CIESC Journal ›› 2020, Vol. 71 ›› Issue (7): 3313-3321.DOI: 10.11949/0438-1157.20191566
• Energy and environmental engineering • Previous Articles Next Articles
Youjian ZHU1( ),Xianxian ZHANG1,Yiming CHEN1,Xuehong WU1,Haiping YANG2,Hanping CHEN2(
),Xianxian ZHANG1,Yiming CHEN1,Xuehong WU1,Haiping YANG2,Hanping CHEN2( )
)
Received:2019-12-23
Revised:2020-03-22
Online:2020-07-05
Published:2020-07-05
Contact:
Hanping CHEN
朱有健1( ),张显显1,陈奕名1,吴学红1,杨海平2,陈汉平2(
),张显显1,陈奕名1,吴学红1,杨海平2,陈汉平2( )
)
通讯作者:
陈汉平
作者简介:朱有健(1987—),男,博士,讲师,基金资助:CLC Number:
Youjian ZHU, Xianxian ZHANG, Yiming CHEN, Xuehong WU, Haiping YANG, Hanping CHEN. Effect of calcium phosphate monobasic on ash fusion and sintering characteristics of cornstalk[J]. CIESC Journal, 2020, 71(7): 3313-3321.
朱有健, 张显显, 陈奕名, 吴学红, 杨海平, 陈汉平. 磷酸二氢钙对玉米秆灰熔融烧结特性的影响研究[J]. 化工学报, 2020, 71(7): 3313-3321.
Add to citation manager EndNote|Ris|BibTeX
| Sample | Proximate analysis/%(mass, db) | Ultimate analysis/%(mass, db) | HHV/(MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | FC | C | H | N | S | O | ||
| CS | 6.74 | 75.15 | 18.11 | 43.71 | 6.02 | 0.78 | 0.15 | 42.60 | 17.83 |
Table 1 Proximate, ultimate analysis and HHV of CS
| Sample | Proximate analysis/%(mass, db) | Ultimate analysis/%(mass, db) | HHV/(MJ/kg) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A | V | FC | C | H | N | S | O | ||
| CS | 6.74 | 75.15 | 18.11 | 43.71 | 6.02 | 0.78 | 0.15 | 42.60 | 17.83 |
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl2 | K2O | CaO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|
| 3.96 | 11.39 | 3.89 | 29.05 | 3.41 | 3.01 | 11.25 | 23.58 | 9.68 | 0.78 |
Table 2 Chemical composition of CS ash/%(mass)
| Na2O | MgO | Al2O3 | SiO2 | P2O5 | SO3 | Cl2 | K2O | CaO | Fe2O3 |
|---|---|---|---|---|---|---|---|---|---|
| 3.96 | 11.39 | 3.89 | 29.05 | 3.41 | 3.01 | 11.25 | 23.58 | 9.68 | 0.78 |
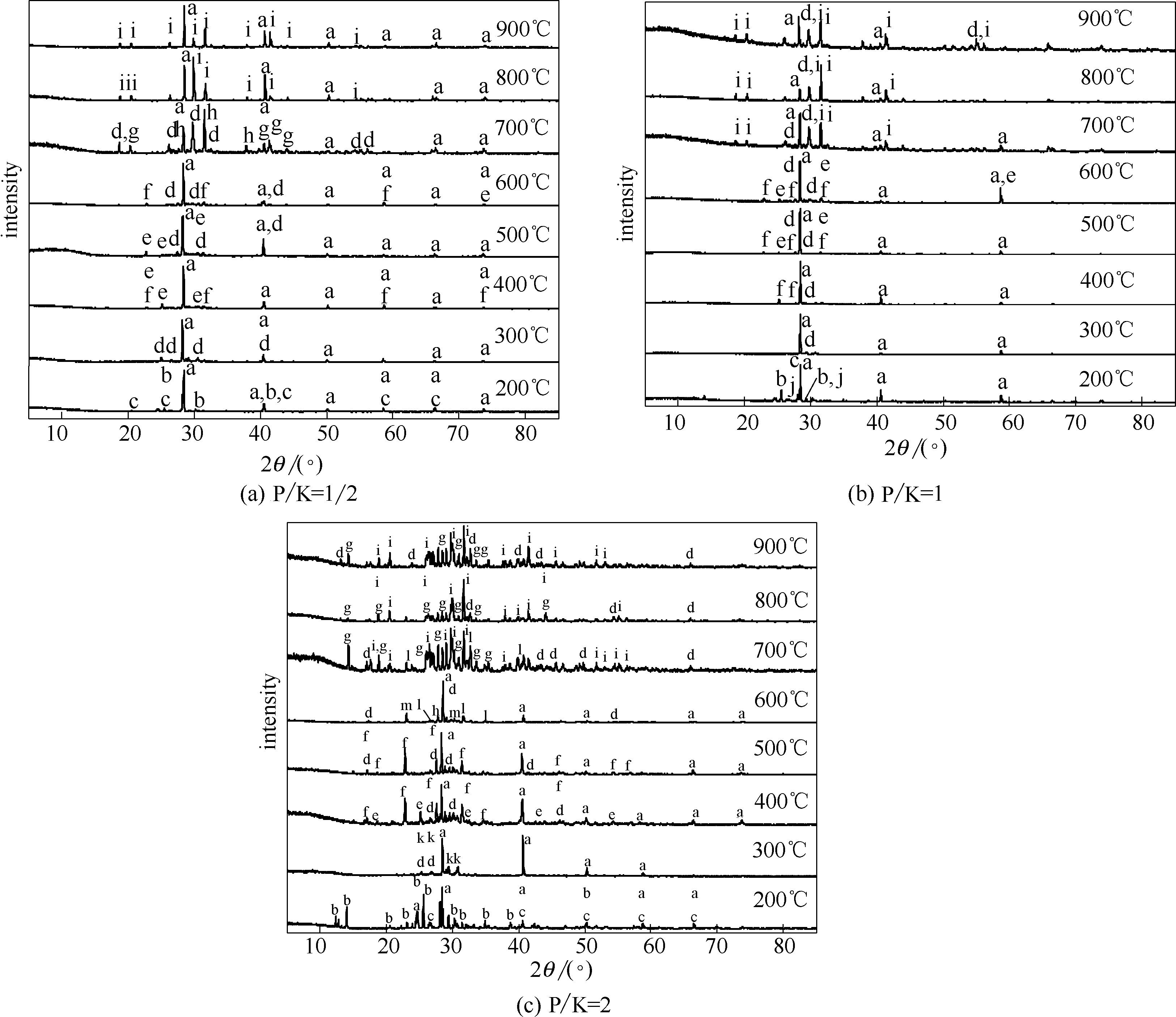
Fig.3 XRD patterns of reaction product of CPM and KCla—KCl; b—Ca(H2PO4)2; c—CaHPO4; d—Ca2P2O7; e—Ca(PO3)2; f—KCa(PO3)3; g—Ca2KP3O10; h—Ca5(PO4)3Cl; i—K2CaP2O7; j—KH2PO4; k—K2H2P2O7·0.5H2O; l—(KPO3)n; m—Ca3(PO4)2

Fig.4 XRD patterns of reaction product of CPM and K2CO3a—K2CO3·1.5H2O; b—CaHPO4; c—Ca(OH)2; d—Ca2P2O7; e—Ca2KP3O10; f—KCa(PO3)3; g—Ca3(PO4)2; h—K2CaP2O7; i—KCaPO4; j—Ca(H2PO4)2; k—K2HPO4; l—Ca(PO3)2; m—(KPO3)n
| Sample | Temperature/℃ | |||
