化工学报 ›› 2025, Vol. 76 ›› Issue (10): 5336-5350.DOI: 10.11949/0438-1157.20250409
宋尚飞1( ), 李匀超1, 吴文宇1, 朱羽墨1, 廖清云2, 廖那伽3, 史博会1(
), 李匀超1, 吴文宇1, 朱羽墨1, 廖清云2, 廖那伽3, 史博会1( ), 宫敬1
), 宫敬1
收稿日期:2025-04-17
修回日期:2025-07-22
出版日期:2025-10-25
发布日期:2025-11-25
通讯作者:
史博会
作者简介:宋尚飞(1993—),男,博士,副教授,song.sf@cup.edu.cn
基金资助:
Shangfei SONG1( ), Yunchao LI1, Wenyu WU1, Yumo ZHU1, Qingyun LIAO2, Najia LIAO3, Bohui SHI1(
), Yunchao LI1, Wenyu WU1, Yumo ZHU1, Qingyun LIAO2, Najia LIAO3, Bohui SHI1( ), Jing GONG1
), Jing GONG1
Received:2025-04-17
Revised:2025-07-22
Online:2025-10-25
Published:2025-11-25
Contact:
Bohui SHI
摘要:
在可燃冰开发过程中,及时分解、清除排采系统中的水合物,是海域可燃冰开发过程中流动安全保障的重要工作。针对液相中游离客体分子及纳米气泡对CO₂-CH₄水合物分解动力学的协同作用机制尚未明晰、亟待系统探究的问题,本研究采用分子动力学方法构建了CO2与CH4分子比例为1∶1且孔穴占有率为100%的CO2-CH4水合物体系,在不同温度条件下,通过在液相中加入不同数量的CO2或CH4分子,模拟并观察其摩尔分数变化以及纳米气泡的出现对水合物分解速率的影响。研究结果表明,液相中存在的高浓度游离客体分子促进了CO2-CH4水合物的分解。游离客体分子在液相中形成较大尺寸的气泡,显著加快了水合物的分解速率。
中图分类号:
宋尚飞, 李匀超, 吴文宇, 朱羽墨, 廖清云, 廖那伽, 史博会, 宫敬. 液相游离客体分子对CO2-CH4水合物分解动力学的影响机理[J]. 化工学报, 2025, 76(10): 5336-5350.
Shangfei SONG, Yunchao LI, Wenyu WU, Yumo ZHU, Qingyun LIAO, Najia LIAO, Bohui SHI, Jing GONG. Influence mechanism of free guest molecules in liquid phase on decomposition kinetics of CO₂-CH₄ hydrates[J]. CIESC Journal, 2025, 76(10): 5336-5350.
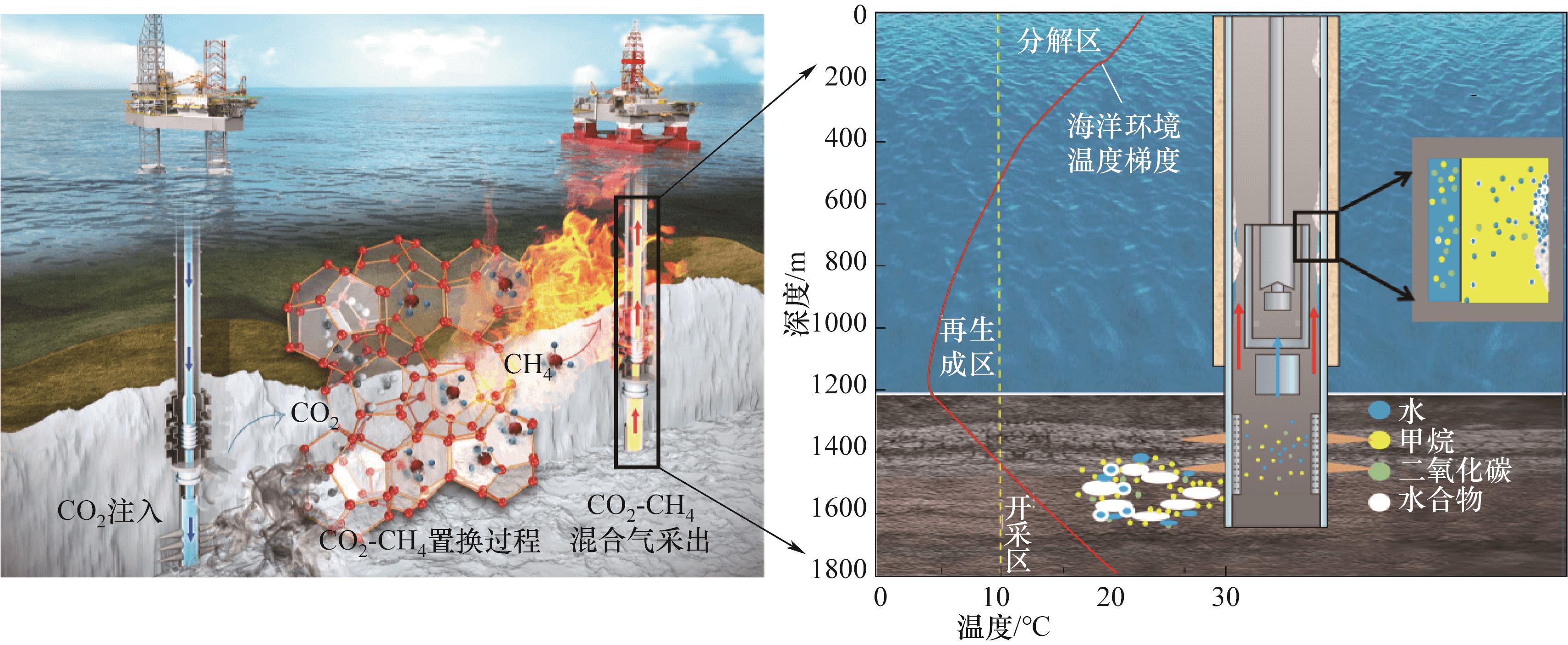
图1 CO2置换法-降压法联用开采可燃冰过程示意图(改编自文献[3])
Fig.1 Schematic diagram of the process of CO2 substitution and depressurization method for mining combustible ice (adapted from Ref.[3] )
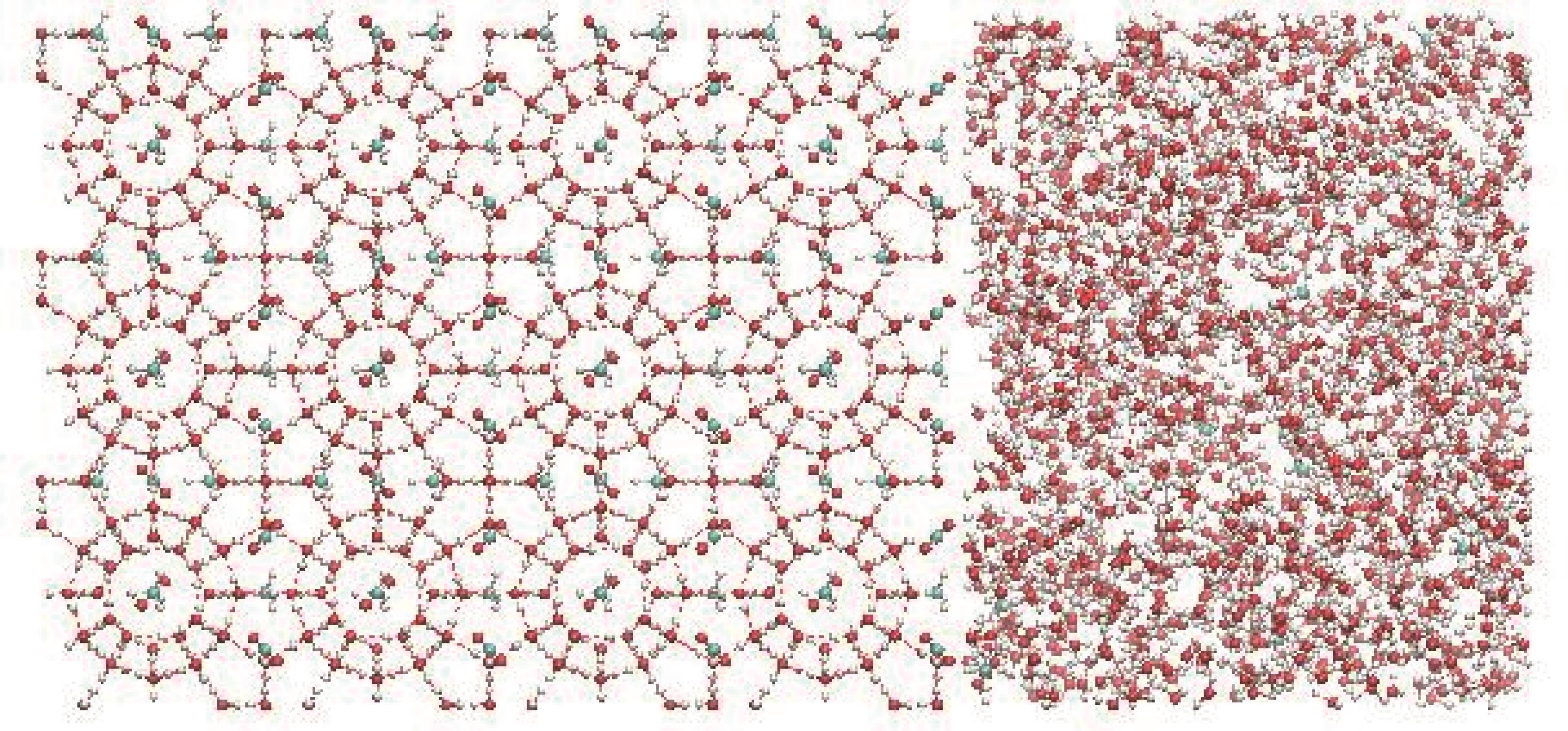
图2 分子数量比为1∶1的CO2-CH4水合物体系(红色球体代表O原子,青色球体代表C原子,白色球体代表H原子)
Fig.2 CO2-CH4 hydrate system with a molecular quantity ratio of 1∶1 (red spheres represent O atom, cyan spheres represent C atom, and white spheres represent H atom)
| 编号 | 模拟编号 | 液相中客体分子种类 | 液相中客体分子摩尔分数/% | 液相中客体分子数量 |
