• •
马明怡1( ), 牛佳宝1, 严鑫1, 李然1, 周高勇1, 朱佳佳1, 王兴宝2, 何孝军1(
), 牛佳宝1, 严鑫1, 李然1, 周高勇1, 朱佳佳1, 王兴宝2, 何孝军1( ), 李宏强1(
), 李宏强1( )
)
收稿日期:2025-08-18
修回日期:2025-10-24
出版日期:2025-12-15
通讯作者:
何孝军,李宏强
作者简介:马明怡(2000—),女,硕士研究生,2382044161@qq.com
基金资助:
Mingyi MA1( ), Jiabao NIU1, Xin YAN1, Ran LI1, Gaoyong ZHOU1, Jiajia ZHU1, Xingbao WANG2, Xiaojun HE1(
), Jiabao NIU1, Xin YAN1, Ran LI1, Gaoyong ZHOU1, Jiajia ZHU1, Xingbao WANG2, Xiaojun HE1( ), Hongqiang LI1(
), Hongqiang LI1( )
)
Received:2025-08-18
Revised:2025-10-24
Online:2025-12-15
Contact:
Xiaojun HE, Hongqiang LI
摘要:
开发高效电催化剂是提升二氧化碳还原(CO2RR)选择性与效率、助力碳中和的关键。针对传统粉末状碳基催化剂易脱落、传质差、活性位点易被覆盖等问题,本研究提出将其稳定于三维多孔碳气凝胶载体的策略。以煤焦油沥青为前驱体,通过冷冻干燥结合热解工艺,成功制备了Ni-N共掺杂碳气凝胶催化剂(Ni-NCA)。该催化剂具有高比表面积和贯通的三维孔道网络,有效均匀分散并稳定Ni-N活性位点,同时促进CO2吸附、电解质渗透和电子传输。将其用于CO2RR,在-0.8 V (vs RHE)下CO法拉第效率(FECO)高达95.6%,且在-0.7至-1.1 V (vs RHE)的宽电位窗口内FECO均超过90%。50小时恒电位(-0.8 V) (vs RHE)测试中电流密度稳定,FECO保持在82%以上,展现出优异的催化活性和稳定性,具有良好的应用前景。
中图分类号:
马明怡, 牛佳宝, 严鑫, 李然, 周高勇, 朱佳佳, 王兴宝, 何孝军, 李宏强. Ni-N共掺杂碳气凝胶的构筑及其电催化还原CO2性能[J]. 化工学报, DOI: 10.11949/0438-1157.20250930.
Mingyi MA, Jiabao NIU, Xin YAN, Ran LI, Gaoyong ZHOU, Jiajia ZHU, Xingbao WANG, Xiaojun HE, Hongqiang LI. Construction of Ni-N co-doped carbon aerogel and its performance in electrocatalytic reduction of CO2[J]. CIESC Journal, DOI: 10.11949/0438-1157.20250930.
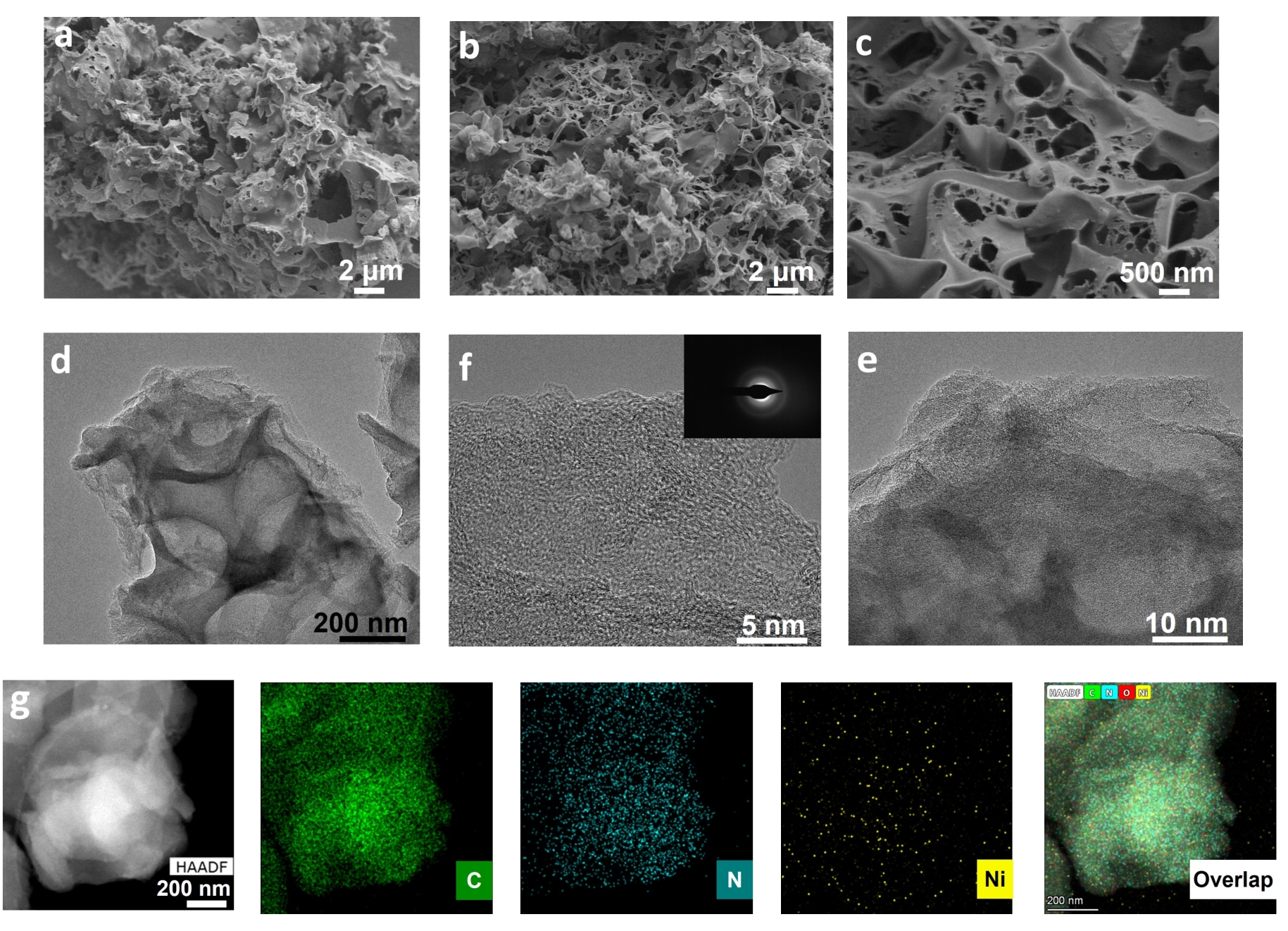
图2 (a)前驱体的SEM图;(b-c)Ni4-NCA在不同放大倍率下的SEM图片;(d-e)Ni4-NCA在不同放大倍率下的TEM图;(f)Ni4-NCA的HR-TEM图片(插图:选取衍射图);(g)Ni4-NCA的元素映射图
Fig.2 (a) SEM image of precursor; (b-c) SEM images of Ni4-NCA at different magnifications; (d-e) TEM images of Ni4-NCA at different magnifications; (f) HR-TEM image of Ni4-NCA (inset: SAED pattern); (g) EDS mapping images of Ni4-NCA
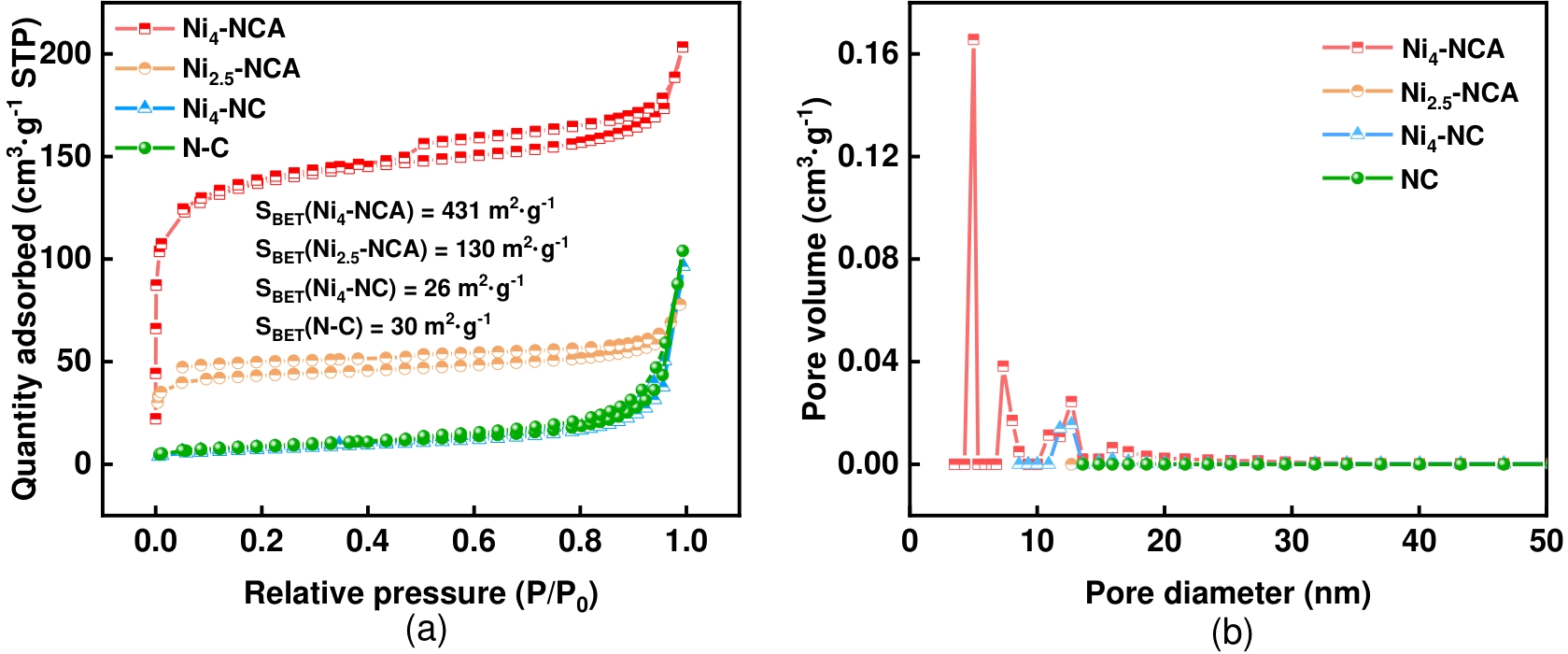
图4 Ni4-NCA、Ni2.5-NCA、Ni4-NC 和 N-C氮气吸脱附等温曲线图(a)和孔径分布图(b)
Fig.4 (a) N2 adsorption-desorption isotherms and (b) pore size distribution of Ni4-NCA、Ni2.5-NCA、Ni4-NC and N-C
