CIESC Journal ›› 2019, Vol. 70 ›› Issue (S2): 25-30.DOI: 10.11949/0438-1157.20190553
• Thermodynamics • Previous Articles Next Articles
Yanjun SUN1( ),Gaolei DI1,Juan XIA2,Xiaopo WANG3(
),Gaolei DI1,Juan XIA2,Xiaopo WANG3( ),Na GONG3
),Na GONG3
Received:2019-05-22
Revised:2019-07-11
Online:2019-09-06
Published:2019-09-06
Contact:
Xiaopo WANG
通讯作者:
王晓坡
作者简介:孙艳军(1983—),男,博士,副教授,基金资助:CLC Number:
Yanjun SUN, Gaolei DI, Juan XIA, Xiaopo WANG, Na GONG. Investigation of solubility behavior between HFO1234yf and lubricants[J]. CIESC Journal, 2019, 70(S2): 25-30.
孙艳军, 邸高雷, 夏娟, 王晓坡, 龚娜. 不同冷冻润滑油对HFO1234yf溶解吸收特性的研究[J]. 化工学报, 2019, 70(S2): 25-30.
Add to citation manager EndNote|Ris|BibTeX
| T/K | x 1 | p/MPa | T/K | x 1 | p/MPa |
|---|---|---|---|---|---|
| 283.15 | 0.0211 | 0.023 | 333.15 | 0.0104 | 0.03 |
| 0.0502 | 0.065 | 0.0298 | 0.083 | ||
| 0.1382 | 0.16 | 0.0755 | 0.21 | ||
| 0.2986 | 0.326 | 0.1611 | 0.449 | ||
| 293.15 | 0.019 | 0.024 | 0.2299 | 0.655 | |
| 0.048 | 0.068 | 0.3212 | 0.916 | ||
| 0.1201 | 0.171 | 0.4745 | 1.352 | ||
| 0.2557 | 0.356 | 343.15 | 0.0087 | 0.031 | |
| 0.3753 | 0.5 | 0.0272 | 0.086 | ||
| 303.15 | 0.0173 | 0.025 | 0.0697 | 0.219 | |
| 0.0421 | 0.072 | 0.1485 | 0.47 | ||
| 0.1015 | 0.183 | 0.2122 | 0.687 | ||
| 0.2202 | 0.383 | 0.2966 | 0.966 | ||
| 0.3226 | 0.546 | 0.4354 | 1.445 | ||
| 0.4656 | 0.734 | 353.15 | 0.0078 | 0.032 | |
| 313.15 | 0.014 | 0.027 | 0.0261 | 0.087 | |
| 0.0382 | 0.075 | 0.0665 | 0.223 | ||
| 0.0933 | 0.191 | 0.1435 | 0.479 | ||
| 0.195 | 0.407 | 0.2046 | 0.702 | ||
| 0.2829 | 0.585 | 0.2864 | 0.99 | ||
| 0.3978 | 0.805 | 0.421 | 1.489 | ||
| 323.15 | 0.0123 | 0.029 | |||
| 0.0333 | 0.079 | ||||
| 0.0832 | 0.201 | ||||
| 0.1757 | 0.429 | ||||
| 0.253 | 0.621 | ||||
| 0.3538 | 0.863 | ||||
| 0.5365 | 1.24 |
Table 1 Experimental data for solubility of HFO1234zyf in squalene
| T/K | x 1 | p/MPa | T/K | x 1 | p/MPa |
|---|---|---|---|---|---|
| 283.15 | 0.0211 | 0.023 | 333.15 | 0.0104 | 0.03 |
| 0.0502 | 0.065 | 0.0298 | 0.083 | ||
| 0.1382 | 0.16 | 0.0755 | 0.21 | ||
| 0.2986 | 0.326 | 0.1611 | 0.449 | ||
| 293.15 | 0.019 | 0.024 | 0.2299 | 0.655 | |
| 0.048 | 0.068 | 0.3212 | 0.916 | ||
| 0.1201 | 0.171 | 0.4745 | 1.352 | ||