|---|---|---|---|---|
| 1 | 1-0 | — | 0 | 0 |
| 2 | M1-1 | CH4 | 1 | 15 |
| 3 | M1-5 | CH4 | 5 | 75 |
| 4 | M1-10 | CH4 | 10 | 150 |
| 5 | M1-12 | CH4 | 12 | 180 |
| 6 | M1-15 | CH4 | 15 | 225 |
| 7 | C1-1 | CO2 | 1 | 15 |
| 8 | C1-5 | CO2 | 5 | 75 |
| 9 | C1-10 | CO2 | 10 | 150 |
| 10 | C1-12 | CO2 | 12 | 180 |
| 11 | C1-15 | CO2 | 15 | 225 |
表1 280 K下CO₂-CH₄水合物分解动力学的模拟体系参数
Table 1 Parameters of simulation systems for CO₂-CH₄ hydrate decomposition kinetic at 280 K
| 编号 | 模拟编号 | 液相中客体分子种类 | 液相中客体分子摩尔分数/% | 液相中客体分子数量 |
|---|---|---|---|---|
| 1 | 1-0 | — | 0 | 0 |
| 2 | M1-1 | CH4 | 1 | 15 |
| 3 | M1-5 | CH4 | 5 | 75 |
| 4 | M1-10 | CH4 | 10 | 150 |
| 5 | M1-12 | CH4 | 12 | 180 |
| 6 | M1-15 | CH4 | 15 | 225 |
| 7 | C1-1 | CO2 | 1 | 15 |
| 8 | C1-5 | CO2 | 5 | 75 |
| 9 | C1-10 | CO2 | 10 | 150 |
| 10 | C1-12 | CO2 | 12 | 180 |
| 11 | C1-15 | CO2 | 15 | 225 |
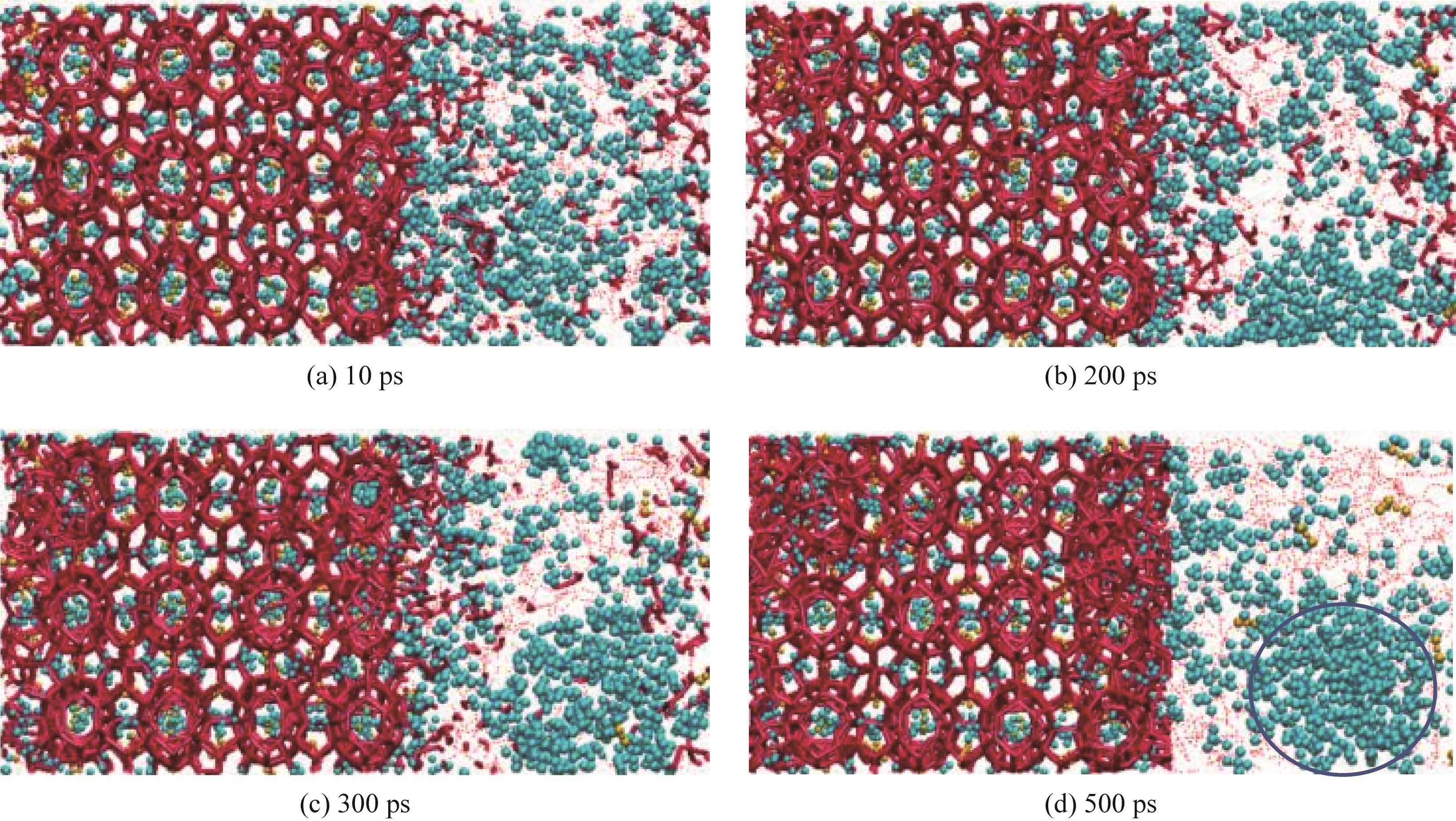
图3 M2-12不同时间点(10~500 ps)下水合物分解过程的模拟快照(蓝色球体代表甲烷分子,黄色球体代表二氧化碳分子,红色粗实线代表水合物的水分子形成的结构,红色虚线代表液相中水分子形成的氢键,蓝色圆框为纳米气泡,所有模拟快照采用相同表示方案)
Fig.3 Simulated snapshot of hydrate decomposition process at different time points (10—500 ps) of M2-12(blue spheres represent methane molecules, yellow spheres represent carbon dioxide molecules, bold solid red lines indicate the structural framework formed by water molecules in the hydrate, and dashed red lines denote hydrogen bonds formed by water molecules in the liquid phase,the blue circle is a nanobubble,all simulation snapshots adopt the same representation scheme )
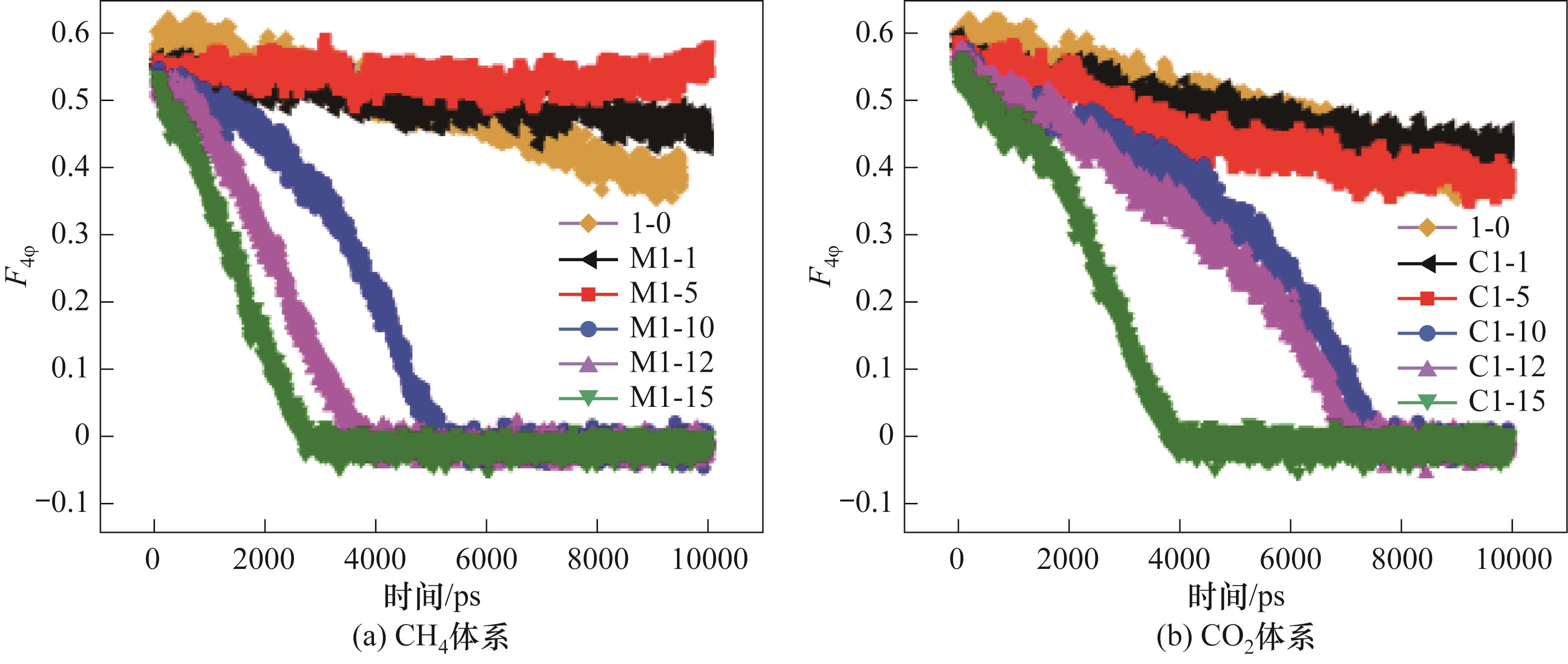
图9 280 K、0.5 MPa下不同体系下液相中含有不同数量的游离客体分子体系中CO2-CH4水合物相的F4φ 值
Fig.9 The F4φ of different systems containing different amounts of guest molecules in the liquid phase at 280 K and 0.5 MPa