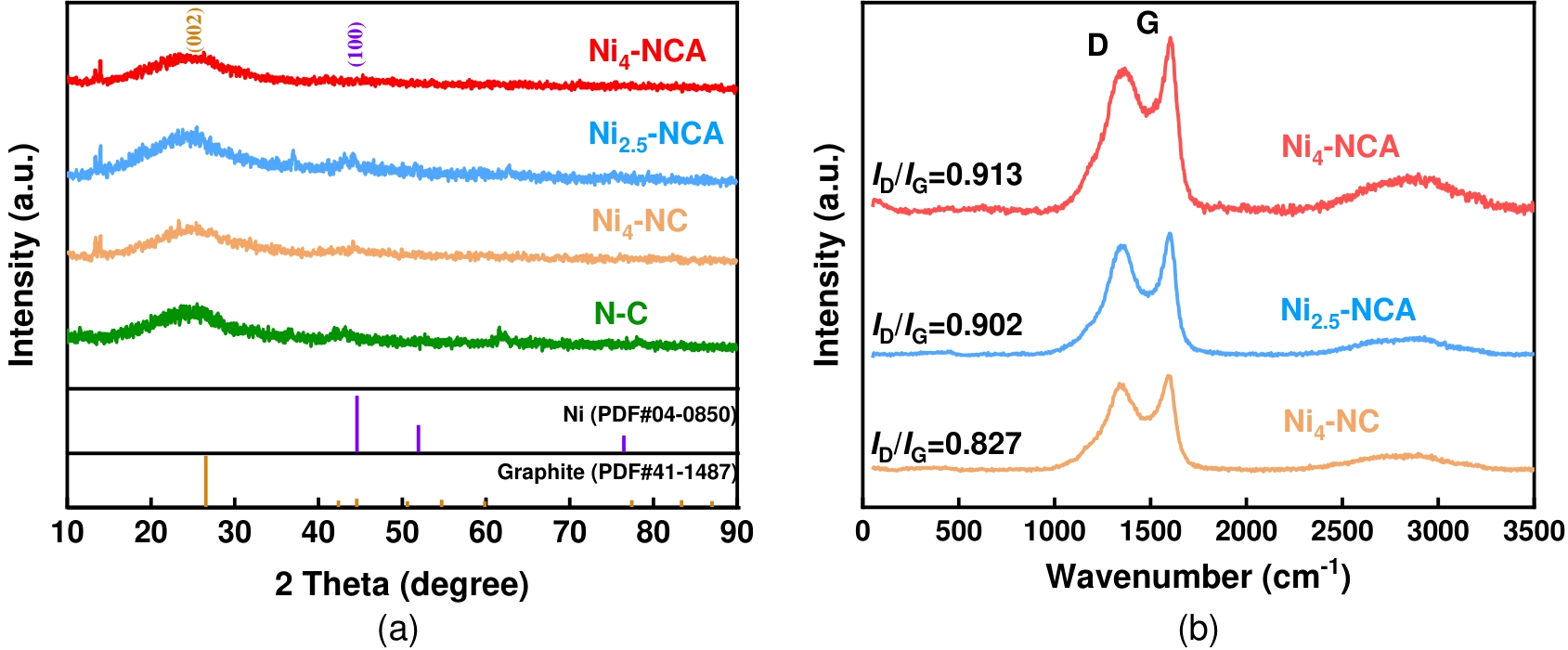
图5 (a)Ni4-NCA、Ni2.5-NCA、Ni4-NC和N-C的XRD谱图;(b)Ni4-NCA、Ni2.5-NCA和Ni4-NC的Raman光谱图
Fig.5 (a) X-ray diffraction patterns of Ni4-NCA; Ni2.5-NCA; Ni4-NC and N-C;(b) Raman spectra of Ni4-NCA, Ni2.5-NCA and Ni4-NC
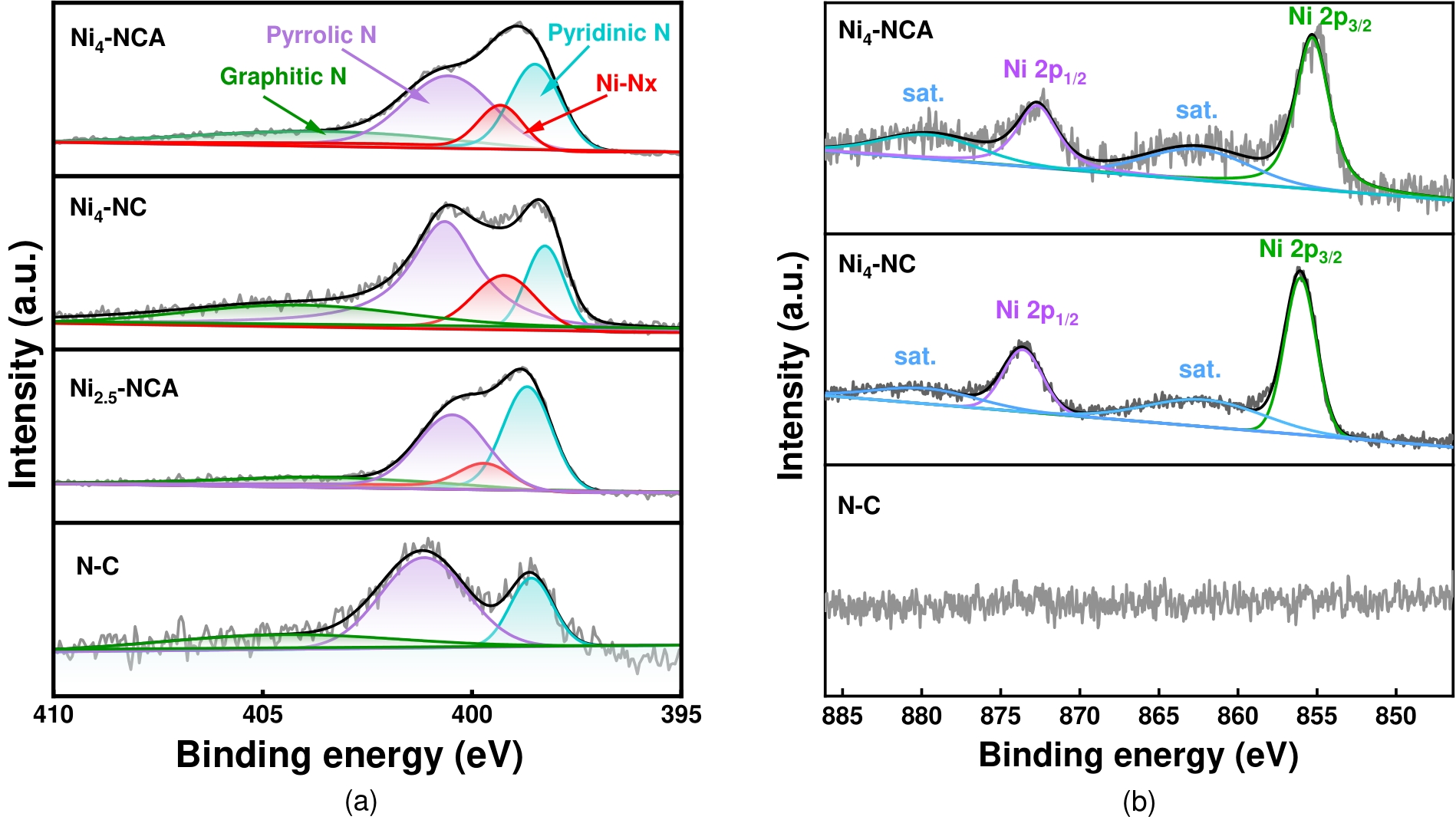
图8 (a)Ni4-NCA、Ni2.5-NCA、Ni4-NC和N-C的高分辨率N 1s 谱图;(b)Ni4-NCA、Ni2.5-NCA和N-C的高分辨率注:Ni 2p 谱图
Fig.8 (a) High resolution N 1s of Ni4-NCA, Ni2.5-NCA, Ni4-NC and N-C; (b) High resolution Ni 2p of Ni4-NCA, Ni4-NC and N-C.
| Catalyst | Ni (at.%) |
|---|---|
Ni4-NCA Ni2.5-NCA Ni4-NC N-C | 0.97% 0.17% 0.46% 0.01% |
表1 XPS 测得的Ni原子含量
Table 1 XPS measurements for the atomic concentration of Ni
| Catalyst | Ni (at.%) |
|---|---|
Ni4-NCA Ni2.5-NCA Ni4-NC N-C | 0.97% 0.17% 0.46% 0.01% |
| Catalyst | Ni (wt.%) |
|---|---|
Ni4-NCA Ni2.5-NCA Ni4-NC N-C | 2.18% 0.96% 1.90% 0.01% |
表2 ICP-OES 测得的Ni质量含量
Table 2 ICP-OES measurements for Ni weight percent.