| 0.2557 | 0.356 | 343.15 | 0.0087 | 0.031 | |
| 0.3753 | 0.5 | 0.0272 | 0.086 | ||
| 303.15 | 0.0173 | 0.025 | 0.0697 | 0.219 | |
| 0.0421 | 0.072 | 0.1485 | 0.47 | ||
| 0.1015 | 0.183 | 0.2122 | 0.687 | ||
| 0.2202 | 0.383 | 0.2966 | 0.966 | ||
| 0.3226 | 0.546 | 0.4354 | 1.445 | ||
| 0.4656 | 0.734 | 353.15 | 0.0078 | 0.032 | |
| 313.15 | 0.014 | 0.027 | 0.0261 | 0.087 | |
| 0.0382 | 0.075 | 0.0665 | 0.223 | ||
| 0.0933 | 0.191 | 0.1435 | 0.479 | ||
| 0.195 | 0.407 | 0.2046 | 0.702 | ||
| 0.2829 | 0.585 | 0.2864 | 0.99 | ||
| 0.3978 | 0.805 | 0.421 | 1.489 | ||
| 323.15 | 0.0123 | 0.029 | |||
| 0.0333 | 0.079 | ||||
| 0.0832 | 0.201 | ||||
| 0.1757 | 0.429 | ||||
| 0.253 | 0.621 | ||||
| 0.3538 | 0.863 | ||||
| 0.5365 | 1.24 |
| 体系 | | | | | |
|---|---|---|---|---|---|
| R1234yf + squalane | 0.42 | 2.2853 | 0.0171 | -2.7626 | 0.0030 |
Table 2 Adjustable parameter
| 体系 | | | | | |
|---|---|---|---|---|---|
| R1234yf + squalane | 0.42 | 2.2853 | 0.0171 | -2.7626 | 0.0030 |
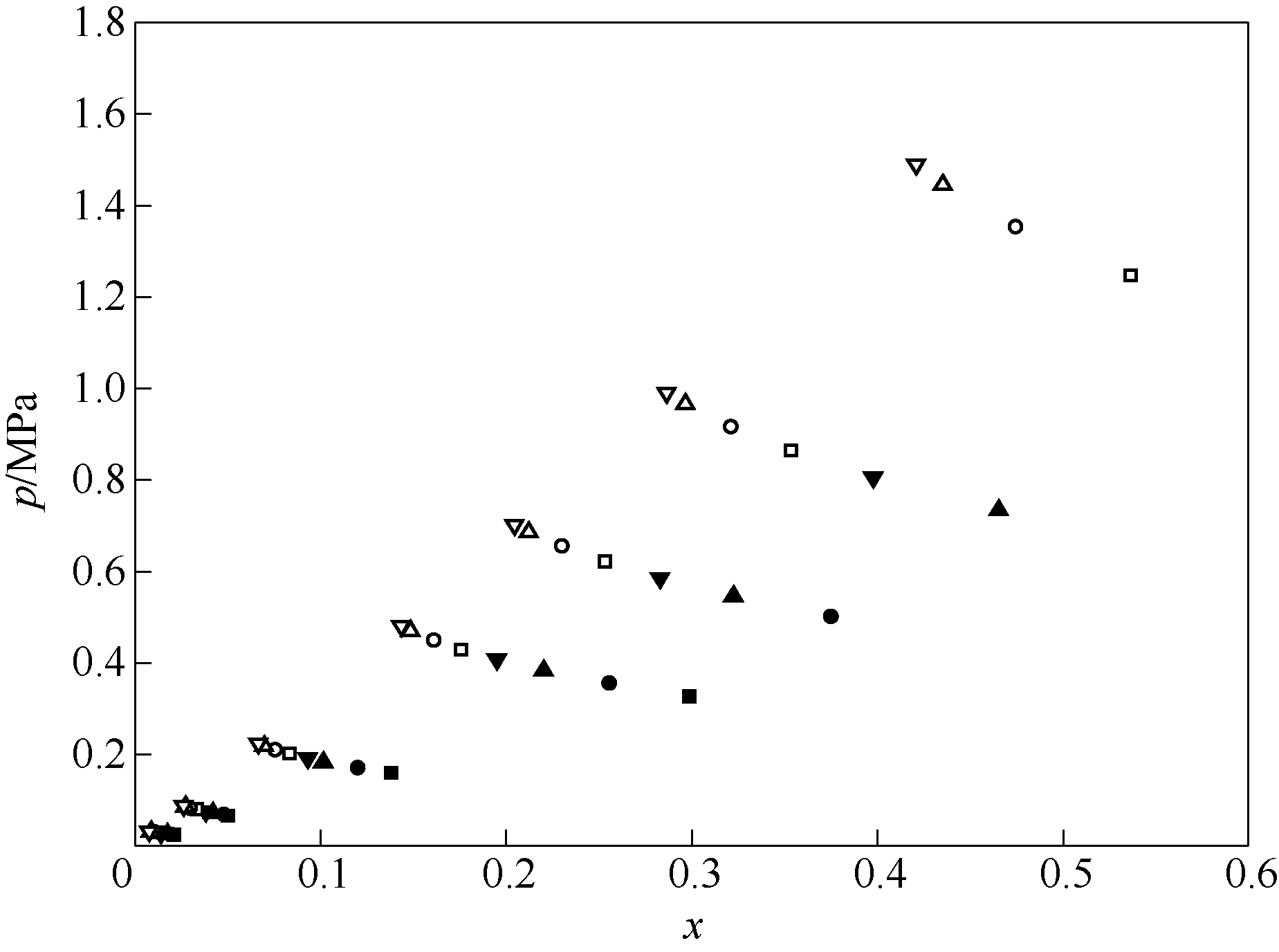
Fig.3 Experimental results of solubility of HFO1234yf in squalane ◆—283.15 K;■—293.15 K;●—303.15 K;▲—313.15 K;▼—323.15 K;□—333.15 K;○—343.15 K;△—348.15 K
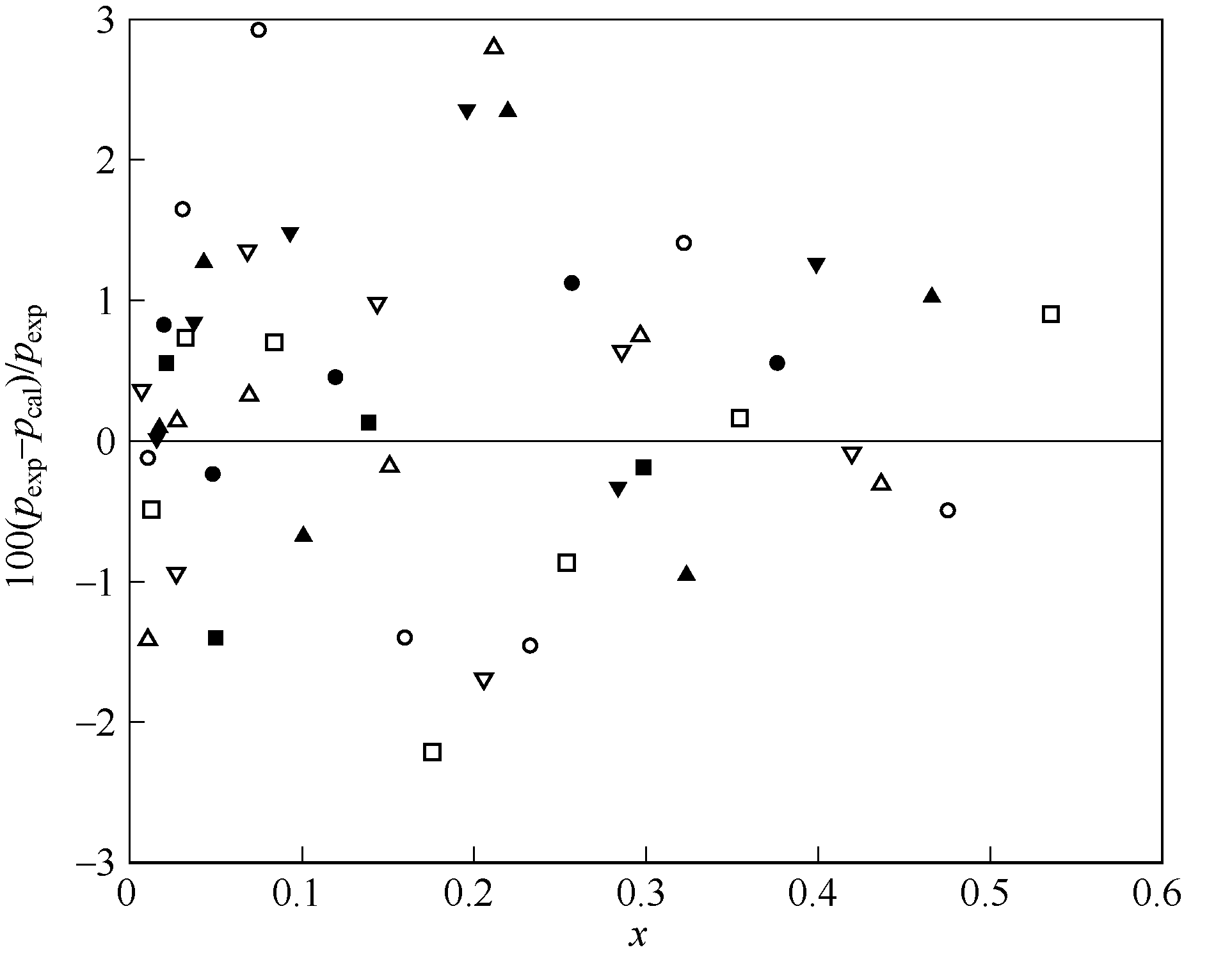
Fig.4 Relative deviation between experimental and calculated values of HFO1234yf in squalane ◆—283.15 K;■—293.15 K;●—303.15 K;▲—313.15 K; ▼—323.15 K;□—333.15 K;○—343.15 K;△—348.15 K
| 1 | Official Journal of the European Union . Directive 2006/40/EC of the European parliament and of the council of 17 May 2006 relating to emissions from air-conditioning systems in motor vehicles and amending council directive 70/156/EEC [R]. European Union: OJ, 2006. |
| 2 | Official Journal of the European Union . Regulation(EU) No. 517/2014 of the European parliament and of the council of 16 April 2014 on fluorinated greenhouse gases and repealing regulation (EC) No 842/2006 [R]. European Union: OJ, 2014. |
| 3 | EI-Sayed A R , Morsi M EI , Mahmoud N A . A review of the potential replacements of HCFC/HFCs using environmental friendly refrigerants[J]. International Journal of Air-Conditioning and Refrigeration, 2018, 26: 18300021-183000224. |
