化工学报 ›› 2025, Vol. 76 ›› Issue (10): 5190-5202.DOI: 10.11949/0438-1157.20250186
董纪广1( ), 谢绍雷2,3, 时东2,3, 李丽娟3, 赵晨宇1, 黄雨婕1, 石成龙1(
), 谢绍雷2,3, 时东2,3, 李丽娟3, 赵晨宇1, 黄雨婕1, 石成龙1( ), 许淘善3(
), 许淘善3( ), 曹大伟2,3(
), 曹大伟2,3( )
)
收稿日期:2025-02-26
修回日期:2025-05-28
出版日期:2025-10-25
发布日期:2025-11-25
通讯作者:
石成龙,许淘善,曹大伟
作者简介:董纪广(2000—),男,硕士研究生,dongjiguang354@163.com
基金资助:
Jiguang DONG1( ), Shaolei XIE2,3, Dong SHI2,3, Lijuan LI3, Chenyu ZHAO1, Yujie HUANG1, Chenglong SHI1(
), Shaolei XIE2,3, Dong SHI2,3, Lijuan LI3, Chenyu ZHAO1, Yujie HUANG1, Chenglong SHI1( ), Taoshan XU3(
), Taoshan XU3( ), Dawei CAO2,3(
), Dawei CAO2,3( )
)
Received:2025-02-26
Revised:2025-05-28
Online:2025-10-25
Published:2025-11-25
Contact:
Chenglong SHI, Taoshan XU, Dawei CAO
摘要:
萃取剂构效关系是溶剂萃取研究的重要内容,但是协萃剂构效关系对萃取性能影响的研究却较少见。本研究合成了两种芳基取代磷酸二酯协萃剂,分别为苯基磷酸二(2-乙基己氧基)酯(BPPO)和对甲基苯基磷酸二(2-乙基己氧基)酯(BTPO)。以邻羟基苯甲酸正辛酯(OHB)作为主萃剂,构建了两个混合萃取体系。研究了有机相组成、水相碱度、相比、水相锂浓度以及有机相饱和负载等因素对萃取实验的影响。结果显示,甲基取代的OHB/BTPO萃取体系萃取性能和分相效果上明显优于无取代的OHB/BPPO体系。将萃取性能较好的OHB/BTPO萃取体系用于沉锂母液提锂。通过采用三级逆流萃取工艺,锂的萃取率ELi可达94%。借助紫外、荧光、红外光谱对萃取机理展开了深入研究,结果表明,在萃取过程中OHB构型发生Keto-Enol转化,当其处于Keto构型时,具有308 nm紫外吸收峰,且无荧光发射;当其处于Enol构型时,具有340 nm紫外吸收峰,在340 nm波长的光激发下产生410 nm的蓝色荧光。同时,红外光谱上C O伸缩振动(νC
O伸缩振动(νC O)、苯环的骨架振动(νPh)等均发生了明显变化。
O)、苯环的骨架振动(νPh)等均发生了明显变化。
中图分类号:
董纪广, 谢绍雷, 时东, 李丽娟, 赵晨宇, 黄雨婕, 石成龙, 许淘善, 曹大伟. 邻羟基苯甲酸正辛酯萃取体系提锂:协萃剂结构变化对萃取性能影响[J]. 化工学报, 2025, 76(10): 5190-5202.
Jiguang DONG, Shaolei XIE, Dong SHI, Lijuan LI, Chenyu ZHAO, Yujie HUANG, Chenglong SHI, Taoshan XU, Dawei CAO. Lithium extraction by n-octyl salicylate extraction system: influence of structural alterations in the synergist on extract performance[J]. CIESC Journal, 2025, 76(10): 5190-5202.