| Catalyst | Ni (wt.%) |
|---|---|
Ni4-NCA Ni2.5-NCA Ni4-NC N-C | 2.18% 0.96% 1.90% 0.01% |
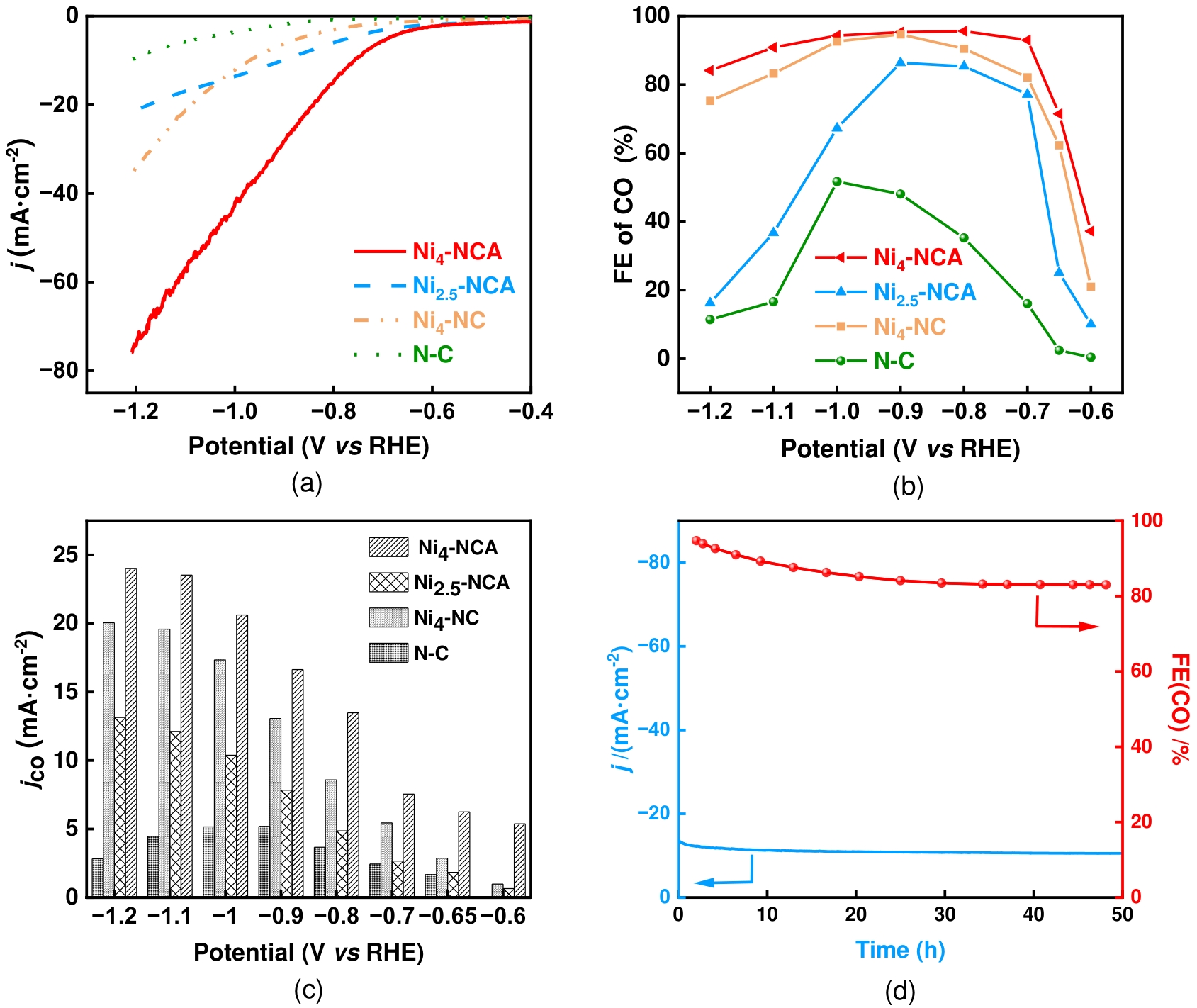
图10 样品Ni4-NCA、Ni2.5-NCA、Ni4-NC和 N-C的电化学测试(a)线性伏安扫描曲线;(b)不同电位下CO法拉第效率曲线;(c)不同电位下CO偏电流密度曲线;(d)在-0.8 V (vs RHE)条件下,样品Ni4-NCA经50小时电解稳定性测试图
Fig.10 (a) Linear sweep voltammetric (LSV) curves; (b) FEco at different potentials; (c) jco at different potentials acquired in CO2-saturated 0.5 M KHCO3 electrolytes for Ni4-NCA, Ni2.5-NCA, Ni4-NC and N-C; (d) Stability of Ni4-NCA: The FECO and current density at a potential of -0.8 V (vs RHE) during 50 h
| 催化剂 | 电解液 | 电压 (vs RHE)/V | CO法拉第效率(%) | 电流密度 (mA · cm-2) | 参考文献 |
|---|---|---|---|---|---|
Ni4-NCA Ni-NC(HPU) | 0.5 M KHCO3 0.5 M KHCO3 | -0.8 -0.8 | 95.6 91 | 14.85 27.1 | This work [ |
Ni@NC SA-Ni@NC | 0.5 M KHCO3 0.1 M KHCO3 | -0.6 ~ -1.3 -0.6 | 80> 86.2 | 16 10.1 | [ [ |
| Ni-N-MEGO | 0.5 M KHCO3 | -0.55~ -0.7 | 92.1 | 28.6 | [ |
| NiSA-NGA | 0.5 M KHCO3 | -0.8 | 90.2 | 6.5 | [ |
| SA-Ni/N-CS | 0.5 M KHCO3 | -0.8 | 95.1 | — | [ |
| Ni-N3-C | 0.5 M KHCO3 | -0.65 | 95.6 | 6.64 | [ |
| Ni-PACN | 0.5 M KHCO3 | -0.7 ~ -1.1 | 95 | 21 | [ |
| Ni–N–C | 0.5 M KHCO3 | -0.65 | 97 | 6.5 | [ |
表3 Ni4-NCA与文献报道的碳基单原子镍催化剂CO2RR性能对比
Table 3 Performance comparison of CO2RR between Ni4-NCA and literature-reported carbon-based single-atom nickel catalysts