| 4 | Tashtoush B , Younes M B . Comparative thermodynamic study of refrigerants to select the best environment-friendly refrigerant for use in a solar ejector cooling system[J]. Arab. J. Sci. Eng., 2019, 44: 1165-1184. |
| 5 | 孟照峰, 张华, 秦延斌, 等 . R1234yf/R134a混合物在汽车空调中替代R134a的实验研究[J]. 化工学报, 2019, 69(6): 2396-2403. |
| Meng Z F , Zhang H , Qin Y B , et al . Experimental study on R1234yf/R134a mixture as alternative to R134a in automobile air conditioner[J]. CIESC Journal, 2019, 69(6): 2396-2403. | |
| 6 | Calm J M . The next generation of refrigerants historical review consideration and outlook[J]. Int. J. Refrig., 2008, 31: 1123-1133. |
| 7 | 杨富方, 段远源, 杨震 . R1234yf + CO2和R1234ze(E) + CO2二元混合物的比容平移Soave-Redlich-Kwong方程[J]. 化工学报, 2016, 67(S2): 14-19. |
| Yang F F , Duan Y Y , Yang Z . Volume translation Soave-Redlich-Kwong equation of state for binary mixtures of R1234yf + CO2 and R1234ze(E) + CO2 [J]. CIESC Journal, 2016, 67(S2): 14-19. | |
| 8 | Tanaka K , Higashi Y . Thermodynamic properties of HFO-1234yf (2,3,3,3-tetrafluoropropene)[J]. Int. J. Refrig., 2010, 33: 474-479. |
| 9 | 邱金友, 张华, 余晓明, 等 . 新型制冷剂R1234ze(E)水平圆管内流动沸腾换热特性[J]. 化工学报, 2016, 67(6): 2255-2262. |
| Qiu J Y , Zhang H , Yu X M , et al . Flow boiling heat transfer characteristic of refrigerant R1234ze(E) in horizontal circular tube[J]. CIESC Journal, 2016, 67(6): 2255-2262. | |
| 10 | Marsh K N , Kandil M E . Review of thermodynamic properties of refrigerants + lubricant oils[J]. Fluid Phase Equilib., 2002, 199: 319-334. |
| 11 | 金梧凤, 于斌, 高攀, 等 . R32与新型PVE 油的互溶性及其对空调性能的影响[J]. 化工学报, 2018, 69(4): 1631-1637. |
| Jin W F , Yu B , Gao P , et al . Effect of solubility between R32 and new PVE oil on performance of air conditioning system[J]. CIESC Journal, 2018, 69(4): 1631-1637. | |
| 12 | Shen B , Groll E . Review article: a critical review of the influence of lubricants on the heat transfer and pressure drop of refrigerants (Ⅰ): Lubricant influence on pool and flow boiling[J]. HVAC & R Research, 2005, 11:341-359. |
| 13 | Chang Y N , Nagashima A . Effect of dissolved lubricating oils on the viscosity of alternative refrigerants [J]. Int. J. Thermophys., 1993, 14: 1007-1019. |
| 14 | 田田, 杨昭, 吴曦, 等 . RE170、RE170/R227ea 与矿物油的互溶性评价[J]. 化工学报, 2015, 66(6): 2005-2010. |