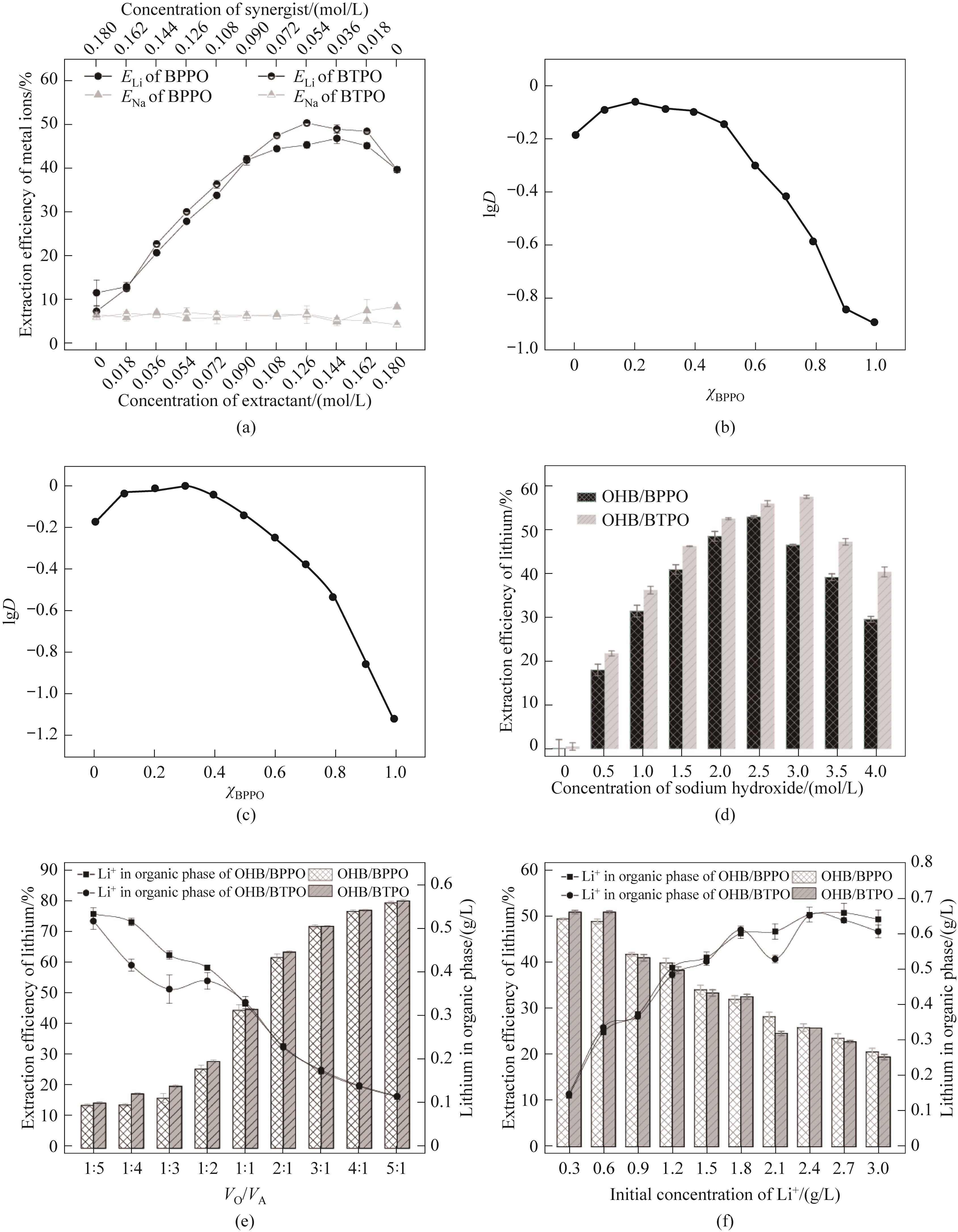
图3 (a)萃取剂和协萃剂浓度对萃取效率的影响;萃取体系(b)OHB/BPPO和(c)OHB/BTPO的协萃图;(d)水相碱度,(e)萃取相比,(f)水相中锂浓度对OHB/BPPO、OHB/BTPO萃取体系提锂的影响
Fig.3 (a) The effect of concentration of extractant and synergist on extraction efficiency; Synergistic extraction diagrams of extraction systems (b) OHB/BPPO and (c) OHB/BTPO; Eeffects of (d) aqueous phase alkalinity, (e) extraction phase ratio, (f) lithium concentration in the aqueous phase on the lithium extraction in the OHB/BPPO and OHB/BTPO extraction systems
| 类型 | Li + | Na + | K + | Ca2+ | Mg2+ | pH |
|---|---|---|---|---|---|---|
| 浓度/(g•L-1) | 2.74 | 44.18 | 0.50 | 2.97×10-3 | 0.75×10-3 | 11.5 |
| 浓度/(mol•L-1) | 0.39 | 1.92 | 2.09×10-3 | 0.74×10-4 | 0.19×10-4 |
表1 沉锂母液主要成分
Table 1 Main components of lithium precipitation mother liquor
| 类型 | Li + | Na + | K + | Ca2+ | Mg2+ | pH |
|---|---|---|---|---|---|---|
| 浓度/(g•L-1) | 2.74 | 44.18 | 0.50 | 2.97×10-3 | 0.75×10-3 | 11.5 |
| 浓度/(mol•L-1) | 0.39 | 1.92 | 2.09×10-3 | 0.74×10-4 | 0.19×10-4 |
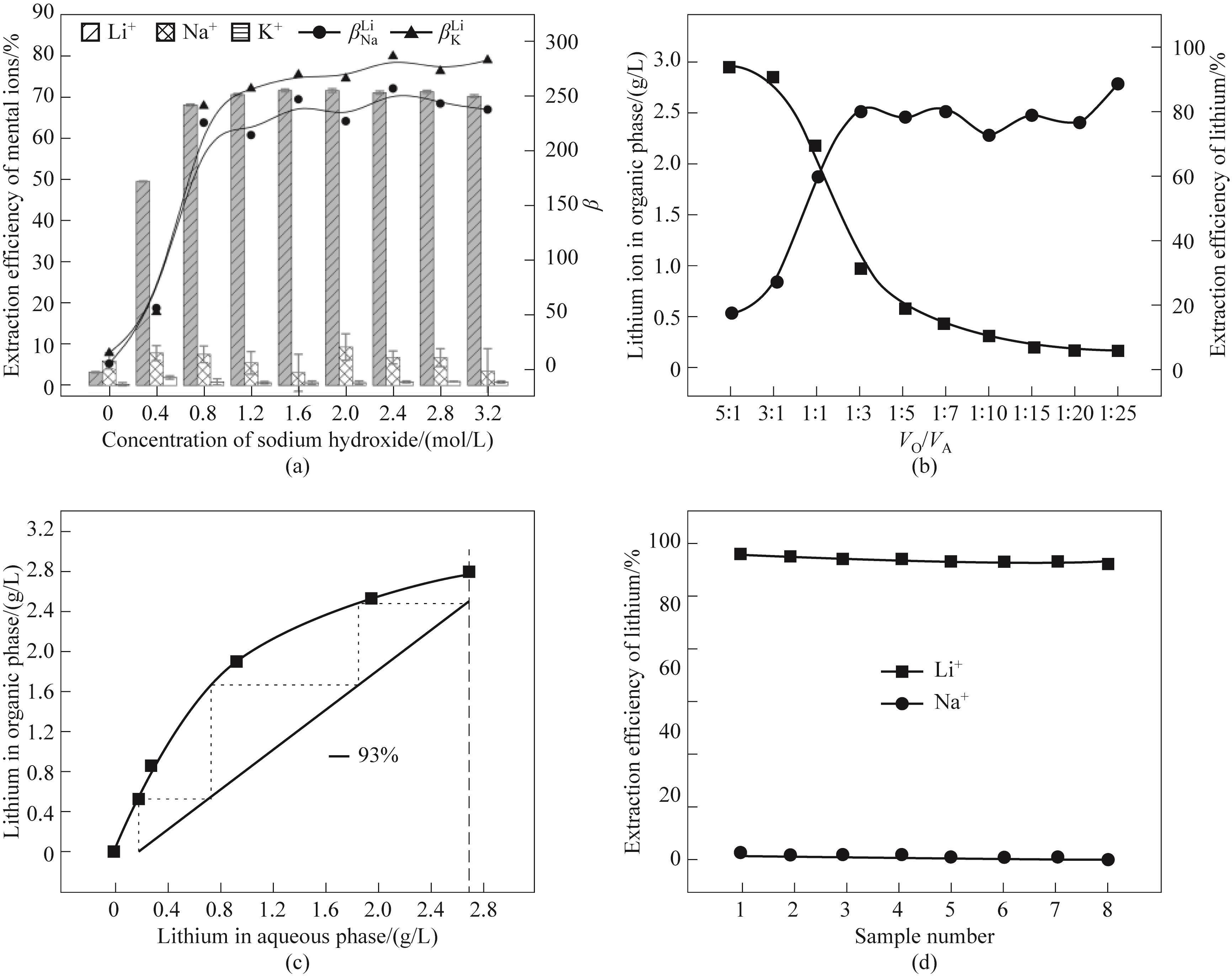
图5 (a)氢氧化钠浓度对OHB/BTPO提锂的影响;(b)相比对锂离子萃取的影响;(c)锂萃取的McCabe Thiele曲线,(d)三级逆流萃取实验结果
Fig.5 (a) Effect of NaOH concentration on the lithium extraction by OHB/BTPO; (b) Effect of phase ratio on lithium extraction; (c) McCabe-Thiele diagram; (d)Three-stage counter-current extraction experiment
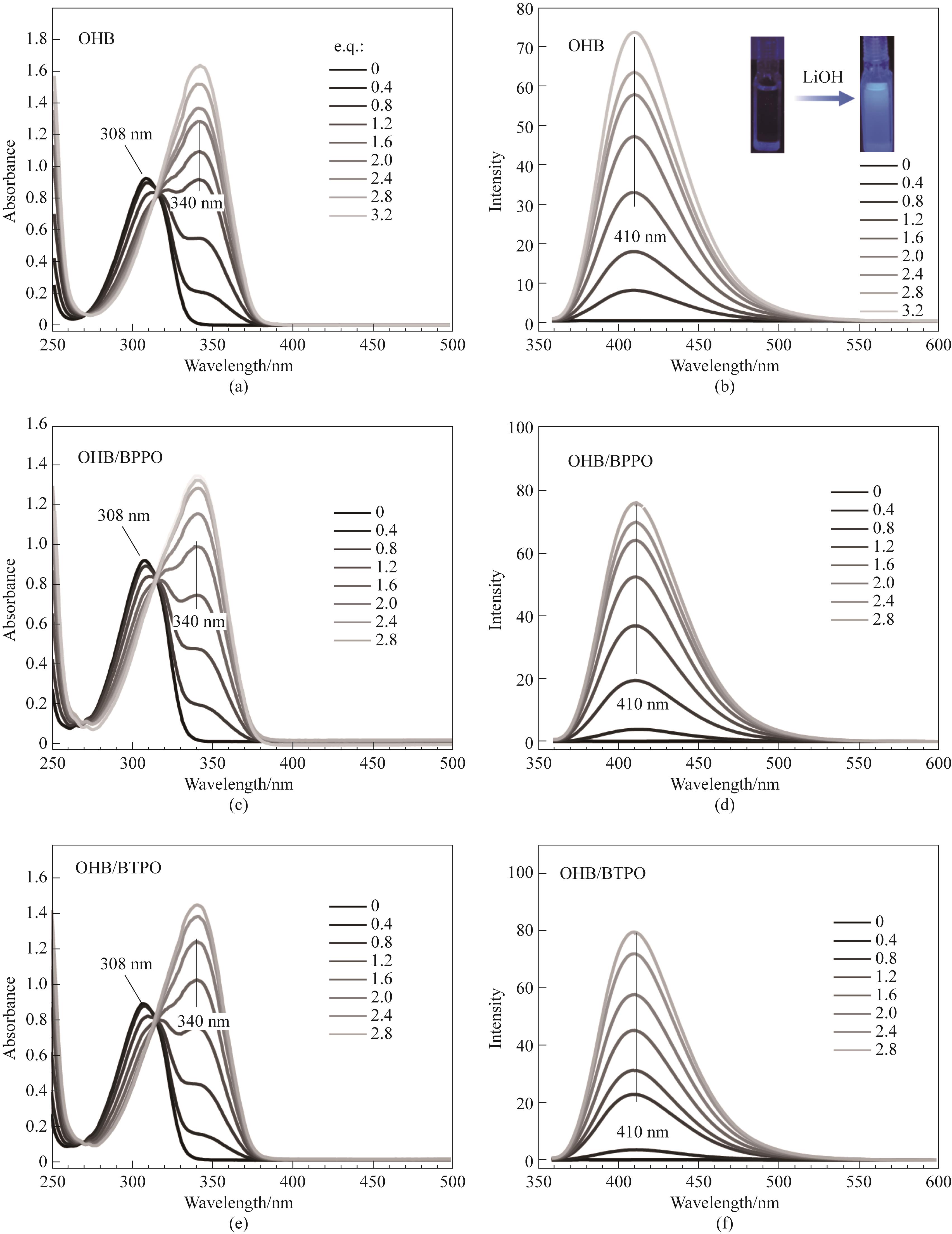
图6 氢氧化锂与OHB、OHB/BPPO、OHB/BTPO滴定实验的(a),(c),(e)紫外光谱和(b),(d),(f)荧光光谱
Fig.6 (a),(c),(e) UV spectra and (b),(d),(f) fluorescence spectra of the titration experiment of lithium hydroxide with OHB, OHB/BPPO and OHB/BTPO

图7 (a)萃取剂OHB主体结构Keto-Enol构型互变;(b)化合物OHB、BPPO与BTPO的红外光谱;(c)氢氧化锂与OHB作用前后红外光谱的变化