| 催化剂 | 电解液 | 电压 (vs RHE)/V | CO法拉第效率(%) | 电流密度 (mA · cm-2) | 参考文献 |
|---|---|---|---|---|---|
Ni4-NCA Ni-NC(HPU) | 0.5 M KHCO3 0.5 M KHCO3 | -0.8 -0.8 | 95.6 91 | 14.85 27.1 | This work [ |
Ni@NC SA-Ni@NC | 0.5 M KHCO3 0.1 M KHCO3 | -0.6 ~ -1.3 -0.6 | 80> 86.2 | 16 10.1 | [ [ |
| Ni-N-MEGO | 0.5 M KHCO3 | -0.55~ -0.7 | 92.1 | 28.6 | [ |
| NiSA-NGA | 0.5 M KHCO3 | -0.8 | 90.2 | 6.5 | [ |
| SA-Ni/N-CS | 0.5 M KHCO3 | -0.8 | 95.1 | — | [ |
| Ni-N3-C | 0.5 M KHCO3 | -0.65 | 95.6 | 6.64 | [ |
| Ni-PACN | 0.5 M KHCO3 | -0.7 ~ -1.1 | 95 | 21 | [ |
| Ni–N–C | 0.5 M KHCO3 | -0.65 | 97 | 6.5 | [ |
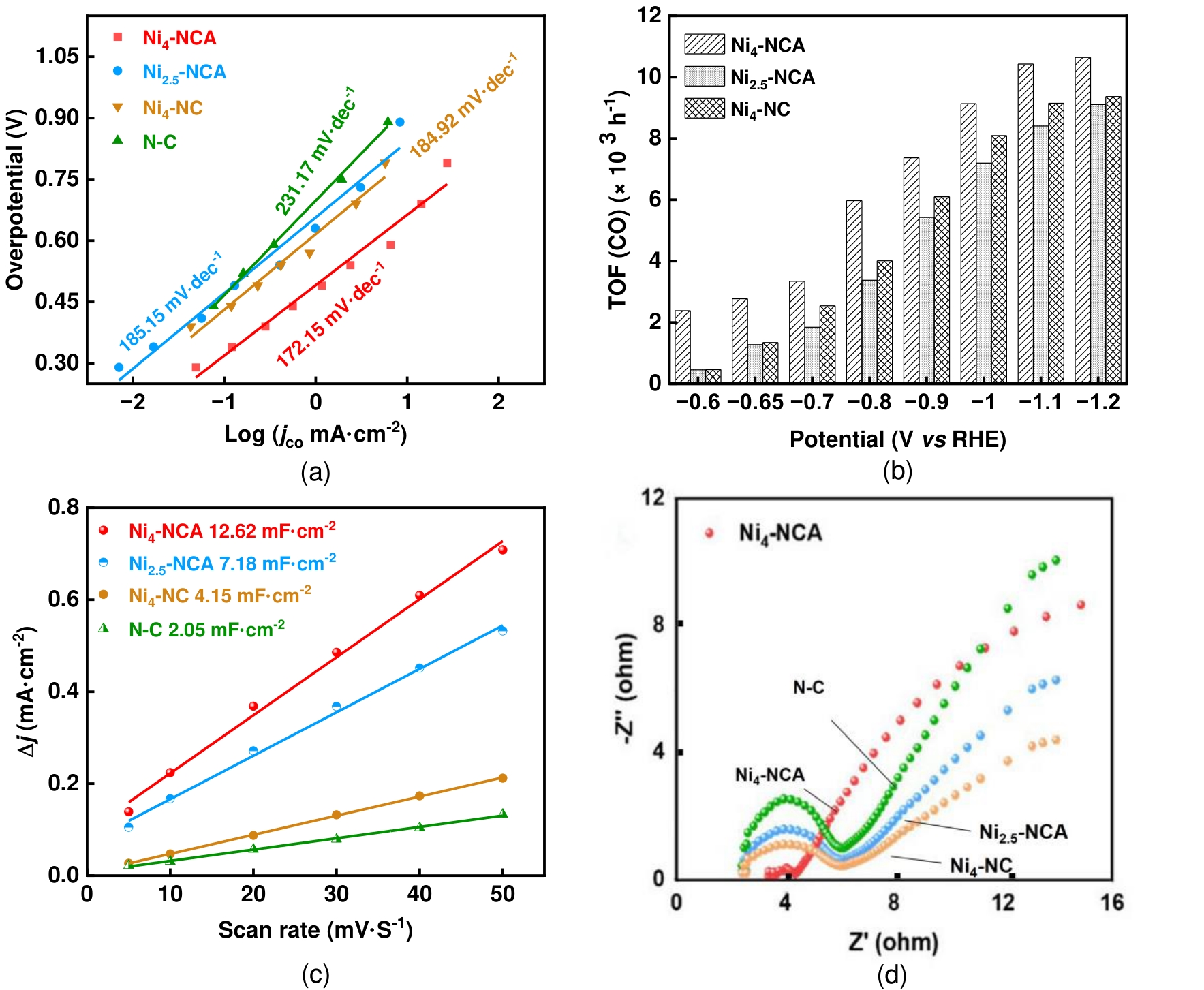
图11 Ni4-NCA、Ni2.5-NCA、Ni4-NC和N-C的(a)Tafel斜率图;(b)TOF图;(c)电流密度差值∆j与扫速的关系图;(d)电化学阻抗图
Fig.11 (a) Tafel curves; (b) TOF; (c) The plot of charging current density differences Δj against the scan rate for the calculation of ECSA acquired in CO2-saturated 0.5 M KHCO3 electrolyte for Ni4-NCA, Ni2.5-NCA, Ni4-NC and N-C; (d) Electrochemical impedance spectra of Ni4-NCA、Ni2.5-NCA、Ni4-NC and N-C
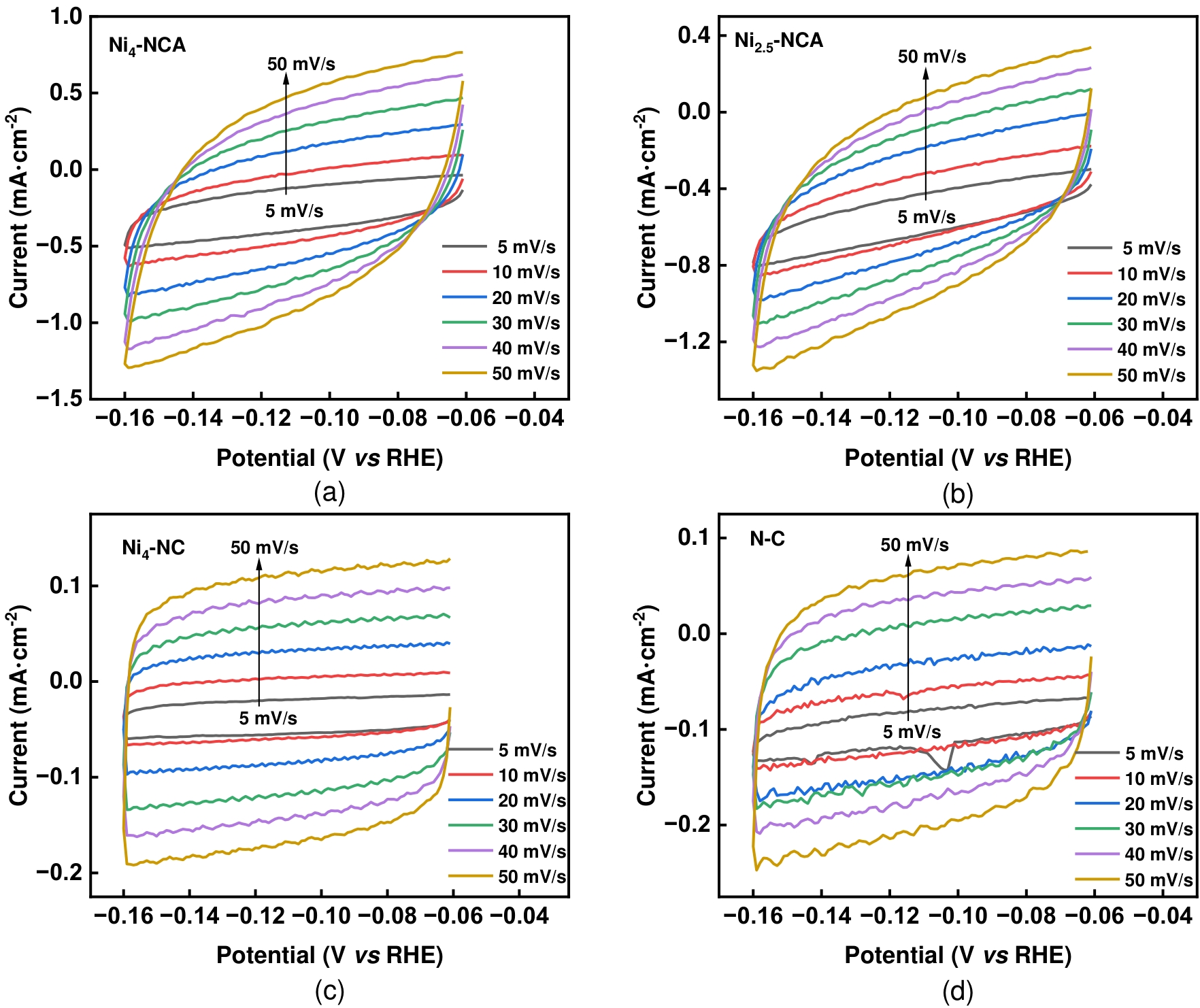
图12 在5到50 mV s-1的扫描速率下(a)Ni4-NCA、(b)Ni2.5-NCA、(c)Ni4-NC、(d)N-C从-0.06 到-0.16 V (vs RHE)的循环伏安曲线;
Fig.12 (a) Cyclic voltammetry curves from -0.06 V to -0.16 V (vs RHE) at scan rates ranging from 5 to 50 mV·s-1 for (a) Ni4-NCA, (b) Ni2.5-NCA (c) Ni4-NC and (d) N-C.