| Tian T , Yang Z , Wu X , et al . Miscibility evaluation of RE170 and RE170/R227ea with a mineral oil[J]. CIESC Journal, 2015, 66(6): 2005-2010. | |
| 15 | Wang X P , Sun Y J , Gong N . Experimental investigations for the phase equilibrium of R1234yf and R1234ze(E) with two linear chained pentaerythritol esters[J]. Int. J. Thermophys., 2016, 92: 66-71. |
| 16 | Bobbo S , Zilio C , Scattolini M , et al . R1234yf as a substitute of R134a in automotive air conditioning. Solubility measurements in two commercial PAG oils [J]. Int. J. Refrig., 2014, 40: 302-308. |
| 17 | Marcelino Neto M A , Franca R M , Barbosa J R . Convection-driven absorption of R-1234yf in lubricating oil[J]. Int. J. Refrig., 2014, 44: 151-160. |
| 18 | Sun Y J , Wang X P , Gong N , et al . Solubility of trans-1,3,3,3-tetrafluoropropene (R1234ze(E)) in pentaerythritol ester heptanoic aid (PEC7) and in pentaerythritol tetranonanoate (PEC9) between 283.15 K and 353.15 K[J]. Fluid Phase Equilib., 2015, 387: 154-159. |
| 19 | Sun Y J , Wang X P , Gong N , et al . Solubility of trans-1,3,3,3-tetrafluoroprop-1-ene in pentaerythritol tetrapentanoate (PEC5) in the temperature from 283.15 to 353.15 K[J]. Int. J. Refrig., 2014, 48: 114-120. |
| 20 | Wang X P , Sun Y J , Kang K . Experimental investigation for the solubility of R1234ze(E) in pentaerythritol tetrahexanoate and pentaerythritol tetraoctanoate[J]. Fluid Phase Equilib., 2015, 400: 38-42. |
| 21 | Sun, Y J, Wang X P , Gong N , et al . Solubility of dimethyl ether in pentaerythritol tetrahexanoate (PEC6) and in pentaerythritol trtraoctanoate(PEC8) between (283.15 and 353.15) K[J]. J. Chem. Eng. Data, 2014, 59: 3791-3797. |
| 22 | Sun Y J , Wang X P , Gong N , et al . Solubility of dimethyl ether in pentaerythritol tetrabutyrate and in pentaerythritol tetrapentanoate. Comparison with other pentaerythritol tetraalkyl esters[J]. Int. J. Thermophys., 2015, 87: 23-28. |
| 23 | Fandino O , Pensado A S , Lugo L , et al . Compressed liquid densities of squalane and pentaerythritol tetra-(2-ethylhexanoate) [J]. J. Chem. Eng. Data, 2005, 50: 939-946. |
| 24 | Lemmon E W , Huber M L , McLinden, M O . NIST Reference Fluid Thermodynamic and Transport Properties - REFPROP, Version 9.1 [CP]. National Institute of Standards and Technology: Boulder, CO, 2013. |