|---|---|---|---|---|
| DT | ST | HT | FT | |
| CS | 963 | 977 | 995 | 1036 |
| CS+CPM1/2 | 1027 | 1181 | 1216 | 1259 |
| CS+CPM1 | 1146 | 1219 | 1226 | 1272 |
| CS+CPM2 | 1172 | 1359 | 1435 | 1446 |
Table 3 Ash fusion characteristic temperature of samples
| Sample | Temperature/℃ | |||
|---|---|---|---|---|
| DT | ST | HT | FT | |
| CS | 963 | 977 | 995 | 1036 |
| CS+CPM1/2 | 1027 | 1181 | 1216 | 1259 |
| CS+CPM1 | 1146 | 1219 | 1226 | 1272 |
| CS+CPM2 | 1172 | 1359 | 1435 | 1446 |

Fig.8 SEM image of morphologies of ash samplesLi H Q, Wang C A, Zhu C Z, et al. Influence of oxy-fuel atmosphere on melting behavior and microscopic physicochemical properties of Zhundong coal ash[J]. CIESC Journal, 2018, 69(6): 2632-2638.[3] Niu Y, Tan H, Hui S E. Ash-related issues during biomass combustion: alkali-induced slagging, silicate melt-induced slagging (ash fusion), agglomeration, corrosion, ash utilization, and related countermeasures[J]. Progress in Energy & Combustion Science, 2016, 52: 1-61.[4] Wang L, Hustad J E, Skreiberg ?, et al. A critical review on additives to reduce ash related operation problems in biomass combustion applications[J]. Energy Procedia, 2012, 20: 20-29.[5] Yan T, Bai J, Kong L, et al. Effect of SiO2/Al2O3 on fusion behavior of coal ash at high temperature[J]. Fuel, 2017, 193: 275-283.[6] Yang W, Zhu Y, Cheng W, et al. Effect of minerals and binders on particulate matter emission from biomass pellets combustion[J]. Applied Energy, 2018, 215: 106-115.[7] Wang G, Jensen P A, Hao W, et al. Potassium capture by kaolin(2): K2CO3, KCl and K2SO4[J]. Energy & Fuels, 2018, 32(3): 3566-3578.[8] Dan B, Skoglund N, Grimm A, et al. Ash transformation chemistry during combustion of biomass[J]. Energy & Fuels, 2012, 26(26): 85-93.Wang Q, Han K H, Li H, et al. Influence of ammonium dihydrogen phosphates additive on potassium fixation capacity and ash fusibility for rice straw combustion in an O2 /CO2 atmosphere[J]. Journal of Fuel Chemistry and Technology, 2015, 43(8): 955-960.Han K H, Qi J H, Li H, et al. Simulation and experiments of removal process of gaseous KCl by ammonium dihydrogen phosphate[J]. CIESC Journal, 2014, 65(3): 1093-1098.Li L N, Ren Q Q, Li S Y, et al. Behavior of alkali metals during combustion of wheat straw with phosphorus-rich additives[J]. Proceedings of the CSEE, 2013, 33(26): 41-47.