Fig.7 (a) The Keto-Enol tautomerism of OHB structure; (b) Infrared spectrum of OHB, BPPO and BTPO; (c) Changes in the Infrared spectrum before and after the reaction of lithium hydroxide with OHB
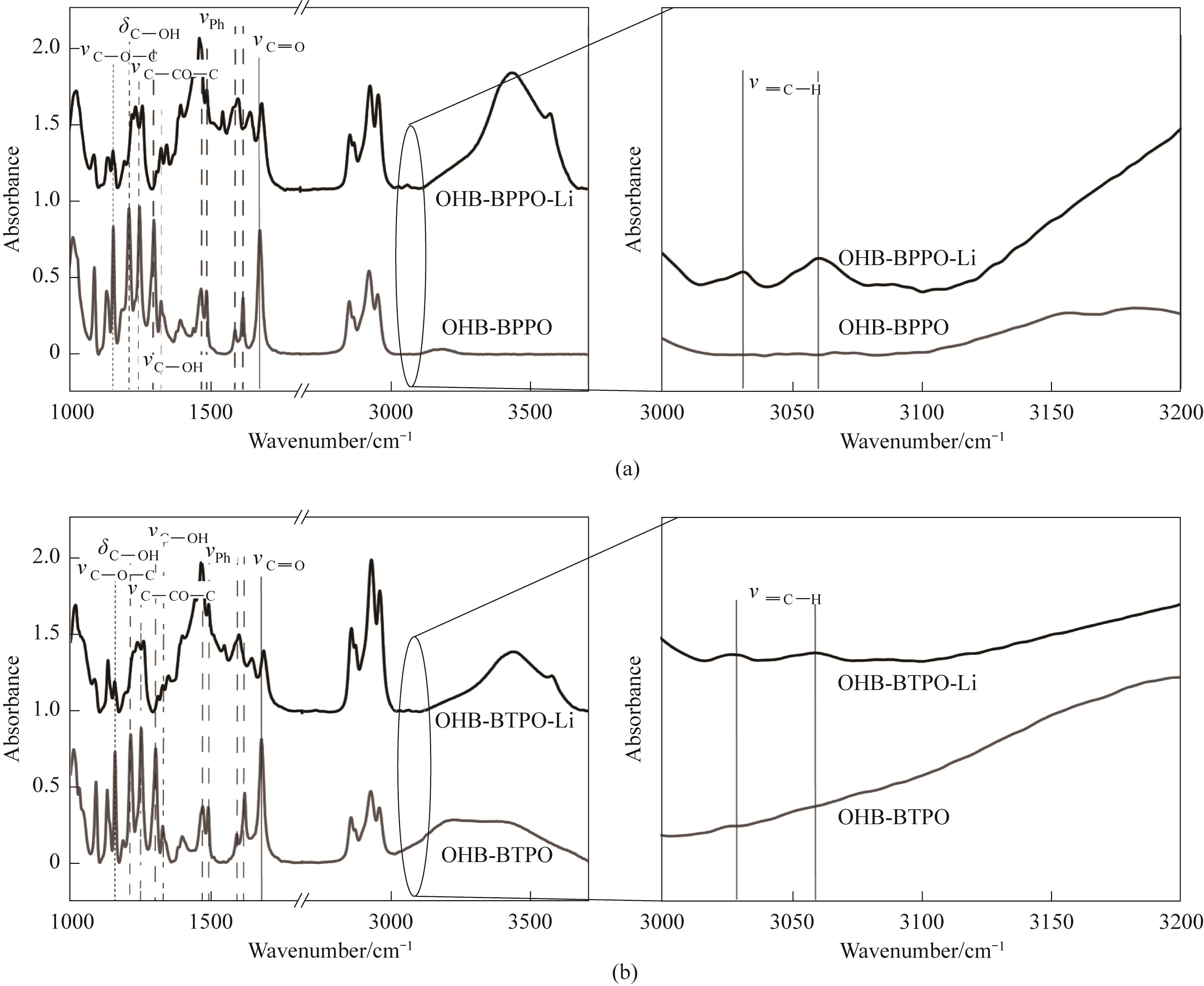
图8 (a)氢氧化锂OHB/BPPO、(b)氢氧化锂OHB/BTPO作用前后红外光谱的变化
Fig.8 Changes in the infrared spectrum before and after the reaction of lithium hydroxide with (a) OHB/BPPO and (b) OHB/BTPO
| [1] | 高峰, 郑绵平, 乜贞, 等. 盐湖卤水锂资源及其开发进展[J]. 地球学报, 2011, 32(4): 483-492. |
| Gao F, Zheng M P, Nie Z, et al. Brine lithium resource in the salt lake and advances in its exploitation[J]. Acta Geoscientica Sinica, 2011, 32(4): 483-492. | |
| [2] | 任世中, 曾英, 李陇岗, 等. 盐湖卤水提锂方法研究进展[J]. 广州化工, 2013, 41(1): 35-37, 50. |
| Ren S Z, Zeng Y, Li L G, et al. Development progress on the extraction of lithium from salt lake brines[J]. Guangzhou Chemical Industry, 2013, 41(1): 35-37, 50. | |
| [3] | Kanagasundaram T, Murphy O, Haji M N, et al. The recovery and separation of lithium by using solvent extraction methods[J]. Coordination Chemistry Reviews, 2024, 509: 215727. |
| [4] | 窦立荣, 刘化清, 常德宽, 等. 全球锂资源分布、产业现状和中国面临的挑战与对策[J]. 中国科学院院刊, 2025, 40(3): 494-510. |
| Dou L R, Liu H Q, Chang D K, et al. Challenges and countermeasures for lithium resources in China and analysis of global distribution and industry status[J]. Bulletin of Chinese Academy of Sciences, 2025, 40(3): 494-510. | |
| [5] | 温汉捷, 罗重光, 杜胜江, 等. 碳酸盐黏土型锂资源的发现及意义[J]. 科学通报, 2020, 65(1): 53-59. |
| Wen H J, Luo C G, Du S J, et al. Carbonate-hosted clay-type lithium deposit and its prospecting significance[J]. Chinese Science Bulletin, 2020, 65(1): 53-59. | |
| [6] | 张照志, 潘昭帅, 车东. 基于中国锂矿床及资源特征的2024—2035年锂供需形势分析[J]. 中国矿业, 2024, 33(6): 26-44. |