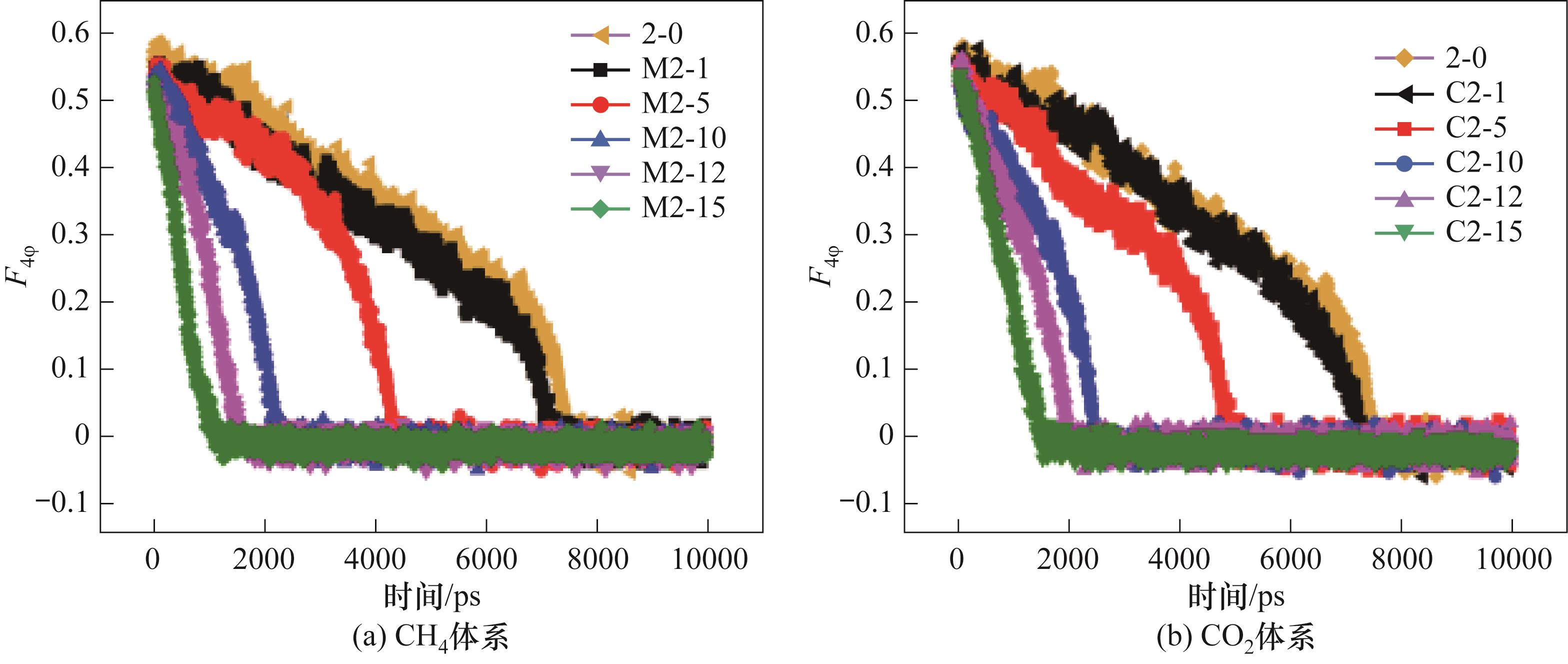
图10 290 K、0.5 MPa下不同体系下液相中含有不同数量的游离客体分子体系中CO2-CH4水合物相的F4φ 值
Fig.10 The F4φ of different systems containing different amounts of guest molecules in the liquid phase at 290 K and 0.5 MPa
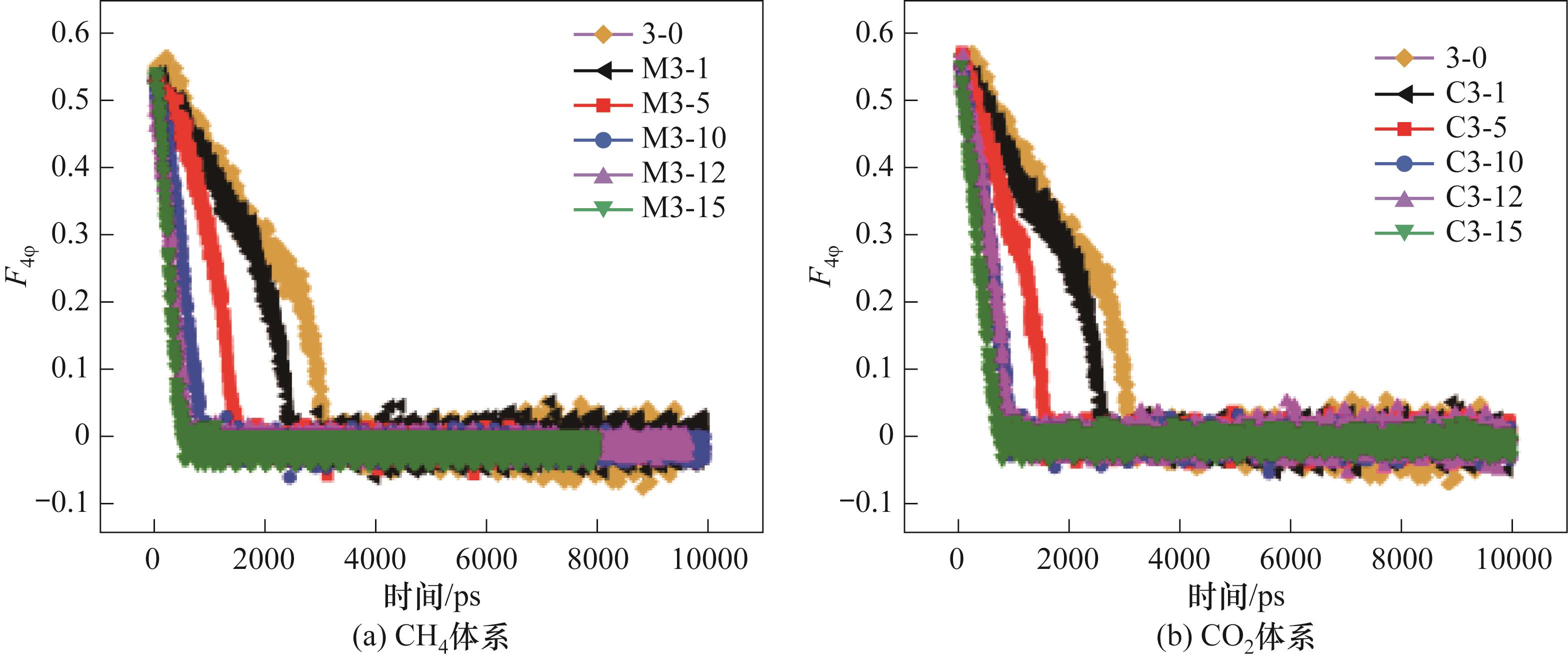
图11 300 K、0.5 MPa下不同体系中液相中含有不同数量的游离客体分子体系中CO2-CH4水合物相的F4φ 值
Fig.11 The F4φ of different systems containing different amounts of guest molecules in the liquid phase at 300 K and 0.5 MPa
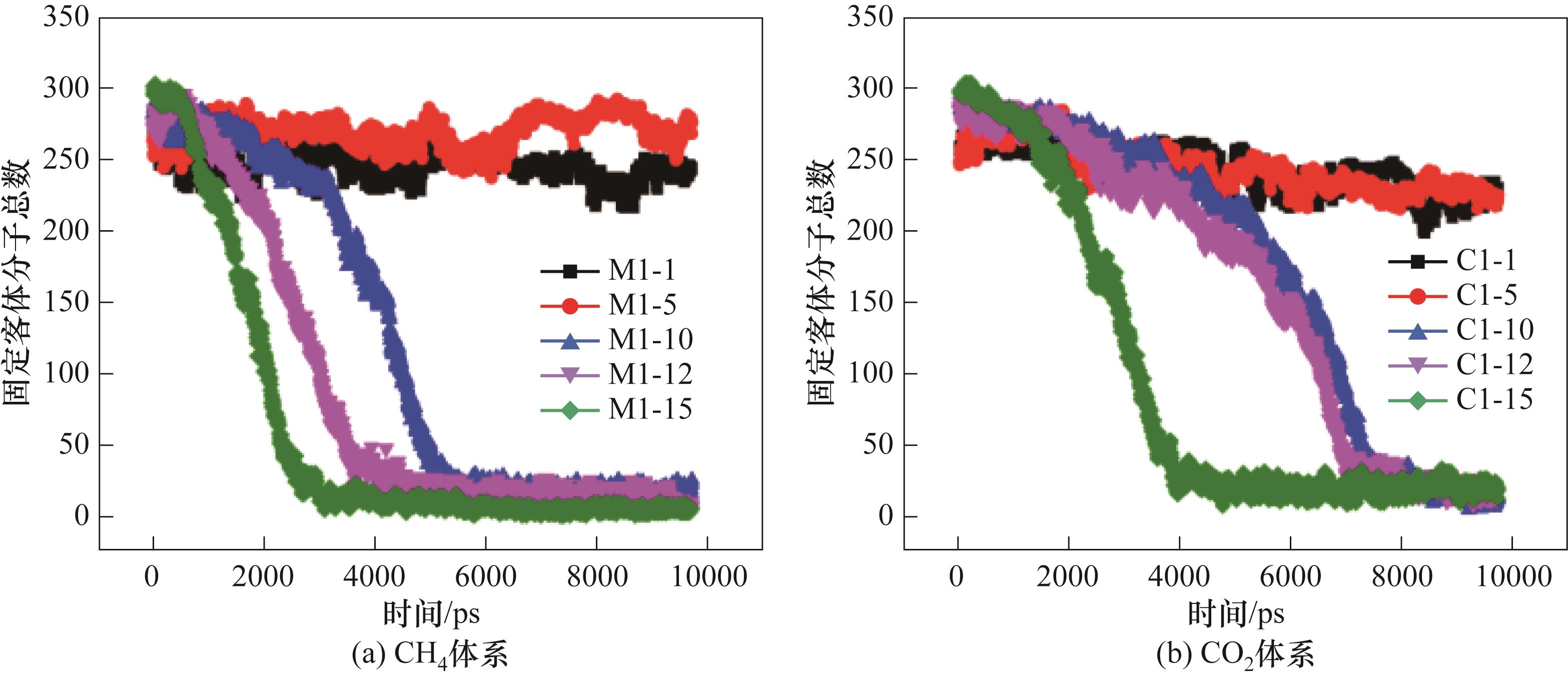
图12 280 K、0.5 MPa下CO2-CH4水合物分解过程的固定客体分子总数
Fig.12 Total number of fixed guest molecules in the decomposition process of CO2-CH4 hydrate at 280 K and 0.5 MPa
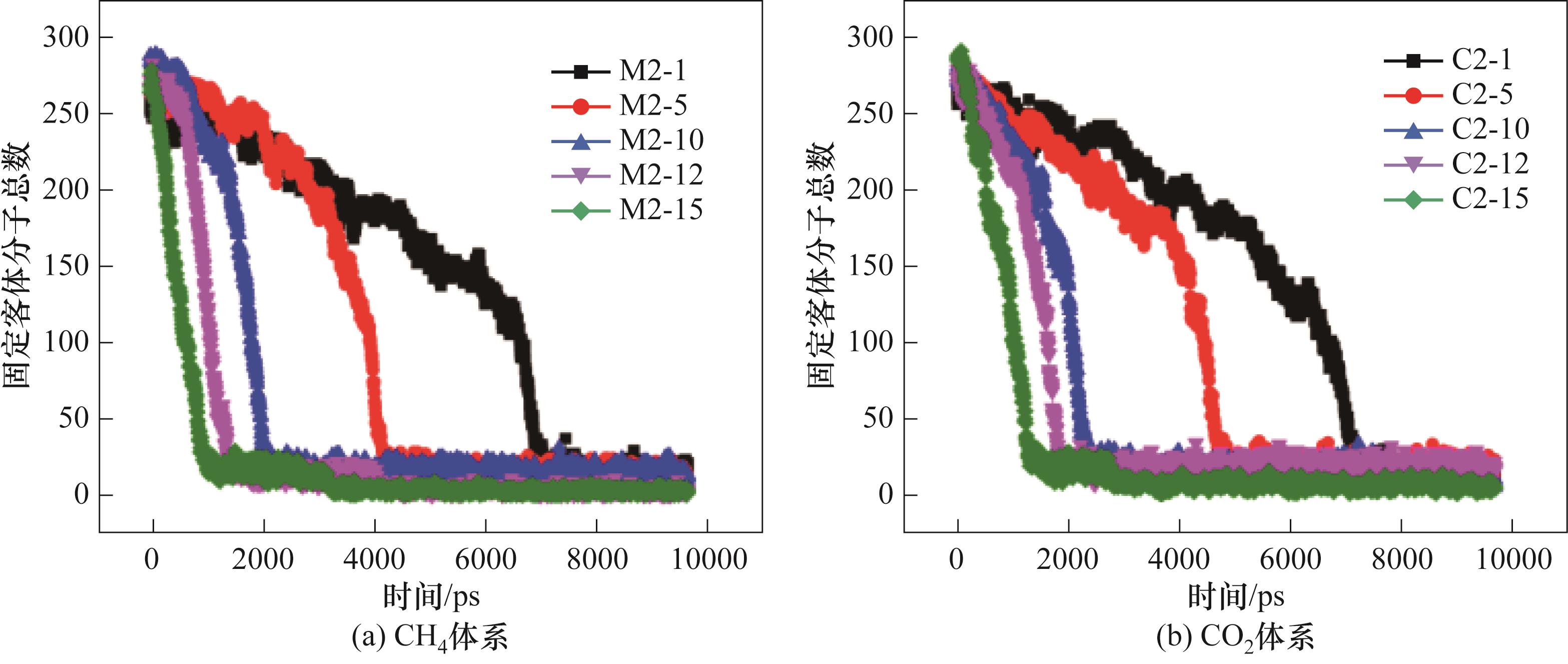
图13 290 K、0.5 MPa下CO2-CH4水合物分解过程的固定客体分子总数
Fig.13 Total number of fixed guest molecules in the decomposition process of CO2-CH4 hydrate at 290 K and 0.5 MPa
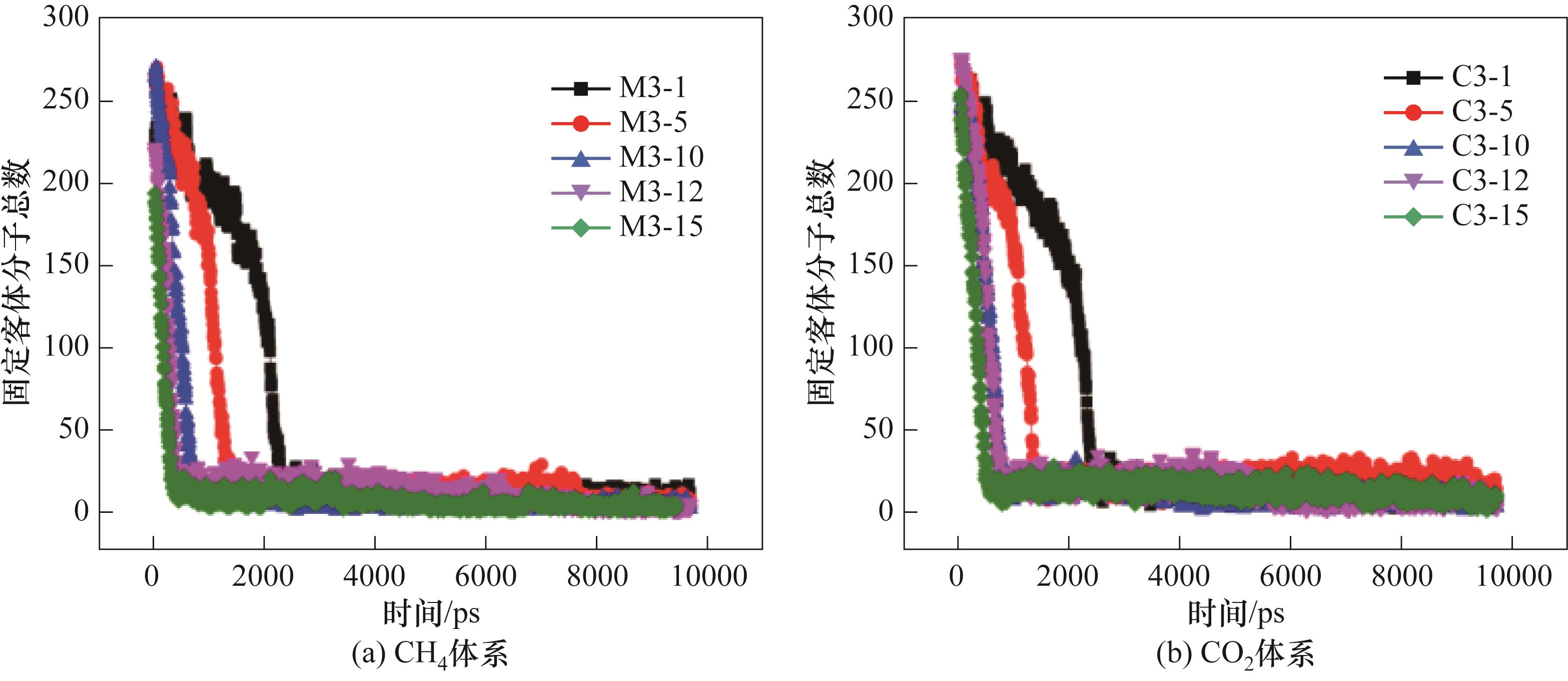
图14 300 K、0.5 MPa下CO2-CH4水合物分解过程的固定客体分子总数
Fig.14 Total number of fixed guest molecules in the decomposition process of CO2-CH4 hydrate at 300 K and 0.5 MPa
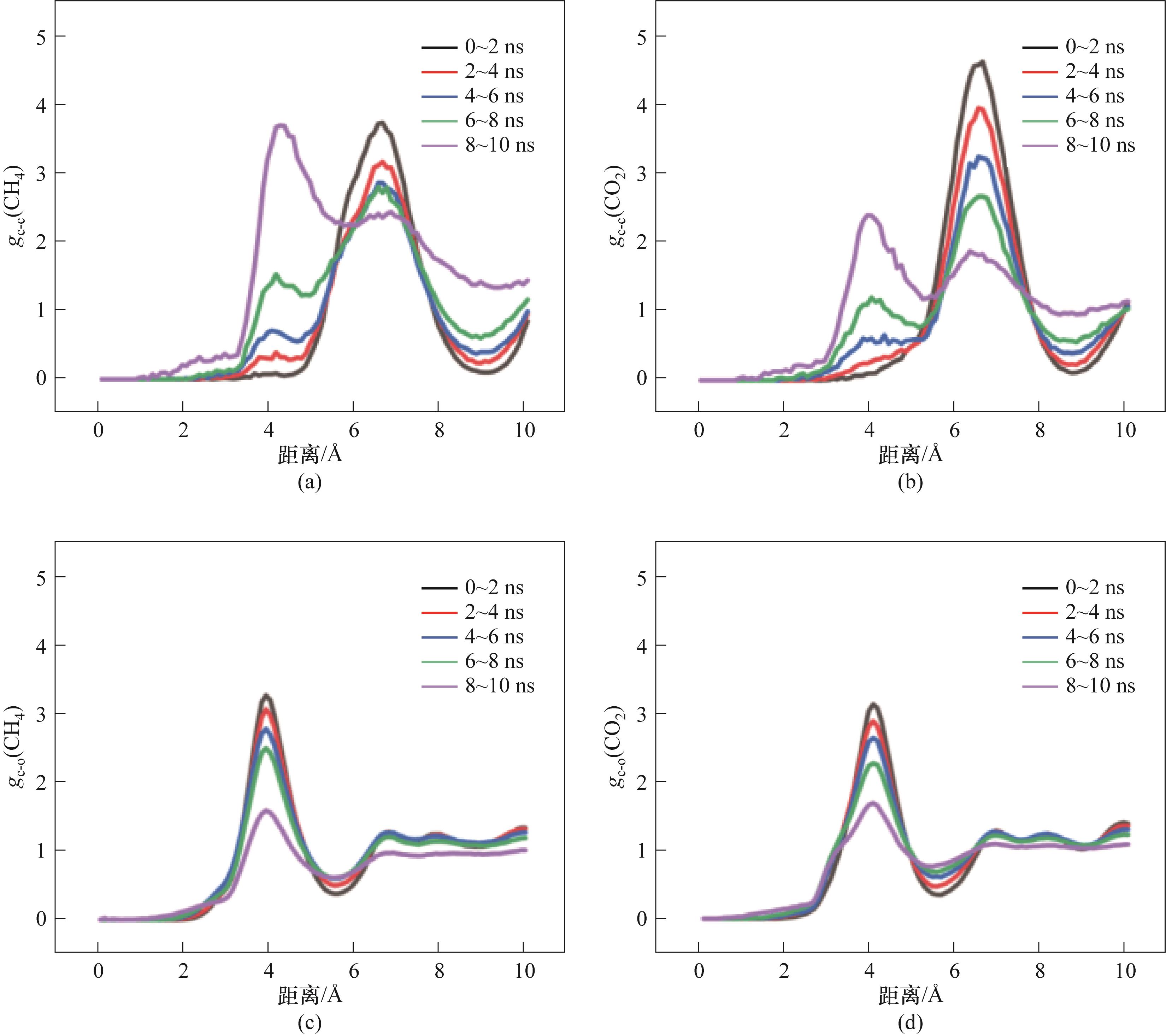
图15 M2-1的RDF参数:(a)CH4分子之间的RDF;(b)CO2分子之间的RDF;(c)CH4分子与H2O分子间的RDF;(d)CO2分子与H2O分子间的RDF
Fig.15 RDF of M2-1:(a) RDF between CH4 molecules; (b) RDF between CO2 molecules; (c) RDF between CH4 molecules and H2O molecules; (d) RDF between CO2 molecules and H2O molecules
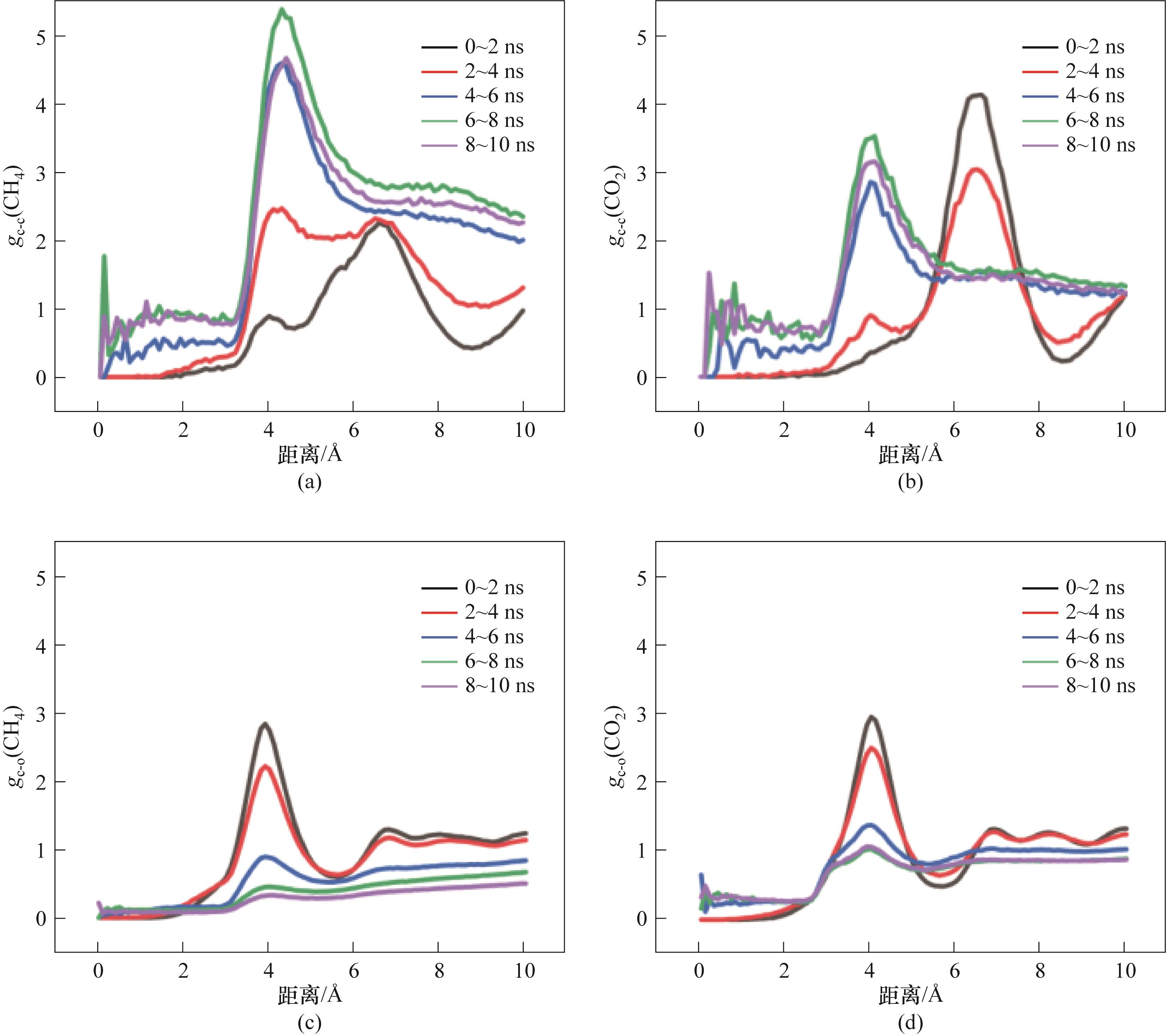
图16 M2-5的RDF参数:(a)CH4分子之间的RDF;(b)CO2分子之间的RDF;(c)CH4分子与H2O分子间的RDF;(d)CO2分子与H2O分子间的RDF
Fig.16 RDF of M2-5:(a) RDF between CH4 molecules; (b) RDF between CO2 molecules; (c) RDF between CH4 molecules and H2O molecules; (d) RDF between CO2 molecules and H2O molecules
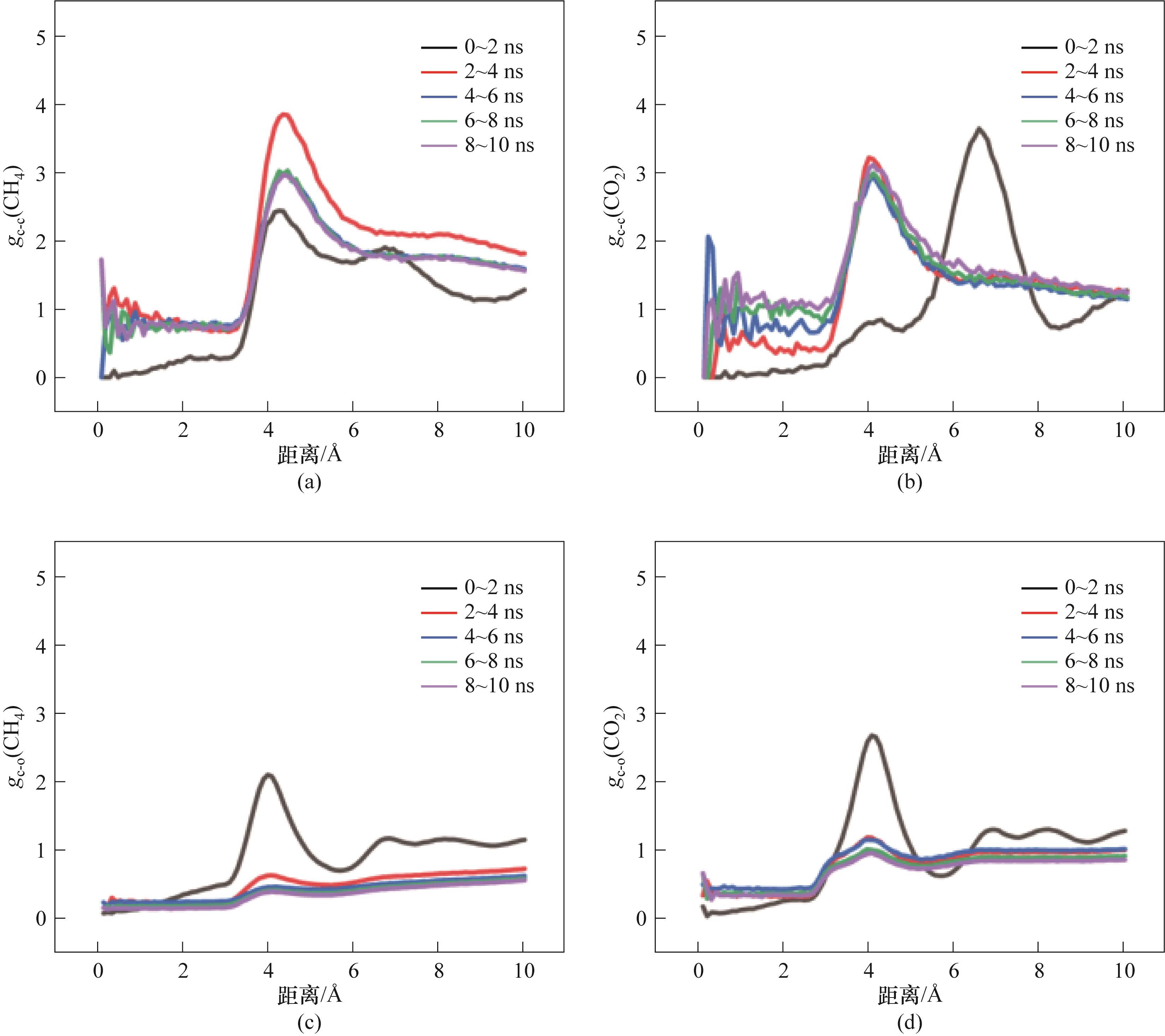
图17 M2-10的RDF参数:(a)CH4分子之间的RDF;(b)CO2分子之间的RDF;(c)CH4分子与H2O分子间的RDF;(d)CO2分子与H2O分子间的RDF