[12] Grimm A, Skoglund N, Bostr?m D, et al. Influence of phosphorus on alkali distribution during combustion of logging residues and wheat straw in a bench-scale fluidized bed[J]. Energy & Fuels, 2012, 26(5): 3012-3023.[13] Zhu Y, Fan J, Yang P, et al. P-based additive for reducing fine particulate matter emissions during agricultural biomass combustion[J]. Energy & Fuels, 2019, 33(11): 11274-11284.[14] Wang Q, Han K, Wang J, et al. Influence of phosphorous based additives on ash melting characteristics during combustion of biomass briquette fuel[J]. Renewable Energy, 2017, 113: 428-437.[15] Werkelin J, Skrifvars B J, Zevenhoven M, et al. Chemical forms of ash-forming elements in woody biomass fuels[J]. Fuel, 2010, 89(2): 481-493.Meng X X, Sun R, Yuan H, et al. Effect of different pyrolysis temperature on alkali metal K and Na emission and existence in semi-char[J]. CIESC Journal, 2017, 68(4): 338-345.[17] van Loo S, Koppejan J. The Handbook of Biomass Combustion and Co-Firing[M]. Earthscan, 2012.[18] Zhu Y, Hu J, Yang W, et al. The ash fusion characteristics and transformation behaviors during bamboo combustion in comparison with straw and poplar[J]. Energy & Fuels, 2018, 32: 5244-5251.[19] Liang W, Skjevrak G, Hustad J E, et al. Investigation of biomass ash sintering characteristics and the effect of additives[J]. Applied Energy, 2015, 28(1): 208-218.[20] Pyldme M, Tynsuaadu K, Paulik F, et al. Dehydrations of Ca(H2PO4)2·H2O and Mg(H2PO4)2·H2O and their reactions with KCl, examined with simultaneous TG, DTF, DTA and EGA[J]. Journal of Thermal Analysis, 1979, 17(2): 479-488.[21] Knudsen J N, Jensen P A, Dam-Johansen K. Transformation and release to the gas phase of Cl, K, and S during combustion of annual biomass[J]. Energy & Fuels, 2004, 18(5): 1385-1399.[22] Tynsuaadu K. Influence of silicic acid and glauconite on thermal dehydration of Ca(H2PO4)2·H2O [J]. Journal of Thermal Analysis, 1990, 36(5): 1785-1793.[23] Larson H W E. Preparation and properties of mono-, di-, and tricalcium phosphates[J]. Industrial & Engineering Chemistry Analytical Edition, 1935, 7(6): 401-406.[24] Wang L, Hustad J E, Gr?nli M. Sintering characteristics and mineral transformation behaviors of corn cob ashes[J]. Energy Fuels, 2012, 26(9): 5905-5916.Li L N, Ren Q Q, Lyu Q G, et al. Behavior of potassium during co-combustion of municilal sewage sludge with wheat straw[J]. Journal of Engineering Thermophysics, 2013, 34(6): 1166-1169.