| Zhang Z Z, Pan Z S, Che D. Analysis of lithium supply and demand situation based on lithium deposits and resources characteristics from 2024 to 2035, China[J]. China Mining Magazine, 2024, 33(6): 26-44. | |
| [7] | 王琪, 赵有璟, 刘洋, 等. 高镁锂比盐湖镁锂分离与锂提取技术研究进展[J]. 化工学报, 2021, 72(6): 2905-2921. |
| Wang Q, Zhao Y J, Liu Y, et al. Recent advances in magnesium/lithium separation and lithium extraction technologies from salt lake brine with high magnesium/lithium ratio[J]. CIESC Journal, 2021, 72(6): 2905-2921. | |
| [8] | 沙亚利, 沈亮, 蒋燕锋, 等. 沉锂母液制备磷酸锂的工艺研究[J]. 当代化工研究, 2024(5): 173-175. |
| Sha Y L, Shen L, Jiang Y F, et al. Process exploration of lithium phosphate from the mother liquor of lithium carbonate[J]. Modern Chemical Research, 2024(5): 173-175. | |
| [9] | 盛怀禹, 李蓓莉, 陈耀焕, 等. 锂的新萃取体系研究[J]. 化学学报, 1995, 53(7): 689-694. |
| Sheng H Y, Li B L, Chen Y H, et al. Study on new extraction system for lithium[J]. Acta Chimica Sinica, 1995, 53(7): 689-694. | |
| [10] | 祝增虎, 朱朝梁, 温现明, 等. 碳酸锂生产工艺的研究进展[J]. 盐湖研究, 2008, 16(3): 64-72. |
| Zhu Z H, Zhu C L, Wen X M, et al. Progress in production processof lithium carbonate[J]. Journal of Salt Lake Research, 2008, 16(3): 64-72. | |
| [11] | 李丽娟, 彭小五, 时东, 等. 含锂卤水中锂资源高效利用与绿色分离的新型萃取体系[J]. 盐湖研究, 2018, 26(4): 1-10. |
| Li L J, Peng X W, Shi D, et al. Eco-friendly separation and effective applications of lithium resources from various brine with lithium: their extractant and extraction system[J]. Journal of Salt Lake Research, 2018, 26(4): 1-10. | |
| [12] | 黄小卫, 李铮, 张正钦, 等. 盐湖原卤提锂研究进展[J]. 有色金属工程, 2024, 14(11): 1-13. |