Fig.17 RDF of M2-10:(a) RDF between CH4 molecules; (b) RDF between CO2 molecules; (c) RDF between CH4 molecules and H2O molecules; (d) RDF between CO2 molecules and H2O molecules
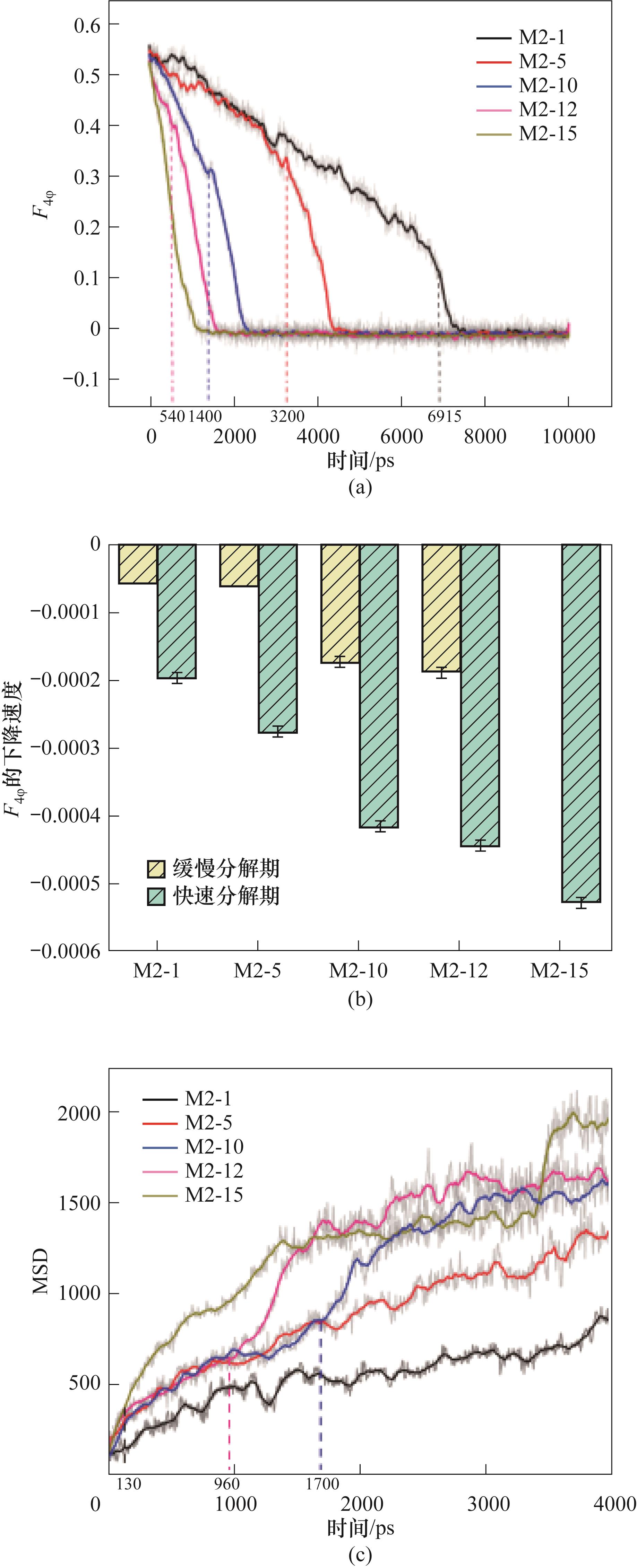
图18 (a)290 K、0.5 MPa下CO2-CH4水合物的分解过程中的F4φ值;(b)用F4φ值的下降速度来表征的CO2-CH4水合物分解速率;(c)290 K、0.5 MPa下CO2-CH4水合物分解过程中的MSD
Fig.18 (a) F4φ value during the decomposition of CO2-CH4 hydrate at 290 K and 0.5 MPa; (b) The decomposition rate of CO2-CH4 hydrate characterized by the decreasing rate of F4φ value; (c) MSD during the decomposition of CO2-CH4 hydrate at 290 K and 0.5 MPa
| 模拟编号 | 液相中客体 分子摩尔 分数/% | 纳米气泡形成时间/ps | 纳米气泡内分子数达到50个的时刻/ps | 纳米气泡内分子数达到100个的时刻/ps |
|---|---|---|---|---|
| M2-1 | 1 | 5750 | 7075 | 7335 |
| M2-5 | 5 | 1245 | 3210 | 4225 |
| M2-10 | 10 | 215 | 740 | 1405 |
| M2-12 | 12 | 0 | 270 | 640 |
| M2-15 | 15 | 0 | 55 | 325 |
表2 纳米气泡形成时间及纳米气泡客体分子数量积累至50、100个所需时间
Table 2 The nucleation time of nanobubbles and the time required for the accumulation of guest molecules to 50 and 100
| 模拟编号 | 液相中客体 分子摩尔 分数/% | 纳米气泡形成时间/ps | 纳米气泡内分子数达到50个的时刻/ps | 纳米气泡内分子数达到100个的时刻/ps |
|---|---|---|---|---|
| M2-1 | 1 | 5750 | 7075 | 7335 |
| M2-5 | 5 | 1245 | 3210 | 4225 |
| M2-10 | 10 | 215 | 740 | 1405 |
| M2-12 | 12 | 0 | 270 | 640 |
| M2-15 | 15 | 0 | 55 | 325 |
| [1] | 欧芬兰, 于彦江, 寇贝贝, 等. 水合物藏的类型、特点及开发方法探讨[J]. 海洋地质与第四纪地质, 2022, 42(1): 194-213. |
| Ou F L, Yu Y J, Kou B B, et al. Gas hydrate reservoir types, characteristics and development methods[J]. Marine Geology & Quaternary Geology, 2022, 42(1): 194-213. | |
| [2] | Goel N. In situ methane hydrate dissociation with carbon dioxide sequestration: current knowledge and issues[J]. Journal of Petroleum Science and Engineering, 2006, 51(3/4): 169-184. |