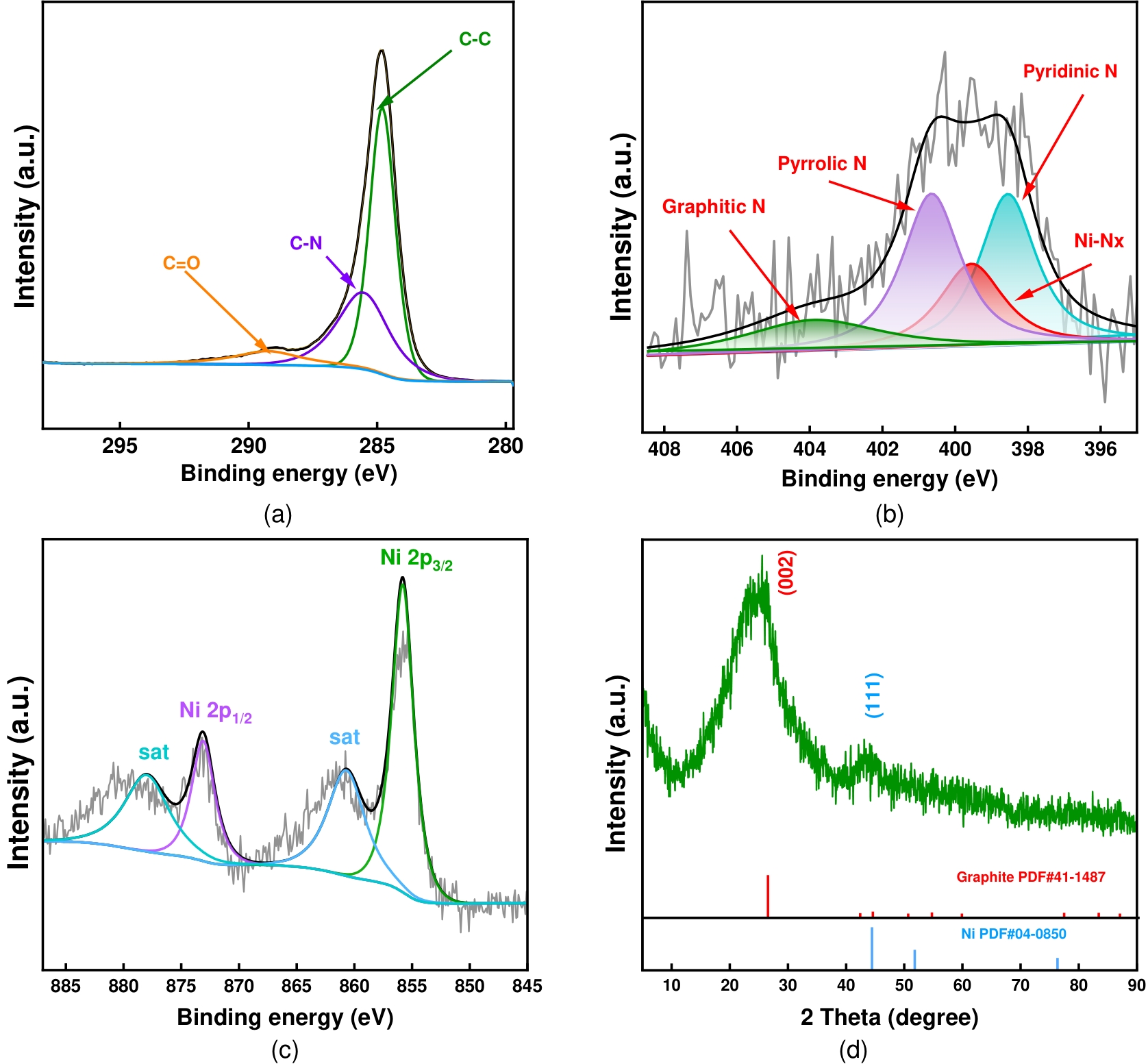
图13 循环50 h后Ni4-NCA的(a)高分辨率C 1s谱图,(b)高分辨率N 1s谱图,(c)高分辨率Ni 2p谱图,(d)XRD谱图
Figure 13 (a) High-resolution C 1s, (b) N 1s, (c) Ni 2p XPS spectrum, and (d) XRD pattern of the Ni₄-NCA sample after 50 h of cycling.
| [1] | Wang C L, Gao W J, Yang H X, et al. Advances and challenges in the electroreduction of carbon dioxide in acidic electrolyte[J]. Journal of Energy Chemistry, 2025, 107: 732-749. |
| [2] | Wang R T, Yang X K, Zhang J P, et al. In-situ characterization technologies and theoretical calculations in carbon dioxide reduction: in-depth understanding of reaction mechanisms and rational design of electrocatalysts[J]. Coordination Chemistry Reviews, 2025, 533: 216541. |
| [3] | Yi W L, Hou C S, Li R Y, et al. Designer electron-reservoir single-atom electrocatalyst for efficient carbon dioxide reduction[J]. Chemical Engineering Journal, 2025, 507: 160387. |
| [4] | Wang J J, Zhu Z Z, Lin Y X, et al. Nano-engineering in zinc-based catalysts for CO2 electroreduction: Advances and challenges[J]. Carbon Neutralization, 2024, 3(3): 423-440. |
| [5] | Guo C, Li N P, Gao S S, et al. Recent advancements in microenvironmental regulation of Single-Atom catalysts for electrochemical conversion of CO2 to CO[J]. Fuel, 2024, 367: 131416. |
| [6] | Gan K N, Li H Q, Li R, et al. Electroreduction of CO2 to syngas with controllable H2/CO ratios in a wide potential range over Ni–N Co-doped ultrathin carbon nanosheets[J]. Inorganic Chemistry Frontiers, 2023, 10(8): 2414-2422. |
| [7] | Guo H Z, Raj J, Wang Z M, et al. Synergistic effects of amine functional groups and enriched-atomic-iron sites in carbon dots for industrial-current–density CO2 electroreduction[J]. Small, 2024, 20(32): 2311132. |
| [8] | Cerrillo M I, Jiménez C, Ortiz M Á, et al. Electrocatalytic reduction of CO2 with N/B Co-doped reduced graphene oxide based catalysts[J]. Journal of Industrial and Engineering Chemistry, 2023, 127: 101-109. |
| [9] | Sun Y, Zhao K, Deng X M, et al. Metal-free Se-based tetra-doped carbon catalyst for high-selective electro-reduction of CO2 into CO[J]. Journal of Environmental Chemical Engineering, 2023, 11(5): 110435. |
| [10] | Qi Z J, Zhou Y, Guan R N, et al. Tuning the coordination environment of carbon-based single-atom catalysts via doping with multiple heteroatoms and their applications in electrocatalysis[J]. Advanced Materials, 2023, 35(38): 2210575. |
| [11] | Tang J Y, Weiss E, Shao Z P. Advances in cutting-edge electrode engineering toward CO2 electrolysis at high current density and selectivity: a mini-review[J]. Carbon Neutralization, 2022, 1(2): 140-158. |
| [12] | Wen M, Sun N N, Jiao L, et al. Microwave-assisted rapid synthesis of MOF-based single-atom Ni catalyst for CO2 electroreduction at ampere-level current[J]. Angewandte Chemie International Edition, 2024, 63(10): e202318338. |
| [13] | Wang T F, Wang J H, Lu C B, et al. Single-atom anchored curved carbon surface for efficient CO2 electro-reduction with nearly 100% CO selectivity and industrially-relevant current density[J]. Advanced Materials, 2023, 35(35): 2205553. |
| [14] | Lin D, Wang T T, Zhao Z L, et al. Molten-salt-assisted synthesis of single-atom iron confined N-doped carbon nanosheets for highly efficient industrial-level CO2 electroreduction and Zn-CO2 batteries[J]. Nano Energy, 2023, 113: 108568. |
| [15] | Liu K Y, Sun Z Y, Chen W X, et al. Ultra-fast pulsed discharge preparation of coordinatively unsaturated asymmetric copper single-atom catalysts for CO2 reduction[J]. Advanced Functional Materials, 2024, 34(16): 2312589. |
| [16] | Yao D Z, Tang C, Vasileff A, et al. The controllable reconstruction of Bi-MOFs for electrochemical CO2 reduction through electrolyte and potential mediation[J]. Angewandte Chemie International Edition, 2021, 60(33): 18178-18184. |
| [17] | Liu H M, Yan T, Tan S D, et al. Observation on microenvironment changes of dynamic catalysts in acidic CO2 reduction[J]. Journal of the American Chemical Society, 2024, 146(8): 5333-5342. |
| [18] | Wang W Y, He X H, Zhang K, et al. Surfactant-modified Zn nanosheets on carbon paper for electrochemical CO2 reduction to CO[J]. Chemical Communications, 2022, 58(33): 5096-5099. |
| [19] | Wu Q, Wu C C. Mechanism insights on single-atom catalysts for CO2 conversion[J]. Journal of Materials Chemistry A, 2023, 11(10): 4876-4906. |