| Huang X W, Li Z, Zhang Z Q, et al. Research progress of lithium extraction from raw brine in salt lake[J]. Nonferrous Metals Engineering, 2024, 14(11): 1-13. | |
| [13] | 李燕, 王敏, 赵有璟, 等. 盐湖卤水锂资源提取技术及开发现状[J]. 盐湖研究, 2023, 31(2): 71-80. |
| Li Y, Wang M, Zhao Y J, et al. Technology and development of lithium extraction from salt lake brine[J]. Journal of Salt Lake Research, 2023, 31(2): 71-80. | |
| [14] | 时东, 李晋峰, 张波, 等. N523-TBP-磺化煤油萃取体系从饱和氯化镁卤水中萃取锂的工艺研究[J]. 盐湖研究, 2013, 21(2): 52-57. |
| Shi D, Li J F, Zhang B, et al. Process study on N523-TBP-sulfonated kerosene extraction system for extraction of lithium from brine saturated by magnesium chloride[J]. Journal of Salt Lake Research, 2013, 21(2): 52-57. | |
| [15] | 张金才, 王敏, 戴静. 卤水提锂的萃取体系概述[J]. 盐湖研究, 2005, 13(1): 42-48, 54. |
| Zhang J C, Wang M, Dai J. Summarization of the lithium extraction system[J]. Journal of Salt Lake Research, 2005, 13(1): 42-48, 54. | |
| [16] | Bai R B, Wang J F, Cui L, et al. Efficient extraction of lithium ions from high Mg/Li ratio brine through the synergy of TBP and hydroxyl functional ionic liquids[J]. Chinese Journal of Chemistry, 2020, 38(12): 1743-1751. |
| [17] | Li Z, Binnemans K. Opposite selectivities of tri-n-butyl phosphate and Cyanex 923 in solvent extraction of lithium and magnesium[J]. AIChE Journal, 2021, 67(7): e17219. |
| [18] | 高振, 黄焜, 杜林, 等. 酸性有机磷类萃取剂单分子膜的气-液界面行为: 亚相pH和铺展溶剂的影响[J]. 化学学报, 2019, 77(6): 506-514. |
| Gao Z, Huang K, Du L, et al. Interfacial behavior of acidic organophosphorus extractant monolayer at air-water interface: subphase pH and spreading solvent effect[J]. Acta Chimica Sinica, 2019, 77(6): 506-514. | |