| [3] | Koh D Y, Kang H, Lee J W, et al. Energy-efficient natural gas hydrate production using gas exchange[J]. Applied Energy, 2016, 162: 114-130. |
| [4] | 陈烨. 天然气水合物降压试采井筒多相流动规律及保障技术研究[D]. 大庆: 东北石油大学, 2020. |
| Chen Y. Study on multiphase flow law and guarantee technology of natural gas hydrate depressurization test production wellbore[D]. Daqing: Northeast Petroleum University, 2020. | |
| [5] | Wu G Z, Tian L Q, Chen D Y, et al. CO2 and CH4 hydrates: replacement or cogrowth?[J]. The Journal of Physical Chemistry C, 2019, 123(22): 13401-13409. |
| [6] | Geng C Y, Wen H, Zhou H. Molecular simulation of the potential of methane reoccupation during the replacement of methane hydrate by CO2 [J]. Journal of Physical Chemistry A, 2009, 113(18): 5463-5469. |
| [7] | Li Z D, Gan B C, Li Z, et al. Kinetic mechanisms of methane hydrate replacement and carbon dioxide hydrate reorganization[J]. Chemical Engineering Journal, 2023, 477: 146973. |
| [8] | Guo P, Song Y L, Liu H, et al. Molecular dynamic simulation study on replacement of methane hydrates with carbon dioxide under different temperatures, pressures, and concentrations of ethylene glycol[J]. ACS Omega, 2024, 9(17): 19031-19042. |
| [9] | Castellani B, Gambelli A M, Nicolini A, et al. Energy and environmental analysis of membrane-based CH4-CO2 replacement processes in natural gas hydrates [J]. Energies, 2019, 12(5): 850. |
| [10] | Li J, Wang Z L. Fluctuation-dissipation analysis of nonequilibrium thermal transport at the hydrate dissociation interface[J]. Physical Chemistry Chemical Physics, 2019, 21(42): 23492-23500. |
| [11] | Liu Y, Zhao J J, Xu J C. Dissociation mechanism of carbon dioxide hydrate by molecular dynamic simulation and ab initio calculation[J]. Computational and Theoretical Chemistry, 2012, 991: 165-173. |