| Sample | Spot | Na | Mg | Al | Si | P | S | Cl | K | Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| CS | C1 | 0 | 6.2 | 1.3 | 49.7 | 3.7 | 0 | 3.7 | 31.5 | 3.9 |
| C2 | 1.1 | 22.1 | 0.2 | 35.5 | 7.5 | 0.6 | 0.6 | 2.2 | 30.3 | |
| C3 | 2.3 | 8.6 | 1.2 | 51.7 | 4.8 | 0 | 0.0 | 26.4 | 5.0 | |
| CS+CPM | P1 | 2.4 | 9.7 | 0.6 | 2.8 | 37.1 | 0 | 2.3 | 12.3 | 32.9 |
| P2 | 2.4 | 12.5 | 0 | 2.42 | 39.0 | 0.7 | 0 | 14.2 | 28.9 | |
| P3 | 3.1 | 9.3 | 1.7 | 21.8 | 27.4 | 0 | 0 | 16.1 | 20.5 |
Table 4 EDS analysis of typical ash particle/%(mol)
| Sample | Spot | Na | Mg | Al | Si | P | S | Cl | K | Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| CS | C1 | 0 | 6.2 | 1.3 | 49.7 | 3.7 | 0 | 3.7 | 31.5 | 3.9 |
| C2 | 1.1 | 22.1 | 0.2 | 35.5 | 7.5 | 0.6 | 0.6 | 2.2 | 30.3 | |
| C3 | 2.3 | 8.6 | 1.2 | 51.7 | 4.8 | 0 | 0.0 | 26.4 | 5.0 | |
| CS+CPM | P1 | 2.4 | 9.7 | 0.6 | 2.8 | 37.1 | 0 | 2.3 | 12.3 | 32.9 |
| P2 | 2.4 | 12.5 | 0 | 2.42 | 39.0 | 0.7 | 0 | 14.2 | 28.9 | |
| P3 | 3.1 | 9.3 | 1.7 | 21.8 | 27.4 | 0 | 0 | 16.1 | 20.5 |
| 1 | Lubwama M, Yiga V A, Kalogirou S A, et al. Characteristics of briquettes developed from rice and coffee husks for domestic cooking applications in Uganda[J]. Renewable Energy, 2018, 118: 43-55. |
| 2 | 李汉卿, 王长安, 朱晨钊, 等. O2/CO2气氛对准东煤灰熔融行为和微观理化特性的影响[J]. 化工学报, 2018, 69(6): 2632-2638. |
| [1] | Chenxi LI, Yongfeng LIU, Lu ZHANG, Haifeng LIU, Jin’ou SONG, Xu HE. Quantum chemical analysis of n-heptane combustion mechanism under O2/CO2 atmosphere [J]. CIESC Journal, 2023, 74(5): 2157-2169. |
| [2] | Jin YU, Binbin YU, Xinsheng JIANG. Study on quantification methodology and analysis of chemical effects of combustion control based on fictitious species [J]. CIESC Journal, 2023, 74(3): 1303-1312. |
| [3] | Jiachen SUN, Chunlei PEI, Sai CHEN, Zhijian ZHAO, Shengbao HE, Jinlong GONG. Advances in chemical-looping oxidative dehydrogenation of light alkanes [J]. CIESC Journal, 2023, 74(1): 205-223. |
| [4] | Yongqian WANG, Ping WANG, Kang CHENG, Chenlin MAO, Wenfeng LIU, Zhicheng YIN, Antonio Ferrante. Stability and NO production of lean premixed ammonia/methane turbulent swirling flame [J]. CIESC Journal, 2022, 73(9): 4087-4094. |