| 25 | Peng D Y , Robinson D B . A new two-constant equation of state[J]. Ind. Eng. Chem., Fundam., 1976, 15: 59-64. |
| 26 | VonNiederhausern D M , Wilson G M , Giles N F . Critical point and vapor pressure measurements at high temperatures by means of a new apparatus with ultralow residence times[J]. J. Chem. Eng. Data, 2000, 45: 157-160. |
| 27 | Nikitin E D , Popov A P . Vapor-liquid critical properties of squalane measured by the pulse-heating technique[J]. Fluid Phase Equilib., 2005, 237: 16-20. |
| 28 | Orbey H , Sandler S I . On the combination of equation of state and excess free energy model[J]. Fluid Phase Equilib., 1995, 111: 53-70. |
| 29 | Orbey H , Sandler S I . A comparison of Huron-Vidal type mixing rules of mixtures of compounds with large size differences, and a new mixing rule[J]. Fluid Phase Equilib., 1997, 132: 1-14. |
| 30 | Renon H , Prausnitz J M . Local compositions in thermodynamic excess functions for liquid mixtures[J]. AIChE Journal, 1968, 14: 135-144. |
| [1] | Chao HU, Yuming DONG, Wei ZHANG, Hongling ZHANG, Peng ZHOU, Hongbin XU. Preparation of high-concentration positive electrolyte of vanadium redox flow battery by activating vanadium pentoxide with highly concentrated sulfuric acid [J]. CIESC Journal, 2023, 74(S1): 338-345. |
| [2] | Xianheng YI, Wu ZHOU, Xiaoshu CAI, Tianyi CAI. Measurable range of nanoparticle concentration using optical fiber backward dynamic light scattering [J]. CIESC Journal, 2023, 74(8): 3320-3328. |
| [3] | Xudong YU, Qi LI, Niancu CHEN, Li DU, Siying REN, Ying ZENG. Phase equilibria and calculation of aqueous ternary system KCl + CaCl2 + H2O at 298.2, 323.2, and 348.2 K [J]. CIESC Journal, 2023, 74(8): 3256-3265. |
| [4] | Ke CHEN, Li DU, Ying ZENG, Siying REN, Xudong YU. Phase equilibria and calculation of quaternary system LiCl+MgCl2+CaCl2+H2O at 323.2 K [J]. CIESC Journal, 2023, 74(5): 1896-1903. |
| [5] | Junxian CHEN, Zhongli JI, Yu ZHAO, Qian ZHANG, Yan ZHOU, Meng LIU, Zhen LIU. Study on online detection method of particulate matter in natural gas pipeline based on microwave technology [J]. CIESC Journal, 2023, 74(3): 1042-1053. |