| [20] | Sun W L, Liu S L, Sun H F, et al. Low-coordinated Ni single atom catalyst with carbon coordination for efficient CO2 electroreduction[J]. Advanced Energy Materials, 2025, 15(26): 2500283. |
| [21] | Xiong R Z, Xu H M, Zhu H R, et al. Recent progress in Cu-based electrocatalysts for CO2 reduction[J]. Chemical Engineering Journal, 2025, 505: 159210. |
| [22] | Liao W, Xiao K, Tian T, et al. Carbon aerogel monoliths from polymers: a review[J]. Journal of Cleaner Production, 2024, 437: 140736. |
| [23] | Godino-Ojer M, Soriano E, Calvino-Casilda V, et al. Metal-free synthesis of quinolines catalyzed by carbon aerogels: Influence of the porous texture and surface chemistry[J]. Chemical Engineering Journal, 2017, 314: 488-497. |
| [24] | Wu K Z, Zhang L, Yuan Y F, et al. Zinc-air batteries: an iron-decorated carbon aerogel for rechargeable flow and flexible Zn–air batteries[J]. Advanced Materials, 2020, 32(32): 2070241. |
| [25] | Ma Z S, Zhang T Y, Lin L L, et al. Ni single-atom arrays as self-supported electrocatalysts for CO2RR[J]. AIChE Journal, 2023, 69(10): e18161. |
| [26] | Yang H Y, Driess M, Menezes P W. Self-supported electrocatalysts for practical water electrolysis[J]. Advanced Energy Materials, 2021, 11(39): 2102074. |
| [27] | Sun X R, Wang S B, Hou Y D, et al. Self-supporting metal–organic framework-based hydrogen and oxygen electrocatalysts[J]. Journal of Materials Chemistry A, 2023, 11(25): 13089-13106. |
| [28] | Wang G T, Li X, Yang X H, et al. Metal-based aerogels catalysts for electrocatalytic CO2 reduction[J]. Chemistry – A European Journal, 2022, 28(64): e202201834. |
| [29] | Zhang F W, Zhang H, Jia Z H, et al. Nickel single atom density-dependent CO2 efficient electroreduction[J]. Small, 2024, 20(16): 2308080. |
| [30] | 陈娅. 改性煤沥青基多孔碳的制备及其在超级电容器中的应用[D]. 贵阳: 贵州大学, 2024. |
| Chen Y. Preparation of Modified Coal Tar Pitch-Based Porous Carbon and Its Application in Supercapacitors[D]. Guiyang: Guizhou University, 2024. | |
| [31] | 黄玉. 煤沥青制备活性炭氮改性及CO2吸附性能研究[D]. 杭州: 浙江大学, 2024. |
| Huang Y. Study on nitrogen modification and CO2 adsorption properties of activated carbon prepared from coal tar pitch[D]. Hangzhou: Zhejiang University, 2024. | |
| [32] | Huang L L, Zhang X W, Wang L, et al. Boosting catalytic performance of N doped porous carbon derived from coal tar pitch: The role of N species and the contribution of 1O2 [J]. Journal of Water Process Engineering, 2025, 72: 107426. |
| [33] | Guo F, Jiang Y Q, Xu Z, et al. Highly stretchable carbon aerogels[J]. Nature Communications, 2018, 9: 881. |
| [34] | Li C, Wang Y W, Xiao N, et al. Nitrogen-doped porous carbon from coal for high efficiency CO2 electrocatalytic reduction[J]. Carbon, 2019, 151: 46-52. |
| [35] | He Q C, Zhu Y P, Li Y, et al. Etch-driven N, P Co-doped hierarchical porous carbon embedded with Ni nanoparticles as an efficient dynamic carrier for room-temperature NaS battery[J]. Journal of Energy Storage, 2023, 74: 109353. |
| [36] | Lee G B, Joo W H, Kang H Y, et al. Fabrication of Ni nanoparticle-embedded porous carbon nanofibers through selective etching of selectively oxidized MgO[J]. Electronic Materials Letters, 2022, 18(2): 198-204. |
| [37] | Yu J, Li J, Xu C Y, et al. Atomically dispersed Ni–N4 species and Ni nanoparticles constructing N-doped porous carbon fibers for accelerating hydrogen evolution[J]. Carbon, 2021, 185: 96-104. |
| [38] | Feng L L, Li Y H, Fu C L, et al. Etching-assisted synthesis of Ni/Ni single atom anchored porous graphitic nanocarbon for improved hydrogen evolution reaction[J]. New Journal of Chemistry, 2023, 47(38): 17657-17665. |
| [39] | Ning H, Guo Z H, Wang W H, et al. Ammonia etched petroleum pitch-based porous carbon as efficient catalysts for CO2 electroreduction[J]. Carbon Letters, 2022, 32(3): 807-814. |
| [40] | Chen X Y, Liu W, Sun Y X, et al. KOH-Enabled axial-oxygen coordinated Ni single-atom catalyst for efficient electrocatalytic CO2 reduction[J]. Small Methods, 2023, 7(3): 2201311. |
| [41] | Shang Y, Ding Y X, Zhang P L, et al. Pyrrolic N or pyridinic N: The active center of N-doped carbon for CO2 reduction[J]. Chinese Journal of Catalysis, 2022, 43(9): 2405-2413. |
| [42] | He C, Zhang Y, Zhang Y F, et al. Molecular evidence for metallic cobalt boosting CO2 electroreduction on pyridinic nitrogen[J]. Angewandte Chemie International Edition, 2020, 59(12): 4914-4919. |