| [12] | Li J, Liang Z J, Wang Z L, et al. Molecular dynamics simulation of decomposition of methane hydrate and interfacial characteristics in nanostructure region[J]. International Journal of Thermophysics, 2020, 41(2): 13. |
| [13] | Ma Y, Gao Q, Guan J, et al. Experimental study on the formation and dissociation characteristics of mixed CO2 + CH4 hydrates in quartz sand[J]. Energy & Fuels, 2023, 37(17): 12934-12945. |
| [14] | Maini B B, Bishnoi P R. Experimental investigation of hydrate formation behaviour of a natural gas bubble in a simulated deep sea environment[J]. Chemical Engineering Science, 1981, 36(1): 183-189. |
| [15] | Yi L Z, Liang D Q, Liang S, et al. Molecular dynamics study of CH4-CO2 mixed hydrate dissociation[J]. Asia-Pacific Journal of Chemical Engineering, 2015, 10(6): 823-832. |
| [16] | Hei Y X, Liu Z L, Shi D, et al. Molecular dynamics simulations to evaluate the decomposition properties of methane hydrate under different thermodynamic conditions[J]. Computational and Theoretical Chemistry, 2024, 1236: 114585. |
| [17] | Ouyang Q, Pandey J S, von Solms N. Critical parameters influencing mixed CH4/CO2 hydrates dissociation during multistep depressurization[J]. Fuel, 2022, 320: 123985. |
| [18] | Ripmeester J A, Alireza S, Hosseini B, et al. Fundamentals of methane hydrate decomposition[C]//Canadian Unconventional Resources and International Petroleum Conference. Calgary, Alberta, Canada: SPE, 2010: SPE-138112-MS. |
| [19] | Bagherzadeh S A, Alavi S, Ripmeester J, et al. Formation of methane nano-bubbles during hydrate decomposition and their effect on hydrate growth[J]. The Journal of Chemical Physics, 2015, 142(21): 214701. |
| [20] | Bagherzadeh S A, Englezos P, Alavi S, et al. Molecular simulation of non-equilibrium methane hydrate decomposition process[J]. The Journal of Chemical Thermodynamics, 2012, 44(1): 13-19. |