| [19] | Shi D, Cui B, Li L J, et al. Lithium extraction from low-grade salt lake brine with ultrahigh Mg/Li ratio using TBP-kerosene-FeCl3 system[J]. Separation and Purification Technology, 2019, 211: 303-309. |
| [20] | Zhou Z Y, Fan J H, Liu X T, et al. Recovery of lithium from salt-lake brines using solvent extraction with TBP as extractant and FeCl3 as co-extraction agent[J]. Hydrometallurgy, 2020, 191: 105244. |
| [21] | Healy T V. Synergism in the solvent extraction of alkali metal ions by thenoyl trifluoracetone[J]. Journal of Inorganic and Nuclear Chemistry, 1968, 30(4): 1025-1036. |
| [22] | Seeley F G, Baldwin W H. Extraction of lithium from neutral salt solutions with fluorinated β-diketones[J]. Journal of Inorganic and Nuclear Chemistry, 1976, 38(5): 1049-1052. |
| [23] | Lee D A, Taylor W L, McDowell W J, et al. Solvent extraction of lithium[J]. Journal of Inorganic and Nuclear Chemistry, 1968, 30(10): 2807-2821. |
| [24] | Ishimori K I, Mori S, Ito Y, et al. Equilibrium and ab initio computational studies on the adduct formation of 1, 3-diketonato-lithium ( Ⅰ ) , -sodium ( Ⅰ ) and-potassium ( Ⅰ ) with 1, 10-phenanthroline and its 2, 9-dimethyl derivatives[J]. Talanta, 2009, 78(4/5): 1272-1279. |
| [25] | Onishi K, Nakamura T, Nishihama S, et al. Synergistic solvent impregnated resin for adsorptive separation of lithium ion[J]. Industrial & Engineering Chemistry Research, 2010, 49(14): 6554-6558. |
| [26] | Pranolo Y, Zhu Z W, Cheng C Y. Separation of lithium from sodium in chloride solutions using SSX systems with LiX 54 and Cyanex 923[J]. Hydrometallurgy, 2015, 154: 33-39. |
| [27] | Zhang L C, Li L J, Rui H M, et al. Lithium recovery from effluent of spent lithium battery recycling process using solvent extraction[J]. Journal of Hazardous Materials, 2020, 398: 122840. |