| [43] | Li H Q, Gan K N, Li R, et al. Highly dispersed NiO clusters induced electron delocalization of Ni-N-C catalysts for enhanced CO2 electroreduction[J]. Advanced Functional Materials, 2023, 33(1): 2208622. |
| [44] | Li Y X, Lu X F, Xi S B, et al. Synthesis of N-doped highly graphitic carbon urchin-like hollow structures loaded with single-Ni atoms towards efficient CO2 electroreduction[J]. Angewandte Chemie International Edition, 2022, 61(18): e202201491. |
| [45] | 龙雪梅. 碳基材料中镍催化剂的配位与形态对电催化二氧化碳还原的影响[D]. 广州: 广州大学, 2025. |
| Long X M. Effect of coordination and morphology of Ni catalysts in carbon-based materials on electrochemical CO2 reduction[D]. Guangzhou: Guangzhou University, 2025. | |
| [46] | Zhang C, Fu Z H, Zhao Q, et al. Single-atom-Ni-decorated, nitrogen-doped carbon layers for efficient electrocatalytic CO2 reduction reaction[J]. Electrochemistry Communications, 2020, 116: 106758. |
| [47] | Cheng Y, Zhao S Y, Li H B, et al. Unsaturated edge-anchored Ni single atoms on porous microwave exfoliated graphene oxide for electrochemical CO2 [J]. Applied Catalysis B: Environmental, 2019, 243: 294-303. |
| [48] | Mou K W, Chen Z P, Zhang X X, et al. Highly efficient electroreduction of CO2 on nickel single-atom catalysts: atom trapping and nitrogen anchoring[J]. Small, 2019, 15(49): 1903668. |
| [49] | Yuan C Z, Zhan L Y, Liu S J, et al. Semi-sacrificial template synthesis of single-atom Ni sites supported on hollow carbon nanospheres for efficient and stable electrochemical CO2 reduction[J]. Inorganic Chemistry Frontiers, 2020, 7(8): 1719-1725. |
| [50] | Zhang Y, Jiao L, Yang W J, et al. Rational fabrication of low-coordinate single-atom Ni electrocatalysts by MOFs for highly selective CO2 reduction[J]. Angewandte Chemie International Edition, 2021, 60(14): 7607-7611. |
| [51] | Koshy D M, Chen S C, Lee D U, et al. Understanding the origin of highly selective CO2 electroreduction to CO on Ni, N-doped carbon catalysts[J]. Angewandte Chemie International Edition, 2020, 59(10): 4043-4050. |
| [52] | Wang J L, Huang Y-C, Wang Y Q, et al. Atomically dispersed metal–nitrogen–carbon catalysts with d-orbital electronic configuration-dependent selectivity for electrochemical CO2-to-CO reduction[J]. ACS Catalysis, 2023, 13(4): 2374-2385. |
| [53] | Ko Y J, Lim C, Jin J, et al. Extrinsic hydrophobicity-controlled silver nanoparticles as efficient and stable catalysts for CO2 electrolysis[J]. Nature Communications, 2024, 15: 3356. |
| [54] | Chen Y J, Ji S F, Wang Y G, et al. Isolated single iron atoms anchored on N-doped porous carbon as an efficient electrocatalyst for the oxygen reduction reaction[J]. Angewandte Chemie International Edition, 2017, 56(24): 6937-6941. |
| [1] | 孙云龙, 徐肖肖, 黄永方, 郭纪超, 陈卫卫. 水平光滑管内CO2流动沸腾的非绝热可视化研究[J]. 化工学报, 2025, 76(S1): 230-236. |
| [2] | 郭纪超, 徐肖肖, 孙云龙. 基于植物工厂中的CO2浓度气流模拟及优化研究[J]. 化工学报, 2025, 76(S1): 237-245. |
| [3] | 孔繁臣, 张硕, 唐明生, 邹慧明, 胡舟航, 田长青. 二氧化碳直线压缩机气体轴承模拟[J]. 化工学报, 2025, 76(S1): 281-288. |
| [4] | 何婷, 张开, 林文胜, 陈利琼, 陈家富. 沼气超临界压力低温脱碳-液化耦合流程研究[J]. 化工学报, 2025, 76(S1): 418-425. |
| [5] | 周怀荣, 伊嘉伟, 曹阿波, 郭奥雪, 王东亮, 杨勇, 杨思宇. 共电解耦合CO2间接加氢制甲醇工艺集成设计与性能评价[J]. 化工学报, 2025, 76(9): 4586-4600. |
| [6] | 佟丽丽, 陈英, 艾敏华, 舒玉美, 张香文, 邹吉军, 潘伦. ZnO/WO3异质结光催化环烯烃[2+2]环加成制备高能量密度燃料[J]. 化工学报, 2025, 76(9): 4882-4892. |
| [7] | 邹家庆, 张肇钰, 张建国, 张博宇, 刘定胜, 毛庆, 王挺, 李建军. 碱水制氢电解槽极板通道中气泡的生成及演化性质[J]. 化工学报, 2025, 76(9): 4786-4799. |
| [8] | 钱慧慧, 王文婕, 陈文尧, 周兴贵, 张晶, 段学志. 聚丙烯定向转化制芳烃:金属-分子筛协同催化机制[J]. 化工学报, 2025, 76(9): 4838-4849. |
| [9] | 张建民, 何美贵, 贾万鑫, 赵静, 金万勤. 聚氧化乙烯/冠醚共混膜及其二氧化碳分离性能[J]. 化工学报, 2025, 76(9): 4862-4871. |
| [10] | 王一飞, 李玉星, 欧阳欣, 赵雪峰, 孟岚, 胡其会, 殷布泽, 郭雅琦. 基于裂尖减压特性的CO2管道断裂扩展数值计算[J]. 化工学报, 2025, 76(9): 4683-4693. |
| [11] | 赵维, 邢文乐, 韩朝旭, 袁兴中, 蒋龙波. g-C3N4基非金属异质结光催化降解水中有机污染物的研究进展[J]. 化工学报, 2025, 76(9): 4752-4769. |
| [12] | 范夏雨, 孙建辰, 李可莹, 姚馨雅, 商辉. 机器学习驱动液态有机储氢技术的系统优化[J]. 化工学报, 2025, 76(8): 3805-3821. |
| [13] | 杨宁, 李皓男, LIN Xiao, GEORGIADOU Stella, LIN Wen-Feng. 从塑料废弃物到能源催化剂:塑料衍生碳@CoMoO4复合材料在电解水析氢反应中的应用[J]. 化工学报, 2025, 76(8): 4081-4094. |
| [14] | 周运桃, 崔丽凤, 张杰, 于富红, 李新刚, 田野. Ga2O3调控CuCeO催化CO2加氢制甲醇的研究[J]. 化工学报, 2025, 76(8): 4042-4051. |
| [15] | 巢欣旖, 陈文尧, 张晶, 钱刚, 周兴贵, 段学志. 甲醇和乙酸甲酯一步法制丙酸甲酯催化剂的可控制备与性能调控[J]. 化工学报, 2025, 76(8): 4030-4041. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号