| [6] | Yuanjing MAO, Zhi YANG, Songping MO, Hao GUO, Ying CHEN, Xianglong LUO, Jianyong CHEN, Yingzong LIANG. Estimation of SAFT-VR Mie equation of state parameters and thermodynamic properties of C6—C10 alcohols [J]. CIESC Journal, 2023, 74(3): 1033-1041. |
| [7] | Jingbo GAO, Qiang SUN, Qing LI, Yiwei WANG, Xuqiang GUO. Hydrate equilibrium model of hydrogen-containing gas considering hydrates structure transformation [J]. CIESC Journal, 2023, 74(2): 666-673. |
| [8] | Wenting CHENG, Jie LI, Li XU, Fangqin CHENG, Guoji LIU. Experiment and prediction for the solubility of AlCl3·6H2O in FeCl3, CaCl2, KCl and KCl-FeCl3 solutions [J]. CIESC Journal, 2023, 74(2): 642-652. |
| [9] | Jin CAI, Xiaohui WANG, Han TANG, Guangjin CHEN, Changyu SUN. Prediction of the phase equilibrium of semi-clathrate hydrate in TBAB aqueous solution [J]. CIESC Journal, 2023, 74(1): 408-415. |
| [10] | Huan ZHOU, Mengli ZHANG, Qing HAO, Si WU, Jie LI, Cunbing XU. Process mechanism and dynamic behaviors of magnesium sulfate type carnallite converting into kainite [J]. CIESC Journal, 2022, 73(9): 3841-3850. |
| [11] | Yongqian WANG, Ping WANG, Kang CHENG, Chenlin MAO, Wenfeng LIU, Zhicheng YIN, Antonio Ferrante. Stability and NO production of lean premixed ammonia/methane turbulent swirling flame [J]. CIESC Journal, 2022, 73(9): 4087-4094. |
| [12] | Qian LIU, Xianglan ZHANG, Zhiping LI, Yulong LI, Mengxing HAN. Screening of deep eutectic solvents and study on extraction performance for oil-hydroxybenzene separation [J]. CIESC Journal, 2022, 73(9): 3915-3928. |
| [13] | Yuxin REN, Runfeng XU, Wanying WANG, Pengzhong CHEN, Xiaojun PENG. Synthesis and stability study of anthraquinone dyes for color photoresist [J]. CIESC Journal, 2022, 73(5): 2251-2261. |
| [14] | Zirui WU, Rui SUN, Lingfeng SHI, Hua TIAN, Xuan WANG, Gequn SHU. A comparative and predictive study of the mixing rules for the vapor-liquid equilibria of CO2-based mixtures [J]. CIESC Journal, 2022, 73(4): 1483-1492. |
| [15] | Biqiang LIU, Haishan CAO. Adsorption measurement method based on flow calibration and its error analysis [J]. CIESC Journal, 2022, 73(4): 1597-1605. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||