| [21] | Li Y C, Song S F, Liao N J, et al. Molecular-level study on decomposition kinetics of CO2–CH4 hydrates[J]. Energy & Fuels, 2024, 38(14): 12875-12887. |
| [22] | Plimpton S. Fast parallel algorithms for short-range molecular dynamics[J]. Journal of Computational Physics, 1995, 117(1): 1-19. |
| [23] | Vega C, Abascal J L F. Simulating water with rigid non-polarizable models: a general perspective[J]. Physical Chemistry Chemical Physics, 2011, 13(44): 19663-19688. |
| [24] | Potoff J J, Siepmann J I. Vapor–liquid equilibria of mixtures containing alkanes, carbon dioxide, and nitrogen[J]. AIChE Journal, 2001, 47(7): 1676-1682. |
| [25] | Harris J G, Yung K H. Carbon dioxide's liquid-vapor coexistence curve and critical properties as predicted by a simple molecular model[J]. The Journal of Physical Chemistry, 1995, 99(31): 12021-12024. |
| [26] | Cygan R T, Romanov V N, Myshakin E M. Molecular simulation of carbon dioxide capture by montmorillonite using an accurate and flexible force field[J]. The Journal of Physical Chemistry C, 2012, 116(24): 13079-13091. |
| [27] | Lu Y, Sun L J, Guan D W, et al. Molecular behavior of CO2 hydrate growth in the presence of dissolvable ionic organics[J]. Chemical Engineering Journal, 2022, 428: 131176. |
| [28] | Parrinello M, Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method[J]. Journal of Applied Physics, 1981, 52(12): 7182-7190. |
| [29] | Nosé S. A molecular dynamics method for simulations in the canonical ensemble[J]. Molecular Physics, 1984, 52(2): 255-268. |
| [30] | Errington J R, Debenedetti P G. Relationship between structural order and the anomalies of liquid water[J]. Nature, 2001, 409(6818): 318-321. |