| [28] | Tsivadze A Y, Bezdomnikov A A, Baulin V E, et al. A new extraction system based on isopropyl salicylate and trioctylphosphine oxide for separating alkali metals[J]. Molecules, 2022, 27(10): 3051. |
| [29] | Niu Z H, Xu T S, Zhang L C, et al. Mechanism and process study of lithium extraction by 2-ethylhexyl salicylate extraction system[J]. Journal of Cleaner Production, 2024, 446: 141351. |
| [30] | Zhang J F, Tanjedrew N, Wenzel D M, et al. Selective separation of lithium, magnesium and calcium using 4-phosphoryl pyrazolones as pH-regulated receptors[J]. Angewandte Chemie International Edition, 2023, 62(13): e202216011. |
| [31] | Zhang J F, Wenzel D M, Steup J, et al. 4-Phosphoryl pyrazolones for highly selective lithium separation from alkali metal ions[J]. Chemistry - A European Journal, 2022, 28(1): e202103640. |
| [32] | 威尔弗雷德 L . F. 阿玛瑞高, 克里斯蒂娜 L . L. 柴. 实验室化学品纯化手册[M]. 林英杰, 刘伟, 王会萍, 等, 译. 5版. 北京: 化学工业出版社, 2006: 148-293. |
| Armarego W L F, Chai C L L. Purification of Laboratory Chemicals[M]. Lin Y J, Liu W, Wang H P, et al, trans. 5th ed. Beijing: Chemical Industry Press, 2006: 148-293. | |
| [33] | 丁贻祥, 袁承业. 有机磷化合物的研究ⅩⅪ. 两可阴离子的区域选择性磷酰化反应[J]. 化学学报, 1987, (8): 785-790. |
| Ding Y X, Yuan C Y. Studies on organophosphorus compounds ⅩⅪ. Regioselective phosphorylation reactions of ambident anions[J]. Acta Chimica Sinica, 1987, 8: 785-790. | |
| [34] | 袁承业, 胡水生. 有机磷化合物的研究ⅩⅩⅣ.辛基膦酸单丁酯的合成[J]. 化学学报, 1988, 46(3): 290-293. |
| Yuan C Y, Hu S S. Studies on organophosphorus compounds ⅩⅩⅣ. Synthesis of monobutyl esters of octylphosphonates[J]. Acta Chimica Sinica, 1988, 46(3): 290-293. | |