| [5] | Nini YUAN, Tuo GUO, Hongcun BAI, Yurong HE, Yongning YUAN, Jingjing MA, Qingjie GUO. Reaction process of CH4 on the surface of Fe2O3/Al2O3 oxygen carrier in chemical looping combustion: ReaxFF-MD simulation [J]. CIESC Journal, 2022, 73(9): 4054-4061. |
| [6] | Kaihong TANG, Xiaofeng HE, Guiqiu XU, Yang YU, Xiaofeng LIU, Tiejun GE, Ailing ZHANG. Review on combustion behavior and flame retardant research of phenolic foams [J]. CIESC Journal, 2022, 73(8): 3483-3500. |
| [7] | Zhidong LI, Jiaqi WAN, Ying LIU, Yixi TANG, Wei LIU, Zhongxian SONG, Xuejun ZHANG. α-MnO2/β-MnO2 catalysts synthesized by one-pot method and their catalytic performance for the oxidation of toluene [J]. CIESC Journal, 2022, 73(8): 3615-3624. |
| [8] | Xinhua LIU, Zhennan HAN, Jian HAN, Bin LIANG, Nan ZHANG, Shanwei HU, Dingrong BAI, Guangwen XU. Principle and technology of low-NO x decoupling combustion based on restructuring reactions [J]. CIESC Journal, 2022, 73(8): 3355-3368. |
| [9] | Cong HE, Wenqi ZHONG, Guanwen ZHOU, Xi CHEN. Study on decomposition characteristics of cement raw meal in suspension furnace at high altitude [J]. CIESC Journal, 2022, 73(5): 2120-2129. |
| [10] | Xue LI, Ming DONG, Huang ZHANG, Jun XIE. Kinetic characteristics of micro-particle impact on a flat surface under humidity conditions [J]. CIESC Journal, 2022, 73(5): 1940-1946. |
| [11] | Yulun ZHANG, Changkun CHEN, Peng LEI. Experimental study on combined burning characteristics of soaked porous media sand bed under different combustible liquid layer heights [J]. CIESC Journal, 2022, 73(4): 1826-1833. |
| [12] | Xuan LIU, Yinjiao SU, Yang TENG, Kai ZHANG, Pengcheng WANG, Lifeng LI, Zhen LI. Selenium transformation in ultra-low-emission coal-fired power units and its enrichment characteristics in fly ash [J]. CIESC Journal, 2022, 73(2): 923-932. |
| [13] | Haolong BAI, Liangliang FU, Guangwen XU, Dingrong BAI. Characteristics of gaseous nitrogen release in coal fluidized bed combustion under different atmospheres [J]. CIESC Journal, 2022, 73(2): 876-886. |
| [14] | Junyi LUO, Shiliang WU, Rui XIAO. Study on combustion characteristics of cycloalkanes mixed with aviation kerosene [J]. CIESC Journal, 2022, 73(2): 847-856. |
| [15] | Xiao YANG, Rui DING, Mohan LI, Zhengchang SONG. Effect of oxygen concentration on homogeneous/heterogeneous coupled reaction characteristics of methane in microchannel [J]. CIESC Journal, 2022, 73(12): 5427-5437. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||