| [31] | Zhou Y B, Huang M Y, Tian F L, et al. Einstein-Stokes relation for small bubbles at the nanoscale[J]. The Journal of Chemical Physics, 2024, 160(5): 054109. |
| [32] | English N J, Johnson J K, Taylor C E. Molecular-dynamics simulations of methane hydrate dissociation[J]. The Journal of Chemical Physics, 2005, 123(24): 244503. |
| [1] | 孙云龙, 徐肖肖, 黄永方, 郭纪超, 陈卫卫. 水平光滑管内CO2流动沸腾的非绝热可视化研究[J]. 化工学报, 2025, 76(S1): 230-236. |
| [2] | 郭纪超, 徐肖肖, 孙云龙. 基于植物工厂中的CO2浓度气流模拟及优化研究[J]. 化工学报, 2025, 76(S1): 237-245. |
| [3] | 孔繁臣, 张硕, 唐明生, 邹慧明, 胡舟航, 田长青. 二氧化碳直线压缩机气体轴承模拟[J]. 化工学报, 2025, 76(S1): 281-288. |
| [4] | 何婷, 张开, 林文胜, 陈利琼, 陈家富. 沼气超临界压力低温脱碳-液化耦合流程研究[J]. 化工学报, 2025, 76(S1): 418-425. |
| [5] | 胡国祥, 朱忆魁, 龙华, 刘晓雯, 熊勤钢. 组分配比影响氯化胆碱-乳酸低共熔溶剂碱木质素溶解度的底层机理研究[J]. 化工学报, 2025, 76(9): 4449-4461. |
| [6] | 张建民, 何美贵, 贾万鑫, 赵静, 金万勤. 聚氧化乙烯/冠醚共混膜及其二氧化碳分离性能[J]. 化工学报, 2025, 76(9): 4862-4871. |
| [7] | 王一飞, 李玉星, 欧阳欣, 赵雪峰, 孟岚, 胡其会, 殷布泽, 郭雅琦. 基于裂尖减压特性的CO2管道断裂扩展数值计算[J]. 化工学报, 2025, 76(9): 4683-4693. |
| [8] | 李相海, 赖德林, 孔纲, 周健. 双仿生表面水下疏油协同机制的分子动力学模拟研究[J]. 化工学报, 2025, 76(9): 4551-4562. |
| [9] | 高正, 汪辉, 屈治国. 数据驱动辅助高通量筛选阴离子柱撑金属有机框架储氢[J]. 化工学报, 2025, 76(8): 4259-4272. |
| [10] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [11] | 李云昊, 徐纯刚, 李小森, 付骏, 王屹, 陈朝阳. 固液复配型促进剂对盐水体系CO2水合物形成影响研究[J]. 化工学报, 2025, 76(8): 4228-4238. |
| [12] | 林嘉豪, 付芳忠, 叶昊辉, 胡金, 姚明灿, 范鹤林, 王旭, 王瑞祥, 徐志峰. NdF3含量对NdF3-LiF熔盐局域结构和输运性质的影响[J]. 化工学报, 2025, 76(8): 3834-3841. |
| [13] | 王小令, 王绍清, 赵云刚, 常方哲, 穆瑞峰. 基于ReaxFF MD模拟的煤加氢热解有机Ca转化机制研究[J]. 化工学报, 2025, 76(8): 4297-4309. |
| [14] | 乔亮, 李尚, 刘新亮, 王明, 张沛, 侯影飞. 三元共聚物稠油降黏剂的合成及分子模拟研究[J]. 化工学报, 2025, 76(7): 3686-3695. |
| [15] | 董泽明, 娄聚伟, 王楠, 陈良奇, 王江峰, 赵攀. 含余热回收的超临界压缩二氧化碳储能系统热力学特性研究[J]. 化工学报, 2025, 76(7): 3477-3486. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号