| [35] | 袁承业, 漆又毛, 向才立. 有机磷化合物的研究: Ⅻ.α-氨基烃基膦酸的合成[J]. 化学学报, 1985, 43(3): 243-249. |
| Yuan C Y, Qi Y M, Xiang C L. Studies on organophosphorus compounds: Ⅻ.Synthesis of α-aminoalkylphosphonic acids[J]. Acta Chimica Sinica, 1985, 43(3): 243-249. |
| [1] | 赵婧, 董书辰, 李高洋, 黄友科, 石浩森, 缪舒文, 谭辰妍, 朱唐琦, 李永帅, 潘慧, 凌昊. 基于电化学模型的电池性能模拟与优化[J]. 化工学报, 2025, 76(9): 4922-4932. |
| [2] | 娄岚浩, 杨立鹏, 杨晓光. 锂离子电池电化学机理模型参数辨识研究综述[J]. 化工学报, 2025, 76(9): 4369-4382. |
| [3] | 王三一, 黄文来. 电化学合成氨流程建模与优化[J]. 化工学报, 2025, 76(9): 4474-4486. |
| [4] | 徐佳琪, 张文君, 余燕萍, 苏宝根, 任其龙, 杨启炜. 热等离子体重整炼厂气制合成气过程数值模拟与实验研究[J]. 化工学报, 2025, 76(9): 4462-4473. |
| [5] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [6] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| [7] | 王御风, 罗小雪, 范鸿亮, 吴白婧, 李存璞, 魏子栋. 耦合电解水制氢的绿色有机电合成——电极界面调控策略综述[J]. 化工学报, 2025, 76(8): 3753-3771. |
| [8] | 吴阿强, 诸葛祥群, 刘通, 王明星, 罗鲲. 纳米普鲁士蓝悬浮电解液对锂氧电池性能的影响[J]. 化工学报, 2025, 76(8): 4310-4317. |
| [9] | 彭梦圆, 李家明, 沙敏, 张丁. 季铵盐氟碳表面活性剂复配体系的性能研究[J]. 化工学报, 2025, 76(8): 4177-4184. |
| [10] | 李欣然, 常龙娇, 罗绍华, 李永兵, 杨瑞芬, 侯增磊, 邹杰. Ho掺杂诱导NCM622局域电子重构抑制阳离子混排的改性机制研究[J]. 化工学报, 2025, 76(7): 3733-3741. |
| [11] | 吴鹂霄, 燕溪溪, 张素娜, 徐一鸣, 钱佳颖, 乔永民, 王利军. 磷掺杂微晶石墨的制备及其在锂离子电池负极材料中的电化学性能研究[J]. 化工学报, 2025, 76(7): 3615-3625. |
| [12] | 孙传付, 胡桂林, 曹俊杰, 左启斌, 陈媚, 夏玉珍. 梯度孔分布ZnO-GA锂离子电池负极材料研究[J]. 化工学报, 2025, 76(7): 3710-3718. |
| [13] | 郭铮铮, 赵一丹, 王辅强, 裴璐, 靳彦岭, 任芳, 任鹏刚. 异质结构MoS2/RGO/NiFe2O4复合材料的构筑及电磁波吸收性能研究[J]. 化工学报, 2025, 76(7): 3719-3732. |
| [14] | 乔亮, 李尚, 刘新亮, 王明, 张沛, 侯影飞. 三元共聚物稠油降黏剂的合成及分子模拟研究[J]. 化工学报, 2025, 76(7): 3686-3695. |
| [15] | 徐鹏国, 孟子衡, 朱干宇, 李会泉, 王晨晔, 孙振华, 田国才. 粗碳酸锂CO2微气泡深度碳化工艺与动力学研究[J]. 化工学报, 2025, 76(7): 3325-3338. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号