory study[J]. The Journal of Physical Chemistry. A, 2021, 125(44): 9680-9690. |
| [31] | Van Der Spoel D, Lindahl E, Hess B, et al. GROMACS: fast, flexible, and free[J]. Journal of Computational Chemistry, 2005, 26(16): 1701-1718. |
| [32] | Zhu Y T, Yan J, Liu C B, et al. Modeling interactions between a β-O-4 type lignin model compound and 1-allyl-3-methylimidazolium chloride ionic liquid[J]. Biopolymers, 2017, 107(8): e23022. |
| [33] | Li R L, Zhang Z P, Wang X, et al. Revealing the mechanism of phenoxyethanol-acid pretreatment for removing lignin from bamboo: kinetic analysis and simulation analysis[J]. Green Chemistry, 2025, 27(11): 3044-3063. |
| [34] | Lee J M, Cheng X, Swails J M, et al. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field[J]. Journal of Chemical Theory and Computation, 2016, 12(1): 405-413. |
| [35] | Martínez L, Andrade R, Birgin E G, et al. PACKMOL: a package for building initial configurations for molecular dynamics simulations[J]. Journal of Computational Chemistry, 2009, 30(13): 2157-2164. |
| [36] | Ji H R, Lv P L. Mechanistic insights into the lignin dissolution behaviors of a recyclable acid hydrotrope, deep eutectic solvent (DES), and ionic liquid (IL)[J]. Green Chemistry, 2020, 22(4): 1378-1387. |
| [37] | Zhang Y L, Ren H W, Li B C, et al. Mechanistic insights into the lignin dissolution behavior in amino acid based deep eutectic solvents[J]. International Journal of Biological Macromolecules, 2023, 242: 124829. |
| [38] | Shi D Y, Zhou F Y, Mu W B, et al. Deep insights into the viscosity of deep eutectic solvents by an XGBoost-based model plus shapley additive explanation[J]. Physical Chemistry Chemical Physics, 2022, 24(42): 26029-26036. |
| [39] | Xu H F, Kong Y, Peng J J, et al. Mechanism of deep eutectic solvent delignification: insights from molecular dynamics simulations[J]. ACS Sustainable Chemistry & Engineering, 2021, 9(20): 7101-7111. |
| [40] | Schutyser W, Renders T, Van den Bosch S, et al. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading[J]. Chemical Society Reviews, 2018, 47(3): 852-908. |
| [1] | 李相海, 赖德林, 孔纲, 周健. 双仿生表面水下疏油协同机制的分子动力学模拟研究[J]. 化工学报, 2025, 76(9): 4551-4562. |
| [2] | 李泽权, 蔡天宇, 刘家骏, 陈奇志, 肖沛文, 徐小飞, 赵双良. 木质素基絮凝剂的合成与应用[J]. 化工学报, 2025, 76(9): 4709-4722. |
| [3] | 高正, 汪辉, 屈治国. 数据驱动辅助高通量筛选阴离子柱撑金属有机框架储氢[J]. 化工学报, 2025, 76(8): 4259-4272. |
| [4] | 张荟钦, 赵泓竣, 付正军, 庄力, 董凯, 贾添智, 曹雪丽, 孙世鹏. 纳滤膜在离子型稀土浸出液提浓中的应用研究[J]. 化工学报, 2025, 76(8): 4095-4107. |
| [5] | 林嘉豪, 付芳忠, 叶昊辉, 胡金, 姚明灿, 范鹤林, 王旭, 王瑞祥, 徐志峰. NdF3含量对NdF3-LiF熔盐局域结构和输运性质的影响[J]. 化工学报, 2025, 76(8): 3834-3841. |
| [6] | 王小令, 王绍清, 赵云刚, 常方哲, 穆瑞峰. 基于ReaxFF MD模拟的煤加氢热解有机Ca转化机制研究[J]. 化工学报, 2025, 76(8): 4297-4309. |
| [7] | 乔亮, 李尚, 刘新亮, 王明, 张沛, 侯影飞. 三元共聚物稠油降黏剂的合成及分子模拟研究[J]. 化工学报, 2025, 76(7): 3686-3695. |
| [8] | 李姿睿, 齐凯, 王军, 夏国栋. 基于Janus纳米通道的脱盐过程分子动力学模拟研究[J]. 化工学报, 2025, 76(7): 3531-3538. |
| [9] | 胡嘉朗, 姜明源, 金律铭, 张永刚, 胡鹏, 纪红兵. 机器学习辅助MOFs高通量计算筛选及气体分离研究进展[J]. 化工学报, 2025, 76(5): 1973-1996. |
| [10] | 齐昊, 王玉杰, 李申辉, 邹琦, 刘轶群, 赵之平. 双金属Co/Zn-ZIFs中C3H6和C3H8吸附和扩散行为分子模拟研究[J]. 化工学报, 2025, 76(5): 2313-2326. |
| [11] | 陈建兵, 常昊, 高明, 邢兵, 张磊, 刘奇磊. 基于反应模板与分子动力学的胺基相变吸收剂分相预测方法[J]. 化工学报, 2025, 76(5): 2387-2396. |
| [12] | 李紫鹃, 谭晓艳, 吴永盛, 杨陈怡, 陈红, 毕小刚, 刘捷, 喻发全. 分子模拟研究三维扭曲催化芳烃-降冰片烯环化聚合物膜的CO2/N2分离机理[J]. 化工学报, 2025, 76(5): 2348-2357. |
| [13] | 石孟琪, 王欢, 王守娟, 席跃宾, 孔凡功. 木质素基炭材料的制备及其在锂硫电池中的研究进展[J]. 化工学报, 2025, 76(4): 1463-1483. |
| [14] | 朱峰, 赵跃, 马凤翔, 刘伟. 改性UIO-66对SF6/N2混合气体及其分解产物的吸附特性[J]. 化工学报, 2025, 76(4): 1604-1616. |
| [15] | 周印洁, 吉思蓓, 何松阳, 吉旭, 贺革. 机器学习辅助高通量筛选金属有机骨架用于富碳天然气中分离CO2[J]. 化工学报, 2025, 76(3): 1093-1101. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号